Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11358
Peer-review started: July 19, 2022
First decision: August 22, 2022
Revised: September 4, 2022
Accepted: September 20, 2022
Article in press: September 20, 2022
Published online: November 6, 2022
Processing time: 99 Days and 20.5 Hours
Hand, foot, and mouth disease (HFMD) has become one of the most common infectious diseases in China. Before 2016, the primary causal serotypes were enterovirus A71 (EV-A71) and coxsackievirus A16 (CV-A16). Following the introduction of EV-A71 vaccines in China since 2016, the situation could change. CV-A6 has recently replaced EV-A71 and CV-A16 in some areas of China. However, the epidemiological characteristics of central China remain unknown.
To investigate the clinical symptoms and pathogen spectrum of HFMD in Shiyan City, central China, in recent years.
The epidemiological, clinical, and laboratory data from HFMD cases reported to the Shiyan Center for Disease Control and Prevention between January 2016 and December 2020 were analyzed. 196 throat swab specimens were collected from hospitalized HFMD patients between January 2018 and December 2020. To detect and genotype enteroviruses, real-time reverse transcription-polymerase chain reaction and sequencing of the 5'-untranslated region were used. In Shiyan, 168 laboratory-confirmed HFMD cases were studied using a logistic regression model to determine the effect of predominant enterovirus serotypes. Based on the logistic regression model, the least absolute shrinkage and selection operator model was used to analyze the correlation between CV-A6 infection and various clinical characteristics in HFMD patients in Shiyan.
From 2016 to 2020, 35840 HFMD cases were reported in Shiyan. The number of cases decreased by 48.4% from 2016 to 2017. Approximately 1.58-fold increases were found in 2018 and 2019 when compared to the previous year, respectively. In 2020, a decrease of about 85.5% was reported when compared to 2019. The most common serotypes shifted from EV-A71 and CV-A16 (about 60%-80% in 2016 and 2018) to others (more than 80.0% in 2017, 2019, and 2020). EV-A71 lost its dominance in 2017 in Shiyan. Among 196 confirmed HFMD cases, 85.7% tested positive for enterovirus, with CV-A6 being the most common serotype (121/168, 72.0%). The positive rates for CV-A16 and CV-A10 were 4.8% and 3.0%, respectively. There was no EV-A71 discovered. Infection with CV-A6 was linked to fever, myocardial damage, increased creatine kinase MB isoenzyme, and lactate dehydrogenase levels.
CV-A6 was the most common enterovirus serotype in Shiyan City, replacing EV-A71 and CV-A16 as the HFMD pathogen. Developing vaccines against CV-A6 or multiple pathogens, as well as rising CV-A6 surveillance, will help prevent HFMD in central China.
Core Tip: Hand, foot, and mouth disease (HFMD) has become China's most common infectious disease since 2008. Enterovirus A71 (EV-A71) and coxsackievirus A16 (CV-A16) were the primary causal serotypes. However, the dominant serotypes shifted recently to other enteroviruses. Nevertheless, there has been no report on the prevalence of the pathogen spectrum in Hubei, central China, since 2017. Our findings revealed that CV-A6 was the most common HFMD pathogen in Shiyan City, displacing EV-A71 and CV-A16. Fever and myocardial damage were more common in HFMD caused by CV-A6.
- Citation: Li JF, Zhang CJ, Li YW, Li C, Zhang SC, Wang SS, Jiang Y, Luo XB, Liao XJ, Wu SX, Lin L. Coxsackievirus A6 was the most common enterovirus serotype causing hand, foot, and mouth disease in Shiyan City, central China. World J Clin Cases 2022; 10(31): 11358-11370
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11358.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11358
Hand, foot, and mouth disease (HFMD) is one of the most widespread infectious diseases in the world. It disproportionately affects children under the age of five[1]. The Chinese Ministry of Health has designated HFMD as a category III notifiable infectious disease since 2008. The Chinese Center for Disease Control and Prevention (China CDC) has documented more than 20 million cases of HFMD from 2008 to 2018. The clinical presentation of HFMD is characterized by low-grade fever, a maculopapular or papulovesicular rash on the palms and soles of the feet, and severe oral ulcerations[2]. The illness is self-limiting, and the majority of children recover in a week without sequelae; however, a small proportion of children who experience neurologic or cardiopulmonary complications could be lethal[2].
As ever reported, the pathogens that caused HFMD are enterovirus-A (EV-A) and enterovirus-B (EV-B) species of the Enterovirus genus of the family Picornaviridae[3]. There are at least 23 serotypes, including nine coxsackievirus-A (CV-A) serotypes, enterovirus-A71 (EV-A71), six CV-B serotypes, six enteric cytopathogenic human orphan (ECHO) virus serotypes, and EV-B84[3]. The most common serotypes are CV-A16 and EV-A71, particularly in Asia-Pacific[4,5]. Furthermore, EV-A71 is also responsible for the most severe and fatal cases[6,7].
However, since the first inactivated EV-A71 whole virus vaccine was launched in China in December 2015, the prevalence of EV-A71 has decreased dramatically[8,9]. A CV-A6-associated HFMD outbreak was reported in Finland in 2008[10]. Since then, CV-A6 has emerged as one of the pathogens responsible for the prevalence of HFMD in America, Europe, Africa, and Asia[11-14]. Between 2009 and 2017, several Chinese provinces reported sporadic HFMD cases caused by CV-A6[15-18]. Furthermore, the CV-A6 epidemic is becoming more widespread[14]. However, etiological data on HFMD in China after 2017 is scarce. There was even no report from Central China after 2017. Nonetheless, monitoring the circulating pathogens following the cumulative coverage of the EV-A71 vaccine is critical because it will help determine how to best implement a preventative strategy.
In this study, we focused on changes in the pathogen spectrum in Shiyan City, Hubei Province, between 2016 and 2020. We also analyzed the relationship between the primary pathogens and the clinical features of HFMD.
We obtained the surveillance data of HFMD in Shiyan city from 2016 to 2020 from Shiyan CDC. Since 2008, all clinically diagnosed and laboratory-confirmed HFMD cases have been reported to the local CDC within 24 h. The diagnostic criteria were based on the People's Republic of China Hygiene Industry Standard for Hand, Foot, and Mouth Disease Diagnosis (WS 588–2018)[19].
From 2018 to 2020, 196 hospitalized HFMD patients were enrolled in this study. The demographics of the patients, clinical data from their hospitalization, and throat swab specimens were all collected. The study was approved by the Institutional Review Board of the Taihe Hospital, Affiliated Hospital of Hubei University of Medicine (2017KS035). Participants' legal guardians signed informed consent forms.
Viral RNAs were extracted from the throat swab specimens using the Tianlong automatic nucleic acid extractor (NP968, Tianlong Science and Technology, Xi'an, China) and its corollary automatic nucleic acid extraction kit according to the manufacturer's instructions. In brief, 200 μL specimens were used. The extracted RNAs were eluted in 100 μL elution buffer and stored at -70 ℃ until used.
Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed to identify the enteroviruses in clinical samples using EV-A71 specific, CV-A16 specific, and pan-enterovirus real-time RT-PCR Kits (Tianlong Science and Technology).
Enterovirus-positive samples were amplified for 5'-untranslated region (5'-UTR) and sequenced to identify the serotypes. The amplification was performed by RT-PCR using the 5'-UTR primers of the enteroviruses as described previously[20]. The primer sequences were as follows: EV-F: AYCYTTGTRCGCCTGTTTT; and EV-R: CCCAAAGTGTCGGTTCCGC. The products of RT-PCR were sequenced using the EV-R primer by Biotecan (Shanghai, China). To identify the serotypes, the detected sequences were compared to reference strains in GenBank from the National Center for Biotechnology Information.
Categorical variables were summarized as frequencies and proportions using descriptive statistics. Chi-square and Fisher's exact tests were used to examine the relationships between categorical variables. Continuous variables were expressed as mean ± SD. For normally distributed data, the means of continuous variables were compared using independent group t-tests, and for non-normally distributed data, the Mann-Whitney test. To determine the relationship between viral pathogens and HFMD characteristics, a logistic regression model was used. The least absolute shrinkage and selection operator (LASSO) method, which is suitable for analyzing high-dimensional data, was used to select the most significant pathogen-related characteristics[21]. In the forward stepwise logistic regression model, features with non-zero coefficients (automatically removing unnecessary/uninfluential covariates in the LASSO regression model were set[22]. All the statistical analyses were performed using R software version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism 7 (GraphPad Software, La Jolla, California, United States).
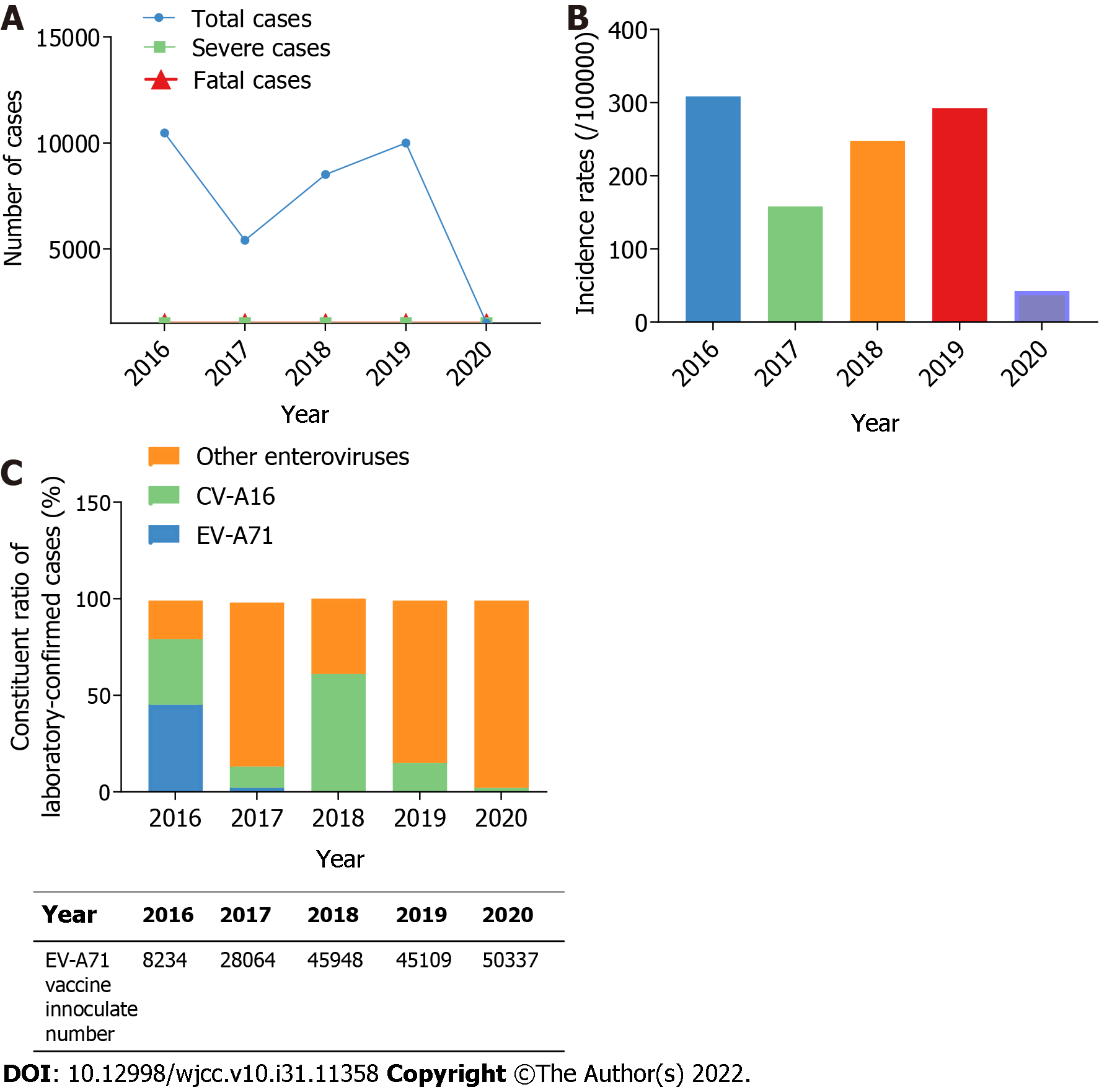
From 2016 to 2020, the Shiyan CDC surveillance system received 35840 HFMD cases, with 1690 (4.7%) laboratory-confirmed cases, 4 (0.01%) severe cases, and 2 (0.06‰) fatal cases. In 2017 and 2020, the temporal distribution showed two low ebbs. The yearly number of HFMD decreased by 48.4% in 2017 (n = 5405) compared to 2016 (n = 10473), following 1.6-fold increases in 2018 (n = 8513) and 2019 (n = 9997). In 2020 (n = 1452), the incidence of HFMD fell by 85.5% compared to 2019 (Table 1 and Figure 1A). When compared to 2016 (308.63/100000), the annual incidence rate of HFMD decreased by 48.8% in 2017 (158.05/100000), then increased by 1.6-fold and 1.2-fold in 2018 (247.78/100000) and 2019 (292.48/100000) respectively. In 2020, the incidence of HFMD reduced by 85.4% (42.73/100000) compared to 2019 (Table 1 and Figure 1B), which could be attributed to the COVID-19 epidemic and China's isolation policy.
