Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11403
Peer-review started: May 8, 2022
First decision: July 12, 2022
Revised: July 26, 2022
Accepted: September 20, 2022
Article in press: September 20, 2022
Published online: November 6, 2022
Processing time: 171 Days and 16.4 Hours
Lipids increase the risk of sleep apnea; however, the causality between them is still inconclusive.
To explore the causal relationship between serum lipids and sleep apnea using two-sample Mendelian randomization (MR) analysis.
Single nucleotide polymorphism (SNP) data related to serum lipids were obtained from the Global Lipids Genetics Consortium study, which included 188578 individuals of European ancestry. Additionally, sleep apnea-related SNP data were collected from the United Kingdom Biobank study, which comprised 463005 individuals of European ancestry. Two-sample MR analysis was performed to assess the causality between serum lipids and sleep apnea based on the above public data.
Genetically predicted low-density lipoprotein (odds ratio [OR] = 0.99, 95% confidence interval [CI] = 0.99 to 1.00; P = 0.58), high-density lipoprotein (OR = 0.99, 95%CI = 0.99 to 1.00; P = 0.91), triglyceride (OR = 1.00, 95%CI = 0.99 to 1.00; P = 0.92), and total cholesterol (OR = 0.99, 95%CI = 0.99 to 1.00; P = 0.33) were causally unrelated to sleep apnea.
Our MR analysis suggests that genetically predicted serum lipids are not risk factors of sleep apnea.
Core Tip: This study had a couple of key advantages. First, compared with other observational studies, the genetic variants can be obtained from different sample of individuals, and genetic associations can be obtained from large genome-wide association studies, which can greatly improve the statistical ability to detect small effects of complex phenotypes. Second, the study excluded more confounding factors, heterogeneity and level pleiotropy, and conducted sensitivity tests to make our results more convincing.
- Citation: Zhang LP, Zhang XX. Relationship between lipids and sleep apnea: Mendelian randomization analysis. World J Clin Cases 2022; 10(31): 11403-11410
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11403.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11403
Snoring during sleep is accompanied by apnea and shallow breathing, resulting in intermittent hypoxemia. Sleep apnea (SA) is a complex disease complicated by cardiovascular diseases such as coronary syndrome, hypertension, congestive heart failure, arrhythmia, and pulmonary hypertension, and neuropsychiatric dysfunction such as inattention, memory, and cognitive impairment. In addition, the incidence of insulin resistance and metabolic disorder is also higher in patients with than without SA[1-5]. As reported, SA can lead to dyslipidemia, obesity, and metabolic syndrome[6].
Traditional studies believe that SA is mainly linked to the anatomical structure of the upper airway[7]. Recent epidemiological studies have demonstrated that male and obesity are the main risk factors for SA[6]. In the past, there has been discussion about how SA affects serum lipids, and several studies have shown that the levels of dyslipidemia including low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), and total cholesterol (TC) increase in patients with obstructive SA (OSA)[8-10]. Nevertheless, it is rarely discussed whether serum lipids can be risk factors for SA. Understanding the effect of serum lipids on SA may assist in reducing relevant risk factors and providing novel ideas for the intervention of SA.
The critical risk factors of OSA are obesity and high body mass index (BMI), which are both associated with abnormal lipid metabolism. However, it remains unclear whether lipids may be directly correlated with OSA. We assumed that there is a correlation between them and analyzed their correlation using the Mendelian randomization (MR) method. MR, a newly developed research method, uses genetic variants as instrumental variables to investigate whether a risk factor causally afflicts a health outcome and is possible to avoid confounding factors in observational studies and clinical trials[11,12].
In our research, LDL, HDL, TG, and TC were utilized as representative lipid markers to probe the causal relationship between lipids and SA.
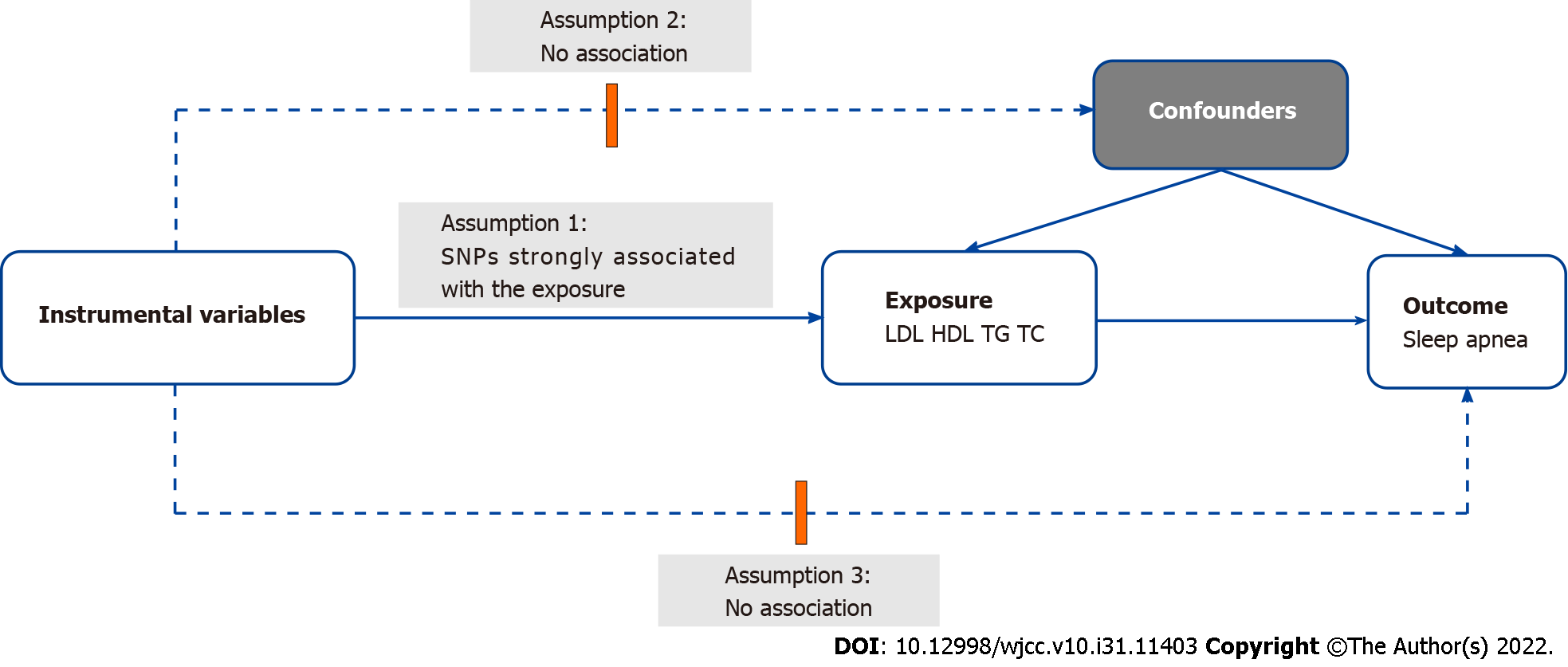
Our data were sourced from published data. The statistical data for genetically predicted LDL (n = 1730820), HDL (n = 187167), TG (n = 177861), and TC (n = 187365) were obtained from the Global Lipids Genetics Consortium study that summarized 45 studies and incorporated 188578 individuals of European ancestry[13]. The outcome data for genetically predicted SA (n = 463010) were harvested from the United Kingdom Biobank study (Table 1). Afterwards, a two-sample MR analysis was conducted to investigate the causal relationship of serum lipids with SA. The complete experimental design is shown in Figure 1.