| Characteristics | Year | ||||
| 2016 | 2017 | 2018 | 2019 | 2020 | |
| Total cases | 10473 | 5405 | 8513 | 9997 | 1452 |
| Incidence rates (/100000) | 308.63 | 158.05 | 247.78 | 292.48 | 42.73 |
| Laboratory-detected cases | 660 | 530 | 700 | 530 | 146 |
| Laboratory-confirmed cases | 367 | 363 | 518 | 370 | 72 |
| Severe cases | 1 | 0 | 2 | 1 | 0 |
| Fatal cases | 2 | 0 | 0 | 0 | 0 |
| Etiological results | |||||
| EV-A71 | 167 (45.5) | 9 (2.5) | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| CV-A16 | 125 (34.1) | 43 (11.8) | 316 (61.0) | 56 (15.1) | 2 (2.8) |
| Other enteroviruses | 75 (20.4) | 311 (85.7) | 202 (39.0) | 313 (84.6) | 70 (97.2) |
Since 2017, the proportion of EV-A71-associated HFMD cases among 1690 laboratory-confirmed HFMD cases in Shiyan has decreased significantly. In 2016, 45.5% of EV-A71-positive cases (167/367) were confirmed; however, this proportion dropped to 2.5% (9/363) in 2017 and nearly zero between 2018 and 2020 (Table 1 and Figure 1C). According to these findings, EV-A71 was the dominant pathogen of HFMD in Shiyan in 2016, but it lost the advantage between 2017 and 2020. The China Food and Drug Administration approved the EV-A71 whole virus vaccine in December 2015[8]. In Shiyan city, there were 8234 inoculated people in 2016, 28064 in 2017, 45948 in 2018, 45109 in 2019, and 50337 in 2020, with the number steadily increasing year after year (Figure 1C). These findings suggest that EV-A71 vaccine inoculation may be linked to the recent decrease in EV-A71 prevalence.
The CV-A16 was another common pathogen in China[5]. Its prevalence in Shiyan fluctuates between 2016 and 2020. In 2016, 34.1% of cases (125/367) tested positive for CV-A16. However, the ratio in 2017 was 11.8% (43/363), 61.0% (316/518) in 2018, 15.1% (56/370) in 2019, and 2.8% (2/72) in 2020 (Table 1 and Figure 1C). These findings indicate that CV-A16 was also a dominant HFMD pathogen in Shiyan in 2016, but that its dominance waned sharply in 2018.
In contrast to EV-A71 and CV-A16, the prevalence of other enteroviruses tends to rise in Shiyan from 2016 to 2020. Other enteroviruses ratios were 20.4% (75/367) in 2016, 85.7%% (311/363) in 2017, 39.0% (202/518) in 2018, 84.6% (313/370) in 2019, and 97.2% (70/72) in 2020. 971 laboratory-confirmed cases were linked to other enteroviruses between 2016 and 2020, accounting for 57.5% of all cases (Table 1 and Figure 1C). These results suggest that other enteroviruses will gradually replace EV-A71 and CV-A16 as the most common HFMD pathogens in Shiyan. The specific prevailing serotype, however, remained unknown.
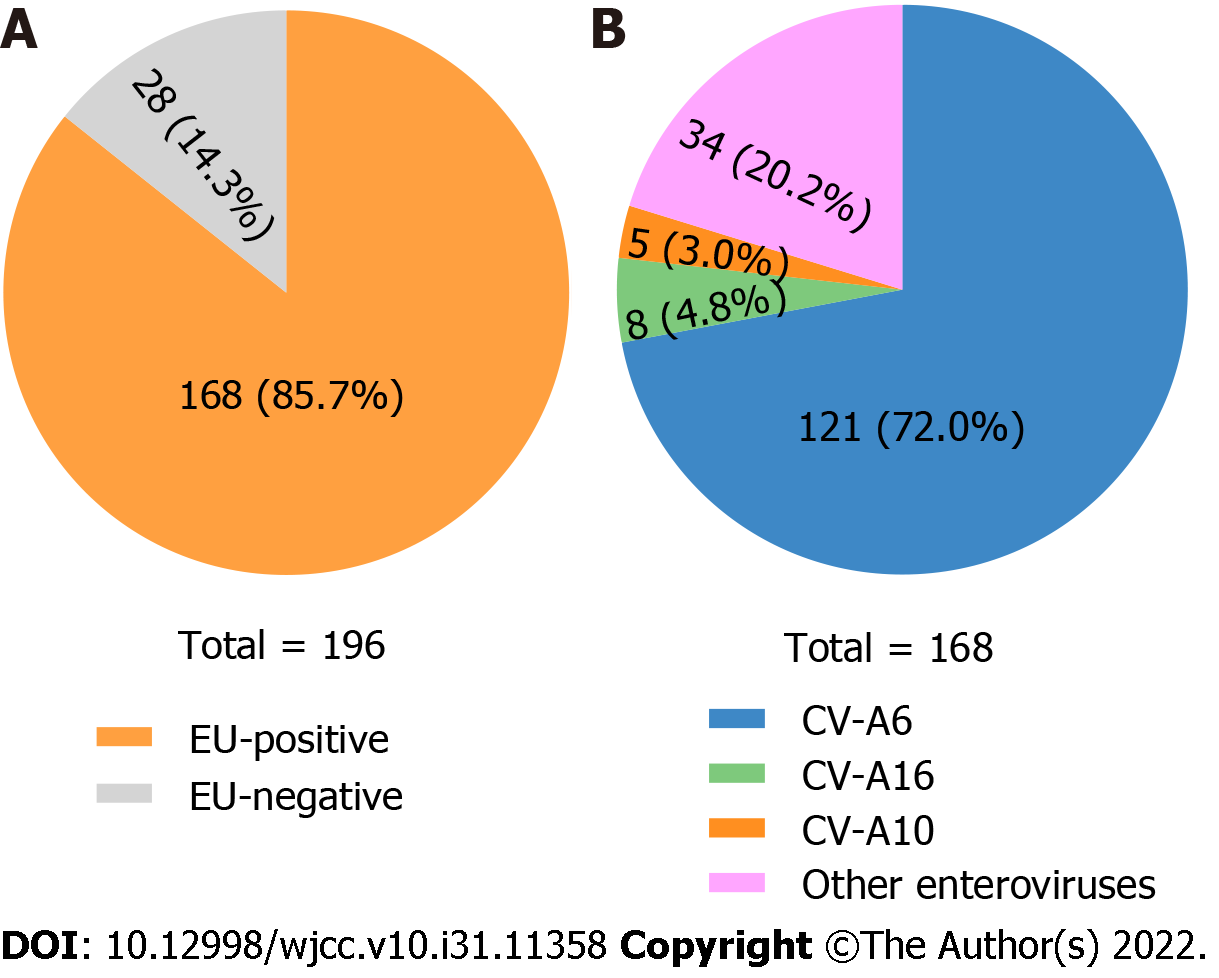
From 2018 to 2020, we collected 196 throat swab samples from hospitalized HFMD patients to investigate the newly prevalent enterovirus serotype (s) in Shiyan. Using EV-A71 specific, CV-A16 specific, and pan-enterovirus real-time RT-PCR Kits, 168 enterovirus-positive specimens were identified (Figure 2A). According to the 5'-UTR sequences of 168 enterovirus-positive samples, 121 (72.0%) were CV-A6 positive, 8 (4.8%) were CV-A16 positive, and 5 (3.0%) were CV-A10. Other enteroviruses were found in 34 of the samples (20.2%) (Figure 2B). From these results, CV-A6 was the newly prevalent serotype contributing to HFMD in Shiyan between 2018 and 2020.