| Variable | Consortium | PMID | Population | Sex |
| Sleep apnea | UKB | - | European | Males and females |
| LDL-C | GLGC | 24097068 | European | Males and females |
| HDL-C | GLGC | 24097068 | European | Males and females |
| TG | GLGC | 24097068 | European | Males and females |
| TC | GLGC | 24097068 | European | Males and females |
To select appropriate instrumental variables, assumption 1 that instrumental variables are strongly correlated with exposure was first satisfied according to our experimental design. Subsequent to separate extraction of SNPs associated with LDL, HDL, TG, and TC with genome-wide significance (P < 5 × 10−8), the clumping process (R2 < 0.001, window size = 10000 kb) was conducted to remove the linkage disequilibrium[14]. Second, assumption 2 was fulfilled to ensure no association between the instrumental SNPs and confounding factors (BMI, male sex, and obesity were identified as confounders[15] in this study). SNPs related to confounding factors were excluded using a website (http://www.phenoscanner.medschl.cam.ac.uk/). Last, SNPs related to outcomes were also eliminated by the aforementioned website to meet assumption 3.
An inverse variance weighted (IVW) meta-analysis was carried out to obtain an MR estimate. To enable a more reliable IVW approach, there was no evidence of targeted pleiotropy in the selected IVs (MR-Egger, P > 0.05)[16,17]. Other methods, including MR-Egger method, weighted median method, simple mode method, and weighted mode method, were also conducted to evaluate the stability of the results[18,19]. The weighted median method has the advantage that the results are consistent even when up to 50% of the information comes from invalid instrumental variables[18].
The Cochran’s Q test was utilized to assess the heterogeneity of individual genetic variability we estimated. P value less than 0.05 was regarded as significant heterogeneity. The stability of the results was evaluated using the funnel diagram. Pleiotropy was not found by MR-Egger test. The leave-one-out sensitivity analysis was performed to observe whether the results changed after each SNP was eliminated. The results are presented in Supplementary Figures 1-4. The package “two-sample-MR” (version 0.5.6; Bristol, United Kingdom) in R (version 4.1.2; Vienna, Austria) was utilized in our analysis.
In MR, the IVW method manifested that the level of serum lipids including LDL (odds ratio [OR] = 0.99, 95% confidence interval [CI] = 0.99 to 1.00, P = 0.58), HDL (OR = 0.99, 95%CI = 0.99 to 1.00, P = 0.91), TG (OR = 1.00, 95%CI = 0.99 to 1.00, P = 0.92), and TC (OR = 0.99, 95%CI = 0.99 to 1.00, P = 0.33) was not causally associated with SA. Another four approaches were also applied including MR Egger, weighted median, simple mode, and weighted mode. No obvious heterogeneity and horizontal pleiotropy was observed. The detailed results are displayed in Tables 2 and 3. There was no evidence of heterogeneity in the IVW analysis as demonstrated by funnel plots (Supplementary Figures 5-8). The effect size of SNPs on exposure (serum lipids) and outcome (SA) was found in scatter plots (Supplemen
| Lipid | Method | Number of SNPs | P value | OR | 95%CI |
| LDL and Sleep apnea | MR Egger | 42 | 0.58 | 0.99 | 0.99-1.00 |
| Weighted median | 42 | 0.97 | 0.99 | 0.99-1.00 | |
| Inverse variance weighted | 42 | 0.85 | 1.00 | 0.99-1.00 | |
| Simple mode | 42 | 0.31 | 1.00 | 0.99-1.00 | |
| Weighted mode | 42 | 0.74 | 0.99 | 0.99-1.00 | |
| HDL and sleep apnea | MR Egger | 55 | 0.91 | 0.99 | 0.99-1.00 |
| Weighted median | 55 | 0.35 | 1.00 | 0.99-1.00 | |
| Inverse variance weighted | 55 | 0.29 | 1.00 | 0.99-1.00 | |
| Simple mode | 55 | 0.22 | 1.00 | 0.99-1.00 | |
| Weighted mode | 55 | 0.33 | 1.00 | 0.99-1.00 | |
| TG and Sleep apnea | MR Egger | 35 | 0.92 | 1.00 | 0.99-1.00 |
| Weighted median | 35 | 0.82 | 0.99 | 0.99-1.00 | |
| Inverse variance weighted | 35 | 0.57 | 1.00 | 0.99-1.00 | |
| Simple mode | 35 | 0.59 | 0.99 | 0.99-1.00 | |
| Weighted mode | 35 | 0.94 | 1.00 | 0.99-1.00 | |
| TC and Sleep apnea | MR Egger | 51 | 0.33 | 0.99 | 0.99-1.00 |
| Weighted median | 51 | 0.68 | 0.99 | 0.99-1.00 | |
| Inverse variance weighted | 51 | 0.82 | 1.00 | 0.99-1.00 | |
| Simple mode | 51 | 0.20 | 1.00 | 0.99-1.00 | |
| Weighted mode | 51 | 0.70 | 1.00 | 0.99-1.00 |
| Lipid | Heterogeneity test, inverse variance weighted | Level pleiotropy test | ||
| Q | df | P value | P value | |
| LDL | 33.04 | 41 | 0.807 | 0.459 |
| HDL | 67.98 | 54 | 0.096 | 0.408 |
| TG | 34.87 | 34 | 0.427 | 0.823 |
| TC | 48.34 | 50 | 0.540 | 0.247 |
This study carefully selected SNPs as effective instrumental variables and excluded known risk factors (obesity and high BMI, as well as other known risk factors including male neck circumference greater than 17 inches [43 cm], female neck circumference greater than 15 inches [38 cm], male sex, age over 50 years, and smoking[15]). MR analysis showed that genetically predicted LDL, HDL, TG, and TC had no causal relationship with SA.
Numerous studies have dissected the relationship between serum lipids and SA, but the results have been varied. Can et al[20] showed the higher levels of TC, LDL, TG, and apolipoprotein B in the OSA group than in the control group. A study of Japanese working men elucidated a positive correlation between the respiratory disorder index and TG[21]. Tan et al[22] noted that HDL was diminished and oxidized LDL was elevated in patients with OSA. In a study of patients in eastern China, the authors observed that LDL was independently associated with OSA[23]. The inconsistency of the above results may be related to the small sample size and the involvement of confounding factors.
Our results revealed that genetically predicted LDL, HDL, TG, and TC were not causally correlated with SA. This result can be explained by the following mechanisms. First, the deposition of excessive fat in the neck may increase airway resistance and resultant susceptibility to SA. Nonetheless, the study comparing the distribution of neck soft tissues and fat between normal men and women by magnetic resonance imaging elaborated that the difference of fat deposition might not substantially damage the anatomical structure of the airway[24]. Second, SA is a complex disease and not simple pathogenesis of mechanical load. The factors involved also consist of the neurohumoral and metabolic inflammatory environment[25,26]. Apneas and hypopneas are classified into obstructive or central types[27], with OSA as the most common type. In OSA, airway anatomy is critically implicated in the influence of airway collapsing pressure in patients with the hypotonic airway. However, some evidence illustrated that neuromuscular factors are a pivotal cause of airway collapse during sleep, including upper airway dilator dysfunction, increased chemical sensitivity, and low arousal threshold (premature sleep arousal contributes to unstable ventilation control)[28,29]. Dong et al[30] delved into the relationship between lipid accumulation products (LAPs) and OSA in patients with type 2 diabetes mellitus, which revealed that after the same confounding factors were adjusted, neither TG nor waist circumference, as constituents of LAPs, was signally associated with apnea-hypopnea indexes and OSA. BMI, visceral fat, and neck circumference are the principal predictors of clinical expression in OSA. After exclusion of these known risk factors, our calculated results unveiled that genetically predicted lipids did not directly correlate to SA. Multiple studies have concluded lipids as a risk factor for SA, most likely because dyslipidemia metabolism may cause obesity and obesity is the primary risk factor for OSA. However, the direct correlation between lipids and SA was discussed in our study based on big data research, not the real world. For the aforementioned reasons, there might not be a causal relationship between serum lipids and SA.