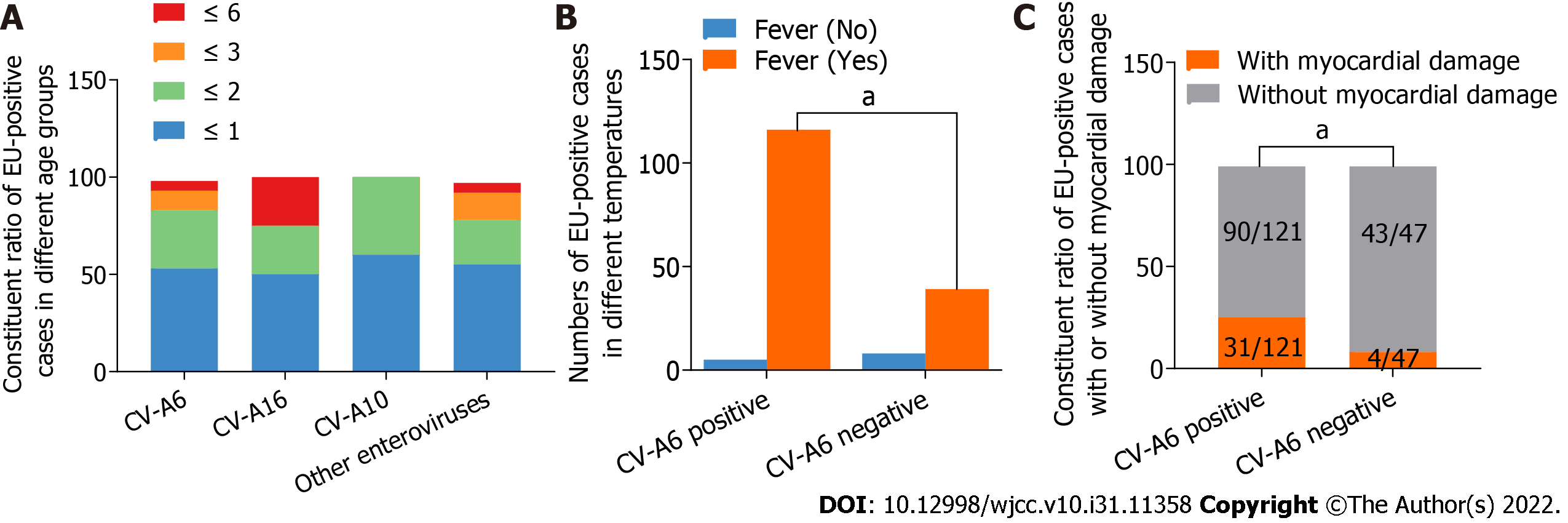
As shown in Table 2 and Figure 3A, the male-to-female ratio among the 168 enterovirus-positive cases was 1.67:1. 91 cases (54.2%) were under one year old, and no case was older than six years old. All patients had oral mucosal or skin rashes on the hand, foot, crissum, or hip. However, no significant differences in gender, age, or rashes were found between CV-A6 and other enteroviruses (CV-A16, CV-A10, and others). Fever was present in 95.9% of CV-A6-positive HFMD patients and 83.0% of CV-A6-negative HFMD patients. Fever levels differed significantly between HFMD with CV-A6 infection and HFMD without CV-A6 infection, according to univariate and multivariate logistic regression analyses (Table 2 and Figure 3B). A subsequent study using the LASSO regression model discovered that CV-A6 infection was more likely to result in fever (P = 0.023 < 0.05, Table 3).
| HFMD pathogens | Univariate analysis | Multivariate analysis | |||||
| CV-A6 (n = 121) | CV-A16 (n = 8) | CV-A10 (n = 5) | Other enteroviruses (n = 34) | P value | Coefficient (SD) | P value | |
| Gender | 1 | -0.224 (0.451) | 0.619 | ||||
| Male (n = 105) | 76 (62.8) | 5 (62.5) | 4 (80.0) | 20 (58.8) | |||
| Female (n = 63) | 45 (37.2) | 3 (37.5) | 1 (20.0) | 14 (41.2) | |||
| Age (yr) | 0.794 | ||||||
| ≤ 1 (n = 91) | 65 (53.7) | 4 (50.0) | 3 (60.0) | 19 (55.9) | |||
| ≤ 2 (n = 49) | 37 (30.6) | 2 (25.0) | 2 (40.0) | 8 (23.5) | 0.131 (0.497) | 0.792 | |
| ≤ 3 (n = 18) | 13 (10.7) | 0 (0.0) | 0 (0.0) | 5 (14.7) | 0.236 (0.717) | 0.742 | |
| ≤ 6 (n = 10) | 6 (5.0) | 2 (25.0) | 0 (0.0) | 2 (5.9) | -0.371 (0.785) | 0.637 | |
| Fever | 0.013a | 2.124 (0.747) | 0.004b | ||||
| Yes | 116 (95.9) | 5 (62.5) | 5 (100.0) | 29 (85.3) | |||
| No | 5 (4.1) | 3 (37.5) | 0 (0.0) | 5 (14.7) | |||
| Clinical manifestations | |||||||
| Skin or oral mucosal rashes | 121 (100.0) | 8 (100.0) | 5 (100.0) | 34 (100.0) | |||
| Respiratory system syndromes1 | 24 (19.8) | 3 (37.5) | 1 (20.0) | 3 (8.8) | 0.603 | 0.811 (0.589) | 0.168 |
| Myocardial damage | 31 (25.6) | 1 (12.5) | 1 (20.0) | 2 (5.9) | 0.025a | 1.040 (0.678) | 0.125 |
| Vomit | 0 (0.0) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 0.623 | -15.923 (882.744) | 0.986 |
| Laboratory findings, n (%, mean ± SD) | |||||||
| WBC increased | 69 (56.6, 14.07 ± 3.42) | 4 (50.0, 19.82 ± 10.74) | 5 (100.0, 20.68 ± 7.67) | 15 (44.1, 13.88 ± 4.38) | 0.042a | -0.058 (0.050) | 0.247 |
| CRP increased | 79 (64.8, 24.64 ± 26.90) | 5 (62.5, 25.28 ± 26.64) | 5 (100.0, 47.68 ± 29.52) | 15 (44.1, 25.97 ± 43.35) | 0.431 | -0.007 (0.006) | 0.271 |
| CK-MB increased | 56 (45.9, 33.77 ± 13.78) | 2 (25.0, 30.00 ± 4.24) | 4 (80.0, 28.00 ± 6.48) | 17 (50.0, 30.94 ± 7.04) | 0.262 | 0.045 (0.027) | 0.097 |
| LDH increased | 98 (80.3, 313.08 ± 59.37) | 6 (75.0, 298.17 ± 38.97) | 5 (100.0, 275.00 ± 24.81) | 27 (79.4, 334.52 ± 59.64) | 0.487 | -0.009 (0.004) | 0.043a |
| ALT increased | 20 (16.4) | 1 (12.5) | 1 (20.0) | 3 (8.8) | 0.333 | -0.575 (1.328) | 0.665 |
| AST increased | 29 (23.8) | 1 (12.5) | 1 (20.0) | 3 (8.8) | 0.086 | 1.778 (1.196) | 0.137 |
| Radiographic evaluation | |||||||
| Abnormal chest X-ray2 | 48 (39.3) | 4 (50.0) | 1 (20.0) | 8 (23.5) | 0.267 | 0.275 (0.449) | 0.540 |
| Abnormal electrocardiogram3 | 24 (19.7) | 1 (12.5) | 1 (20.0) | 5 (14.7) | 0.389 | 0.236 (0.566) | 0.677 |
There were no severe or fatal cases among the 168 enterovirus-positive cases. However, some patients had respiratory inflammation (such as bronchopneumonia, bronchitis, other upper respiratory tract diseases, or abnormal chest X-ray), and vomited. Nonetheless, there were no significant differences in these clinical manifestations between CV-A6 and other enteroviruses (CV-A16, CV-A10, and others) (Table 2). Myocardial damage, on the other hand, was significantly more common in CV-A6 infected patients than in other enterovirus infections (presenting as polyhedrosis, flustered, electrocardiographic abnormality, increased CK, CK-MB, and LDH). Myocardial damage was present in approximately 25.6% (31/121) of CV-A6-positive HFMD cases, whereas it was present in 8.5% (4/47) of CV-A6-negative HFMD patients (Table 2, Figure 3C). Furthermore, the LASSO regression model revealed that myocardial damage (P = 0.037 < 0.05) was significantly associated with CV-A6 infection. Additionally, elevated CK-MB (P = 0.015 < 0.05) and LDH (P = 0.001), two myocardial damage markers, were strongly linked with CV-A6 infection (Table 3), implying that myocardial damage is more frequent in HFMD caused by CV-A6.