This study had several advantages. First, different from other observational studies, genetic variants can be collected from different samples of individuals and genetic associations can be attained from large genome-wide association studies, which can remarkably improve the statistical ability to detect the small effects of complex phenotypes[31]. Second, the present study excluded more confounding factors, heterogeneity and level pleiotropy, and conducted sensitivity tests to strengthen the conviction of our results.
This study also has limitations. First, the sample population included in our study was all from Europe. In this context, it cannot be confirmed that the same conclusion is obtained from non-European populations. In addition, in terms of the selection of serum lipid markers, only four markers were included in our research. It remains unknown whether other biomarkers are causally related to SA.
Genetically predicted LDL, HDL, TG, and TC may not have a causal relationship with SA. More pathogenesis of SA needs to be studied.
Obstructive sleep apnea (OSA) has a negative effect on serum lipids, but the relationship between serum lipids and OSA is still uncertain.
We explored the direct effect of serum lipids on OSA.
We observed that lipids are not related to OSA, and we need to further look for other markers to predict OSA in the future.
First, compared with other observational studies, the genetic variants can be obtained from different sample of individuals, and genetic associations can be obtained from large genome-wide association studies, which can greatly improve the statistical ability to detect small effects of complex phenotypes. Second, the study excluded more confounding factors, excluded heterogeneity and level pleiotropy, and conducted sensitivity tests to make our results more convincing.
In Mendelian randomization, the inverse variance weighted method manifested that the level of serum lipids including low-density lipoprotein (odds ratio [OR] = 0.99, 95% confidence interval [CI] = 0.99 to 1.00, P = 0.58), high-density lipoprotein (OR = 0.99, 95%CI = 0.99 to 1.00, P = 0.91), triglyceride (OR = 1.00, 95%CI = 0.99 to 1.00, P = 0.92), and total cholesterol (OR = 0.99, 95%CI = 0.99 to 1.00, P = 0.33) was not causally associated with sleep apnea (SA).
Through MR analysis, this study concludes that serum lipids are not associated with SA.
We need to find other markers to predict SA in the future.
| 1. | Wheaton AG, Perry GS, Chapman DP, Croft JB. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005-2008. Sleep. 2012;35:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Chen YH, Keller JK, Kang JH, Hsieh HJ, Lin HC. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med. 2013;9:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 3. | Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Gonzaga C, Bertolami A, Bertolami M, Amodeo C, Calhoun D. Obstructive sleep apnea, hypertension and cardiovascular diseases. J Hum Hypertens. 2015;29:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 6. | Nadeem R, Singh M, Nida M, Waheed I, Khan A, Ahmed S, Naseem J, Champeau D. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. 2014;10:475-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 580] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 8. | Barceló A, Barbé F, Llompart E, Mayoralas LR, Ladaria A, Bosch M, Agustí AG. Effects of obesity on C-reactive protein level and metabolic disturbances in male patients with obstructive sleep apnea. Am J Med. 2004;117:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med. 2007;175:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Trakada G, Steiropoulos P, Nena E, Gkioka T, Kouliatsis G, Pataka A, Sotiriou I, Anevlavis S, Papanas N, Bouros D. Plasma visfatin levels in severe obstructive sleep apnea-hypopnea syndrome. Sleep Breath. 2009;13:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res Synth Methods. 2019;10:486-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 1207] [Article Influence: 172.4] [Reference Citation Analysis (0)] |
| 12. | Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2788] [Cited by in RCA: 4154] [Article Influence: 180.6] [Reference Citation Analysis (0)] |