In 1957, HFMD was initially reported in New Zealand and Canada[23,24]. Then it spread all over the world, including the United States, Bulgaria, Hungary, and a slew of Asia countries[25-33]. For a long time, little attention was paid to this disease due to its mild clinical manifestations and self-limiting nature. Nonetheless, over the last two decades, an increasing number of outbreaks with atypical manifestations and expanding pathogen spectrums have been reported worldwide, attracting public attention[3,34,35]. Because of the widespread prevalence of HFMD in China, the Chinese Ministry of Health has also designated it as a category III notifiable infectious disease since 2008. The main pathogens in the pathogen spectrums were CV-A16 and EV-A71[36]. The disease caused by CV-A16 was typically mild, with classical rashes. EV-A71, on the other hand, can cause severe neurological manifestations such as polio-like paralysis, fatal encephalitis, and cardiopulmonary complications in addition to rashes[37]. As a result, developing effective pathogen-specific vaccines is a wise decision.
Since 2016, three inactivated, whole EV-A71 vaccines have been launched in China[38]. And the EV-A71 vaccines were proved to be effective in a recent epidemiologic survey in Guangxi Province (Southern China). The incidence and severity of EV-A71 cases have both decreased significantly following vaccination with EV-A71 vaccines from 2017 to 2019[9]. A report from Nanchang (Southeastern China) found no EV-A71 after a 2-year implementation of the EV-A71 vaccination[39]. Our findings also showed the efficacy of EV-A71 vaccines. It was discovered that, as the number of inoculated people in Shiyan (Central China) increased year after year, the incidence of HFMD decreased sharply in 2017 (Table 1 and Figure 1A-B). Furthermore, EV-A71 prevalence in Shiyan decreased sharply in 2017 and will almost completely disappear between 2018 and 2020 (Table 1 and Figure 1C), indicating that EV-A71 vaccine inoculation may be beneficial. However, the HFMD incidence increased again in Shiyan in 2018 and 2019, indicating that, while EV-A71 was under control, other pathogens may be contributing to the increased HFMD cases. In fact, the EV-A71 vaccine provided no cross-protection against other serotypes. Then, which pathogen(s) were responsible for the rise in HFMD cases in Shiyan between 2018 and 2020?
In reality, a shift in HFMD etiology has been reported in recent years. CV-A6, CV-A10, and CV-A12 have emerged as important HFMD pathogens, except for CV-A16 and EV-A71[40-42]. HFMD caused by CV-A6 has been reported in countries around the world, including Finland, the United States, Japan, Spain, etc[10,11,43,44]. CV-A6 was also a significant pathogen contributing to HFMD in China. Between 2010 and 2012, CV-A6 surpassed CV-A16 as the second most common serotype in southern China (Guangdong Province)[45,46]. In 2013, CV-A6 replaced EV-A71 and CV-A16 as the primary pathogen of HFMD in northern (Beijing, Tianjin), northeastern (Jilin), eastern (Taizhou), and middle China (Chongqing)[47-51]. In 2015, CV-A6 outbreaks were reported in northern (Beijing) and middle (Chongqing) China[52,53]. During 2018-2019, CV-A6 was found to be the most common cause of HFMD in eastern (Shanghai), northern (Beijing, 2018, and 2020), southeastern (Nanchang, 2019), and southern (Kunming, 2018) China[39,54-56]. In our study, CV-A6 surpassed EV-A71 and CV-A16 as the leading cause of HFMD in central China (Shiyan) between 2018 and 2020 (Table 2 and Figure 2), demonstrating that CV-A6 has become the most common serotype recently in China. Intriguingly, CV-A6 nonetheless overtook CV-A16 and EV-A71 as the primary causal agent in some regions in 2013 despite the absence of EV-A71 vaccines. The reason remained unknown.
It has been reported that CV-A6 can cause severe HFMD in children and atypical HFMD in adults[57-59]. Clinical manifestations involved vesiculobullous exanthema, palm and sole desquamation, and onychomadesis[14,60]. A study of 8 confirmed HFMD children found severe clinical features such as 100% fever (higher than 39 ℃), 87.5% herpangina, 100% meningitis, and 25% myocardial damage caused by CV-A6[57]. In our collected samples, we found that more than half of the cases occurred in boys and children under the age of one year. No patient was older than the age of six (Table 2). As a result, the prevention of infant HFMD should be prioritized. Because we collected samples from the pediatric department, no adult patients were found. In addition, we showed that CV-A6 was more likely to cause fever (95.9% vs 83%) and myocardial damage (25.6% vs 8.5%) (Table 3 and Figure 3), which was consistent with the previous study[57]. However, no onychomadesis was observed in our study. It might be due to different CV-A6 genotypes. The limitation is that genotypes of prevailing CV-A6 in Shiyan and other regions were not identified. Further study can be done to determine the association between different CV-A6 genotypes and clinical features.
The median R0 (interquartile range) of the major HFMD serotypes was calculated in a recent study. Among EV-A71, CV-A16, and CV-A6, CV-A6 had the highest R0 (5.94, and 25.80 after adjusting for seroprevalences), implying that CV-A6 may be the major challenge in the prevention and control of HFMD in China[61]. As a result, developing vaccines against CV-A6 or multiple pathogens will help prevent HFMD. Recently, an inactivated CV-A6 vaccine candidate based on Vero cells was shown to be effective in mouse models[62]. However, more clinical trials were required to provide evidence of its efficacy. A recent study[63] used cryo-electron microscopy to identify the virion structure of CV-A6, laying the groundwork for the development of novel vaccines and drugs. In addition, multiple enterovirus serotypes, including CV-A6, should be included in long-term HFMD surveillance.
In conclusion, CV-A6 is the most common enterovirus serotype in Shiyan City, replacing EV-A71 and CV-A16 as the HFMD pathogen. Fever and myocardial damage are more common in CV-A6-caused HFMD. CV-A6 prevalence surveillance should be prioritized.
Before 2016, the main causative serotypes of hand, foot, and mouth disease (HFMD) were coxsackievirus A16 (CV-A16) and enterovirus A71 (EV-A71). Some regions in China have shown that CV-A6 has recently supplanted EV-A71 and CV-A16 due to the introduction of EV-A71 vaccines. Finding out Central China's epidemiological characteristics is equally important.
By investigating the clinical symptoms and pathogen spectrum of HFMD in Shiyan City, central China, after 2016, it will provide critical proof for future knowledge of the etiology of HFMD.
To investigate the epidemiological and etiological features of HFMD in Shiyan City, which may provide information on the disease's recent prevalence in central China.
The Shiyan Center for Disease Control and Prevention HFMD data from 2016 to 2020 were examined. Between 2018 and 2020, throat swab specimens were collected from hospitalized HFMD patients in Shiyan. To detect and genotype enteroviruses, real-time reverse transcription-polymerase chain reaction and sequencing of the 5'-untranslated region were used. The effect of predominant enterovirus serotypes and the correlation between CV-A6 infection and various clinical characteristics in Shiyan HFMD patients were studied using a logistic regression model and the least absolute shrinkage and selection operator model.
The frequency of HFMD dramatically dropped in 2017 as the number of immunized persons in Shiyan grew year after year. But the prevalence of HFMD rose once more in 2018 and 2019. Between 2018 and 2020, CV-A6 superseded EV-A71 and CV-A16 as the main cause of HFMD in Shiyan. CV-A6 was associated with an increased risk of fever and myocardial damage. The limitation is that the dominant CV-A6 genotypes in Shiyan and other regions were not identified. More research is needed to determine the relationship between different CV-A6 genotypes and clinical features.
The most common enterovirus serotype in Shiyan City was CV-A6, which replaced EV-A71 and CV-A16 as the HFMD pathogen.
The prevention of HFMD in central China will be aided by the creation of vaccines against CV-A6 or multiple infections as well as increased CV-A6 surveillance.
We are very grateful to Jia CL for his skillful statistical analysis guidance.
| 1. | Esposito S, Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis. 2018;37:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 2. | Saguil A, Kane SF, Lauters R, Mercado MG. Hand-Foot-and-Mouth Disease: Rapid Evidence Review. Am Fam Physician. 2019;100:408-414. [PubMed] |
| 3. | Lei X, Cui S, Zhao Z, Wang J. Etiology, pathogenesis, antivirals and vaccines of hand, foot, and mouth disease. Natl Sci Rev. 2015;2:268-284. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Noisumdaeng P, Korkusol A, Prasertsopon J, Sangsiriwut K, Chokephaibulkit K, Mungaomklang A, Thitithanyanont A, Buathong R, Guntapong R, Puthavathana P. Longitudinal study on enterovirus A71 and coxsackievirus A16 genotype/subgenotype replacements in hand, foot and mouth disease patients in Thailand, 2000-2017. Int J Infect Dis. 2019;80:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Huang J, Liao Q, Ooi MH, Cowling BJ, Chang Z, Wu P, Liu F, Li Y, Luo L, Yu S, Yu H, Wei S. Epidemiology of Recurrent Hand, Foot and Mouth Disease, China, 2008-2015. Emerg Infect Dis. 2018;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 6. | Messacar K, Spence-Davizon E, Osborne C, Press C, Schreiner TL, Martin J, Messer R, Maloney J, Burakoff A, Barnes M, Rogers S, Lopez AS, Routh J, Gerber SI, Oberste MS, Nix WA, Abzug MJ, Tyler KL, Herlihy R, Dominguez SR. Clinical characteristics of enterovirus A71 neurological disease during an outbreak in children in Colorado, United States, in 2018: an observational cohort study. Lancet Infect Dis. 2020;20:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 107] [Reference Citation Analysis (0)] |
| 7. | Liu SL, Pan H, Liu P, Amer S, Chan TC, Zhan J, Huo X, Liu Y, Teng Z, Wang L, Zhuang H. Comparative epidemiology and virology of fatal and nonfatal cases of hand, foot and mouth disease in mainland China from 2008 to 2014. Rev Med Virol. 2015;25:115-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Mao QY, Wang Y, Bian L, Xu M, Liang Z. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev Vaccines. 2016;15:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 9. | Wang J, Jiang L, Zhang C, He W, Tan Y, Ning C. The changes in the epidemiology of hand, foot, and mouth disease after the introduction of the EV-A71 vaccine. Vaccine. 2021;39:3319-3323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Centers for Disease Control and Prevention (CDC). Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6 - Alabama, Connecticut, California, and Nevada, November 2011-February 2012. MMWR Morb Mortal Wkly Rep. 2012;61:213-214. [PubMed] |
| 12. | Horsten HH, Kemp M, Fischer TK, Lindahl KH, Bygum A. Atypical Hand, Foot, and Mouth Disease Caused by Coxsackievirus A6 in Denmark: A Diagnostic Mimicker. Acta Derm Venereol. 2018;98:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Gopalkrishna V, Ganorkar N. Epidemiological and molecular characteristics of circulating CVA16, CVA6 strains and genotype distribution in hand, foot and mouth disease cases in 2017 to 2018 from Western India. J Med Virol. 2021;93:3572-3580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Zhao TS, Du J, Sun DP, Zhu QR, Chen LY, Ye C, Wang S, Liu YQ, Cui F, Lu QB. A review and meta-analysis of the epidemiology and clinical presentation of coxsackievirus A6 causing hand-foot-mouth disease in China and global implications. Rev Med Virol. 2020;30:e2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Xu Y, Zheng Y, Shi W, Guan L, Yu P, Xu J, Zhang L, Ma P. Pathogenic characteristics of hand, foot and mouth disease in Shaanxi Province, China, 2010-2016. Sci Rep. 2020;10:989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Xu S, Li H, Qiao P, Xu G, Zhao D, Lin X, Qin Y, Yu H, Zhang X, Zhang W, Huang L. Neonatal hand, foot, and mouth disease due to coxsackievirus A6 in Shanghai. BMC Pediatr. 2020;20:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Song Y, Zhang Y, Ji T, Gu X, Yang Q, Zhu S, Xu W, Xu Y, Shi Y, Huang X, Li Q, Deng H, Wang X, Yan D, Yu W, Wang S, Yu D. Persistent circulation of Coxsackievirus A6 of genotype D3 in mainland of China between 2008 and 2015. Sci Rep. 2017;7:5491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Lu QB, Zhang XA, Wo Y, Xu HM, Li XJ, Wang XJ, Ding SJ, Chen XD, He C, Liu LJ, Li H, Yang H, Li TY, Liu W, Cao WC. Circulation of Coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One. 2012;7:e52073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Li XW, Ni X, Qian SY, Wang Q, Jiang RM, Xu WB, Zhang YC, Yu GJ, Chen Q, Shang YX, Zhao CS, Yu H, Zhang T, Liu G, Deng HL, Gao J, Ran XG, Yang QZ, Xu BL, Huang XY, Wu XD, Bao YX, Chen YP, Chen ZH, Liu QQ, Lu GP, Liu CF, Wang RB, Zhang GL, Gu F, Xu HM, Li Y, Yang T. Chinese guidelines for the diagnosis and treatment of hand, foot and mouth disease (2018 edition). World J Pediatr. 2018;14: 437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Tang S, Xie Z, Liu D, Yuan Y, Chen M, Fan X, Ding X, Yu N. Evaluation of 5′-untranslated region amplification and sequencing for enterovirus serotypes identification diagnosis. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2018;32:488-491. [DOI] [Full Text] |
| 21. | Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512-5528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 861] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 22. | Kidd AC, McGettrick M, Tsim S, Halligan DL, Bylesjo M, Blyth KG. Survival prediction in mesothelioma using a scalable Lasso regression model: instructions for use and initial performance using clinical predictors. BMJ Open Respir Res. 2018;5:e000240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 23. | Seddon JH, Duff MF. Hand-foot-and-mouth disease: Coxsackie virus types A 5, A 10, and A 16 infections. N Z Med J. 1971;74:368-373. [PubMed] |
| 24. | Robinson CR, Doane FW, Rhodes AJ. Report of an outbreak of febrile illness with pharyngeal lesions and exanthem: Toronto, summer 1957; isolation of group A Coxsackie virus. Can Med Assoc J. 1958;79:615-621. [PubMed] |
| 25. | Khanh TH, Sabanathan S, Thanh TT, Thoa le PK, Thuong TC, Hang Vt, Farrar J, Hien TT, Chau Nv, van Doorn HR. Enterovirus 71-associated hand, foot, and mouth disease, Southern Vietnam, 2011. Emerg Infect Dis. 2012;18:2002-2005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis. 2003;9:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 185] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | McMinn P, Stratov I, Nagarajan L, Davis S. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin Infect Dis. 2001;32:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 315] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP, Ho KK, Han LL, Pallansch MA, Suleiman AB, Jegathesan M, Anderson LJ. Deaths of children during an outbreak of hand, foot, and mouth disease in sarawak, malaysia: clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin Infect Dis. 2000;31:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 427] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | Centers for Disease Control and Prevention (CDC). Deaths among children during an outbreak of hand, foot, and mouth disease--Taiwan, Republic of China, April-July 1998. MMWR Morb Mortal Wkly Rep. 1998;47:629-632. [PubMed] |
| 30. | Nagy G, Takátsy S, Kukán E, Mihály I, Dömök I. Virological diagnosis of enterovirus type 71 infections: experiences gained during an epidemic of acute CNS diseases in Hungary in 1978. Arch Virol. 1982;71:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 202] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Ishimaru Y, Nakano S, Yamaoka K, Takami S. Outbreaks of hand, foot, and mouth disease by enterovirus 71. High incidence of complication disorders of central nervous system. Arch Dis Child. 1980;55:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 169] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Chumakov M, Voroshilova M, Shindarov L, Lavrova I, Gracheva L, Koroleva G, Vasilenko S, Brodvarova I, Nikolova M, Gyurova S, Gacheva M, Mitov G, Ninov N, Tsylka E, Robinson I, Frolova M, Bashkirtsev V, Martiyanova L, Rodin V. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol. 1979;60:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 241] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | FLEWETT TH, WARIN RP, CLARKE SK. 'Hand, foot, and mouth disease' associated with Coxsackie A5 virus. J Clin Pathol. 1963;16:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Nassef C, Ziemer C, Morrell DS. Hand-foot-and-mouth disease: a new look at a classic viral rash. Curr Opin Pediatr. 2015;27:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Koh WM, Bogich T, Siegel K, Jin J, Chong EY, Tan CY, Chen MI, Horby P, Cook AR. The Epidemiology of Hand, Foot and Mouth Disease in Asia: A Systematic Review and Analysis. Pediatr Infect Dis J. 2016;35:e285-e300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 36. | Nishimura Y, Shimizu H. Cellular receptors for human enterovirus species a. Front Microbiol. 2012;3:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Chang PC, Chen SC, Chen KT. The Current Status of the Disease Caused by Enterovirus 71 Infections: Epidemiology, Pathogenesis, Molecular Epidemiology, and Vaccine Development. Int J Environ Res Public Health. 2016;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Li ML, Shih SR, Tolbert BS, Brewer G. Enterovirus A71 Vaccines. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | He F, Rui J, Deng Z, Zhang Y, Qian K, Zhu C, Yu S, Tu J, Xia W, Zhu Q, Chen S, Chen T, Zhou X. Surveillance, Epidemiology and Impact of EV-A71 Vaccination on Hand, Foot, and Mouth Disease in Nanchang, China, 2010-2019. Front Microbiol. 2021;12:811553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Bian L, Gao F, Mao Q, Sun S, Wu X, Liu S, Yang X, Liang Z. Hand, foot, and mouth disease associated with coxsackievirus A10: more serious than it seems. Expert Rev Anti Infect Ther. 2019;17:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 41. | Puenpa J, Mauleekoonphairoj J, Linsuwanon P, Suwannakarn K, Chieochansin T, Korkong S, Theamboonlers A, Poovorawan Y. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PLoS One. 2014;9:e98888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 42. | Liu X, Mao N, Yu W, Chai Q, Wang H, Wang W, Wang L, Wang Z, Xu W. Genetic characterization of emerging coxsackievirus A12 associated with hand, foot and mouth disease in Qingdao, China. Arch Virol. 2014;159:2497-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Montes M, Artieda J, Piñeiro LD, Gastesi M, Diez-Nieves I, Cilla G. Hand, foot, and mouth disease outbreak and coxsackievirus A6, northern Spain, 2011. Emerg Infect Dis. 2013;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Fujimoto T, Iizuka S, Enomoto M, Abe K, Yamashita K, Hanaoka N, Okabe N, Yoshida H, Yasui Y, Kobayashi M, Fujii Y, Tanaka H, Yamamoto M, Shimizu H. Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012;18:337-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 45. | Di B, Zhang Y, Xie H, Li X, Chen C, Ding P, He P, Wang D, Geng J, Luo L, Bai Z, Yang Z, Wang M. Circulation of Coxsackievirus A6 in hand-foot-mouth disease in Guangzhou, 2010-2012. Virol J. 2014;11:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | He YQ, Chen L, Xu WB, Yang H, Wang HZ, Zong WP, Xian HX, Chen HL, Yao XJ, Hu ZL, Luo M, Zhang HL, Ma HW, Cheng JQ, Feng QJ, Zhao DJ. Emergence, circulation, and spatiotemporal phylogenetic analysis of coxsackievirus a6- and coxsackievirus a10-associated hand, foot, and mouth disease infections from 2008 to 2012 in Shenzhen, China. J Clin Microbiol. 2013;51:3560-3566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Wang SH, Wang A, Liu PP, Zhang WY, Du J, Xu S, Liu GC, Zheng BS, Huan C, Zhao K, Yu XF. Divergent Pathogenic Properties of Circulating Coxsackievirus A6 Associated with Emerging Hand, Foot, and Mouth Disease. J Virol. 2018;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Tan X, Li L, Zhang B, Jorba J, Su X, Ji T, Yang D, Lv L, Li J, Xu W. Molecular epidemiology of coxsackievirus A6 associated with outbreaks of hand, foot, and mouth disease in Tianjin, China, in 2013. Arch Virol. 2015;160:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Zha J, Ma Z. Epidemiological and genetic analysis concerning the coxsackievirus A6 related endemic outbreak of hand-foot-mouth disease in Taizhou, China, during 2013. J Med Virol. 2015;87:2000-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Yan X, Zhang ZZ, Yang ZH, Zhu CM, Hu YG, Liu QB. Clinical and Etiological Characteristics of Atypical Hand-Foot-and-Mouth Disease in Children from Chongqing, China: A Retrospective Study. Biomed Res Int. 2015;2015:802046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Hongyan G, Chengjie M, Qiaozhi Y, Wenhao H, Juan L, Lin P, Yanli X, Hongshan W, Xingwang L. Hand, foot and mouth disease caused by coxsackievirus A6, Beijing, 2013. Pediatr Infect Dis J. 2014;33:1302-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Yu FY, Zhu RN, Deng J, Song QW, Jia LP, Liu LY, Qian Y. [Pathogen spectrum in enteroviral infections among children in Beijing from 2010 to 2016]. Zhonghua Er Ke Za Zhi. 2018;56:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 53. | Tao J, He XY, Shi Y, Zhu G, Liu S, Zhang Z, Tang S, Zhang R, Peng B, Liu Z, Tan J, Chen Q, Wang X, Bao L, Zou L, Zhang P. Epidemiology of 45,616 suspect cases of Hand, Foot and Mouth Disease in Chongqing, China, 2011-2015. Sci Rep. 2017;7:45630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Hu L, Maimaiti H, Zhou L, Gao J, Lu Y. Changing serotypes of hand, foot and mouth disease in Shanghai, 2017-2019. Gut Pathog. 2022;14:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Cui Y, Yang YN, Zheng RR, Xie MZ, Zhang WX, Chen LY, Du J, Yang Y, Xi L, Li H, Li HJ, Lu QB. Epidemiological characteristics of hand, foot, and mouth disease clusters during 2016-2020 in Beijing, China. J Med Virol. 2022;94:4934-4943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Jiang H, Zhang Z, Rao Q, Wang X, Wang M, Du T, Tang J, Long S, Zhang J, Luo J, Pan Y, Chen J, Ma J, Liu X, Fan M, Zhang T, Sun Q. The epidemiological characteristics of enterovirus infection before and after the use of enterovirus 71 inactivated vaccine in Kunming, China. Emerg Microbes Infect. 2021;10:619-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Yang F, Yuan J, Wang X, Li J, Du J, Su H, Zhou B, Jin Q. Severe hand, foot, and mouth disease and coxsackievirus A6-Shenzhen, China. Clin Infect Dis. 2014;59:1504-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Ramirez-Fort MK, Downing C, Doan HQ, Benoist F, Oberste MS, Khan F, Tyring SK. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Harris PNA, Wang AD, Yin M, Lee CK, Archuleta S. Atypical hand, foot, and mouth disease: eczema coxsackium can also occur in adults. Lancet Infect Dis. 2014;14:1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 107] [Reference Citation Analysis (0)] |
| 60. | Justino MCA, da S Mesquita D, Souza MF, Farias FP, Dos S Alves JC, Ferreira JL, Lopes DP, Tavares FN. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol. 2020;126:104307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Zhang Z, Liu Y, Liu F, Ren M, Nie T, Cui J, Chang Z, Li Z. Basic Reproduction Number of Enterovirus 71 and Coxsackievirus A16 and A6: Evidence From Outbreaks of Hand, Foot, and Mouth Disease in China Between 2011 and 2018. Clin Infect Dis. 2021;73:e2552-e2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Qian SS, Wei ZN, Jin WP, Wu J, Zhou YP, Meng SL, Guo J, Wang ZJ, Shen S. Efficacy of a coxsackievirus A6 vaccine candidate in an actively immunized mouse model. Emerg Microbes Infect. 2021;10:763-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Büttner CR, Spurný R, Füzik T, Plevka P. Cryo-electron microscopy and image classification reveal the existence and structure of the coxsackievirus A6 virion. Commun Biol. 2022;5:898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chisthi MM, India; Hoveidaei AH, Iran; Enomoto H, Japan S-Editor: Wang LL L-Editor: A P-Editor: Wang LL