| 13. | Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson Å, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen LP, Magnusson PKE, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O'Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney ASF, Döring A, Elliott P, Epstein SE, Ingi Eyjolfsson G, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJP, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin SY, Lindström J, Loos RJF, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TVM, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stančáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YI, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin MR, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PEH, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BHR, Ordovas JM, Boerwinkle E, Palmer CNA, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Kathiresan S, Mohlke KL, Ingelsson E, Abecasis GR; Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2340] [Cited by in RCA: 2389] [Article Influence: 183.8] [Reference Citation Analysis (0)] |
| 14. | Park S, Lee S, Kim Y, Lee Y, Kang MW, Kim K, Kim YC, Han SS, Lee H, Lee JP, Joo KW, Lim CS, Kim YS, Kim DK. Atrial fibrillation and kidney function: a bidirectional Mendelian randomization study. Eur Heart J. 2021;42:2816-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 15. | Abbasi A, Gupta SS, Sabharwal N, Meghrajani V, Sharma S, Kamholz S, Kupfer Y. A comprehensive review of obstructive sleep apnea. Sleep Sci. 2021;14:142-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 16. | Yang J, Ferreira T, Morris AP, Medland SE; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium, Madden PA, Heath AC, Martin NG, Montgomery GW, Weedon MN, Loos RJ, Frayling TM, McCarthy MI, Hirschhorn JN, Goddard ME, Visscher PM. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44:369-375, S1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1280] [Cited by in RCA: 1243] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 17. | Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. 2017;14:577-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 524] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 18. | Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4015] [Cited by in RCA: 6510] [Article Influence: 651.0] [Reference Citation Analysis (0)] |
| 19. | Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985-1998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 643] [Cited by in RCA: 2505] [Article Influence: 313.1] [Reference Citation Analysis (0)] |
| 20. | Can M, Açikgöz Ş, Mungan G, Bayraktaroğlu T, Koçak E, Güven B, Demirtas S. Serum cardiovascular risk factors in obstructive sleep apnea. Chest. 2006;129:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Toyama Y, Chin K, Chihara Y, Takegami M, Takahashi KI, Sumi K, Nakamura T, Nakayama-Ashida Y, Minami I, Horita S, Oka Y, Wakamura T, Fukuhara SI, Mishima M, Kadotani H. Association between sleep apnea, sleep duration, and serum lipid profile in an urban, male, working population in Japan. Chest. 2013;143:720-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Tan KC, Chow WS, Lam JC, Lam B, Wong WK, Tam S, Ip MS. HDL dysfunction in obstructive sleep apnea. Atherosclerosis. 2006;184:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Xu H, Guan J, Yi H, Zou J, Meng L, Tang X, Zhu H, Yu D, Zhou H, Su K, Wang Y, Wang J, Yin S; Shanghai Sleep Health Study Research Group. Elevated low-density lipoprotein cholesterol is independently associated with obstructive sleep apnea: evidence from a large-scale cross-sectional study. Sleep Breath. 2016;20:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Whittle AT, Marshall I, Mortimore IL, Wraith PK, Sellar RJ, Douglas NJ. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 179] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132:325-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 348] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 26. | Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 457] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 27. | Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez-Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S, Somers VK. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J Am Coll Cardiol. 2017;69:841-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 998] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 28. | Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1480] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 29. | Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1169] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 30. | Dong L, Lin M, Wang W, Ma D, Chen Y, Su W, Chen Z, Wang S, Li X, Li Z, Liu C. Lipid accumulation product (LAP) was independently associatedwith obstructive sleep apnea in patients with type 2 diabetes mellitus. BMC Endocr Disord. 2020;20:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Lawlor DA. Commentary: Two-sample Mendelian randomization: opportunities and challenges. Int J Epidemiol. 2016;45:908-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 571] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Dziegielewska-Gesiak S, Poland; Papadopoulos VP, Greece S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH