Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11313
Peer-review started: May 14, 2022
First decision: June 19, 2022
Revised: July 5, 2022
Accepted: September 20, 2022
Article in press: September 20, 2022
Published online: November 6, 2022
Processing time: 159 Days and 6.1 Hours
Refractory ascites has a 1-year survival rate of 50%. In selected patients, treatment options include liver transplantation (LT) or transjugular intrahepatic porto
To assess the outcomes of patients who underwent a TIPSS compared to large volume paracentesis (LVP).
Retrospective study of patients who underwent a covered TIPSS or LVP for refractory or recurrent ascites over 7 years. Primary outcome was transplant-free survival (TFS). Further analysis was done with propensity score matching (PSM).
There were 150 patients [TIPSS group (n = 75), LVP group (n = 75)]. Seven patients in the TIPSS group underwent LT vs 22 patients in the LVP group. Overall median follow up, 20 (0.47-179.53) mo. In the whole cohort, there was no difference in TFS [hazard ratio (HR): 0.80, 95% confidence interval (CI): 0.54-1.21]; but lower de novo hepatic encephalopathy with LVP (HR: 95%CI: 0.20-0.96). These findings were confirmed following PSM analysis. On multivariate analysis albumin and hepatocellular carcinoma at baseline were associated with TFS.
Covered TIPSS results in similar TFS compared to LVP in cirrhotic patients with advanced liver failure. Liver transplant assessment should be considered in all potential candidates for TIPSS. Further controlled studies are recommended to select appropriate patients for TIPSS.
Core Tip: Refractory ascites is a serious complication of cirrhosis and portal hypertension with a one-year mortality of 50%. The only curative treatment for refractory ascites is liver transplantation, whilst the non-surgical treatments for refractory ascites include large volume paracentesis (LVP) and transjugular intrahepatic portosystemic stent shunt (TIPSS). A randomized controlled trial showed covered TIPSS can improve survival compared to LVP. In our real world cohort of selected patients with cirrhosis and advanced liver failure, we demonstrate that covered TIPSS results in similar transplant free survival compared to LVP following propensity score matching. This suggests that all patients with refractory ascites that are eligible for TIPSS should be considered for liver transplantation.
- Citation: Dhaliwal A, Merhzad H, Karkhanis S, Tripathi D. Covered transjugular intrahepatic portosystemic stent-shunt vs large volume paracentesis in patients with cirrhosis: A real-world propensity score-matched study. World J Clin Cases 2022; 10(31): 11313-11324
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11313.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11313
Liver cirrhosis is a significant cause of morbidity and mortality in the United Kingdom and worldwide[1-3]. Clinically significant portal hypertension leads to decompensation, with ascites often the first evidence of hepatic decompensation[4]. Ascites is initially treated with diuretics however for those with diuretic intractable ascites, frequent large-volume paracentesis with albumin cover is the remaining option. Patients with refractory ascites have a 1-year survival rate of 50%. In a selection of these patients, treatment options include liver transplantation and transjugular intrahepatic portosystemic stent shunt (TIPSS)[5].
Ascites occurs due to two main mechanisms: Portal hypertension and sodium and water retention. Liver cirrhosis alters the normal hepatic architecture by progressive collagen deposition (fibrosis) and nodular regeneration within the hepatocytes and distortion of the hepatic vasculature[6]. As a result, all these changes, hepatic sinusoids have a reduction in their compliance and there is an increase in resistance to portal flow. Splanchnic vasodilation, mediated by nitrous oxide (extra-hepatic production increases in cirrhosis), and soluble guanylyl cyclase dependent protein kinase G signalling, and other vasoactive mediators, contributes to hyperdynamic circulation manifested as increased cardiac output and heart rate, with a decreased systemic vascular resistance and a low arterial blood pressure. This leads to greater blood flow through the portal vein, which in presence of increased resistance, contributes to portal hypertension. Clinically significant portal hypertension, (defined as a hepatic venous portal gradient ≥ 10 mmHg)[7], whereby there is an increase in the hydrostatic pressure within hepatic sinusoids, compounds this. Hence there is further transduction of fluid into the peritoneal cavity and subsequent ascites[8]. The hyperdynamic circulation results in reduced central blood volume due to splanchnic vasodilation. This triggers increases in renal sympathetic activity including activation of renin-angiotensin and aldosterone systems, and antidiuretic hormone to improve central blood volume. This enhances sodium reabsorption within the renal tubules and collecting ducts resulting in increased sodium and water retention[8-11].
The only curative treatment option in refractory ascites is liver transplantation. This is suitable for selected patients through a rigorous screening and assessment process[12-14]. Whilst diuretic therapy and large volume paracenteses provide a therapeutic approach, more definitive treatment with TIPSS is an option in selected patients[14-16]. TIPSS reduces portal pressure with an initial increase in cardiac output, right atrial pressure, and pulmonary artery pressure leading to a secondary reduction in systemic vascular resistance and effective arterial blood volume. These haemodynamic changes have been reported to return to pre TIPSS level with time. Additionally, there is an increase in urinary sodium excretion and glomerular filtration rate[13,17-20]. It is well established that covered TIPSS is superior to non-covered TIPSS with significantly reduced stent dysfunction and recurrence of portal hypertension-related complications[21,22]. Patient selection for TIPSS has several considerations including the severity of liver disease, renal function, vascular anatomy, nutritional status, risk factors for hepatic encephalopathy (HE), and cardiac function[16]. Patient selection is paramount to a beneficial outcome. One of the most important and disabling complications of TIPSS is the development of de novo HE which occurs in 30%-50% of patients[13].
For those with diuretic intractable ascites, and who are unsuitable for TIPSS and liver transplantation, frequent palliative large volume paracentesis (LVP) remains the main course of symptom management. These patients often have end-stage liver disease, with high short-term mortality, so LVP remains a safe and effective management strategy[5,8]. There is recent interest in long term abdominal drains, which may reduce the need for hospital visits but can be complicated by increased infections. Further research is required[14].
This study aims to assess the outcome of those who underwent covered TIPSS vs LVP for the treatment of refractory ascites. We aimed to ascertain the following: (1) Transplant-free survival (TFS); (2) Clinical or biochemical variables that predict survival; and (3) Risk factors for developing HE.
A retrospective cohort study was performed at the Liver Unit, Queen Elizabeth Hospital, University Hospitals Birmingham. Our study groups consisted of two groups. Group 1 comprised patients who underwent a covered TIPSS between April 2010 to November 2017. Group 2 (the standard of care group) comprised patients who underwent frequent LVP with albumin cover between January 2011 to November 2017. Patients were identified using our institute’s electronic information technology informatics system and electronic patient database through coding methods.
We included patients with liver cirrhosis, and an age greater than or equal to 18. For Group 1, we included those who had covered TIPSS for refractory or recurrent ascites as an elective procedure. For Group 2 we included those who had > 1 LVP per month.
We excluded patients who did not have a diagnosis of liver cirrhosis, those who underwent a TIPSS insertion for variceal haemorrhage, those who underwent frequent paracentesis due to malignancy including hepatocellular carcinoma (HCC) with malignant spread or underwent liver transplantation before first LVP.
The primary outcome measure of this study was TFS. We had several secondary endpoints which included effectiveness of TIPSS compared with LVP as quantified by the number of LVP per month and complications of TIPSS in the form of de novo HE. Follow up was carried out until 2017 or until death had occurred.
TIPSS group: After the patient’s informed consent, TIPSS (Viatorr® stent-graft; GORE, Flagstaff, AR, United States) was placed in the standard method described previously, and the tract between right hepatic and portal veins was dilated to 8-10 mm[16]. Pre-TIPSS (either portal pressure or hepatic venous pressure gradient) and post-TIPSS pressures were measured. Patients were monitored for any immediate complications for at least 48 h post-procedure. None of these patients received anticoagulation post-TIPSS. The patency was routinely assessed by follow up 6-12 mo doppler US at clinic review and venography if indicated by the US scan or clinical deterioration.
LVP group: LVP was performed according to the local guidelines with 20 g of albumin administered for every 2 L to 3 L ascites fluid drained, in concordance with the local and national guidance. The procedures were performed as a day case for most patients. All patients were followed up routinely in the outpatient clinic at 3-6 mo intervals.
We compared biochemical and clinical parameters for each group. The clinical parameters were obtained from clinical medical electronic records. These included mortality rates, complications of portal hypertension (HE and gastric or oesophageal varices), development of HCC, and use of non-selective beta-blockers (NSBB). We collected laboratory records to assess severity staging in the form of model for end-stage liver disease (MELD) and United Kingdom model for end-stage liver disease (UKELD), liver function, hematology and renal function parameters. These were recorded at the time of the index intervention [i.e. TIPSS or first LVP (1 ± 1 d)].
Categorical variables such as gender were expressed as a number and percentage. Numerical data were expressed as a mean ± standard deviation for normally distributed data. Data was also expressed as median with a range where appropriate.
Comparisons between the two groups were performed using an unpaired t test or chi-squared test. Both univariate and multivariable analyses were used to control for differences in selected independent parameters such as MELD score. A Cox regression analysis was used to identify clinical and biochemical variables predicting survival. Actuarial probability survival curves were constructed using the Kaplan Meier method and compared with the log-rank test. To confirm the validity of the results in matched cohorts, a propensity score analysis was performed. Propensity score matching (1:1) with matched tolerance of 0.02 was performed to account for covariates (platelet count, MELD, gender, sodium and age). Further supplemental sensitivity analysis was done using the propensity score weighting method[23]. Statistical significance was established at a P < 0.05. SPSS statistical software (version 27) was used to perform the analyses.
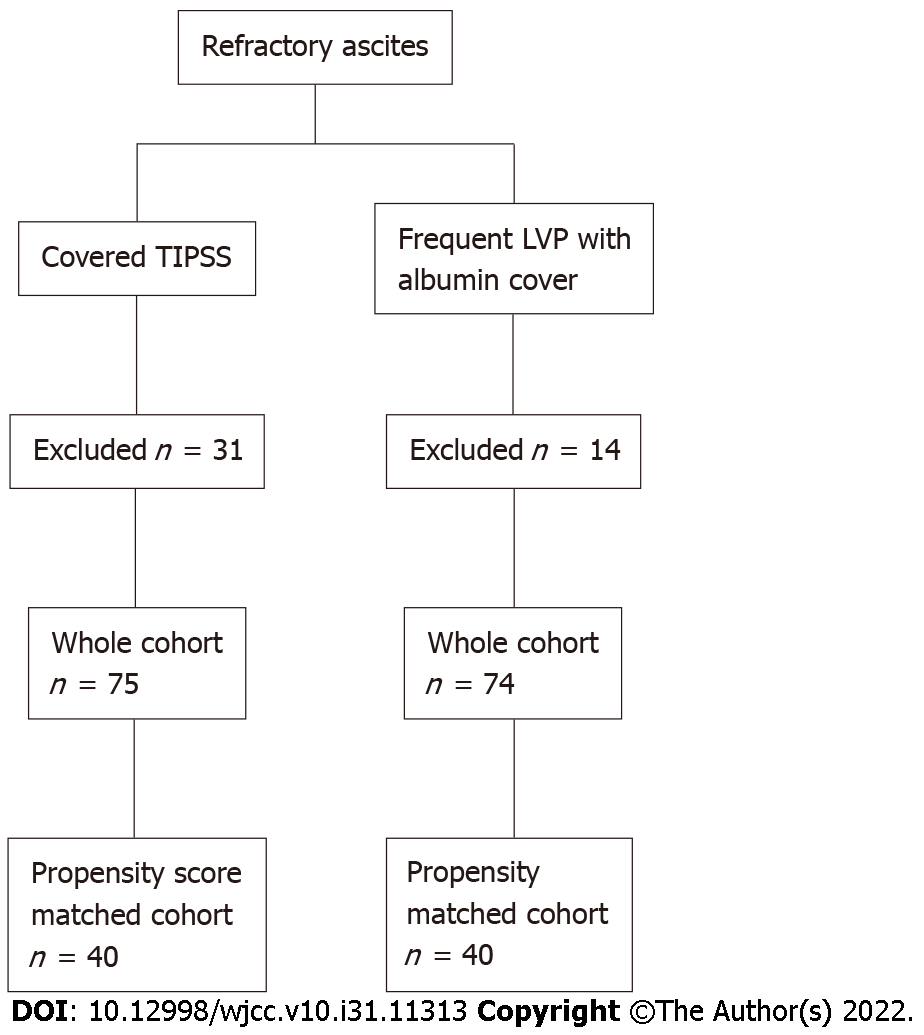
There were 106 patients identified as receiving a covered TIPSS. We excluded 31 patients due to other indications such as a repeated procedure with an existing TIPSS in situ, and who did not fulfil the inclusion criteria. Thus, the TIPSS group comprised 75 patients. There were 89 patients with liver cirrhosis who underwent frequent LVP with albumin cover within our hospital, however, 14 were excluded as they met the exclusion criteria. Hence, the LVP group comprised of n = 75 (Figure 1, Table 1).
| Whole cohort | Propensity score - matched cohort | ||||||
| TIPSS (n = 75) | LVP (n = 75) | P value | TIPSS (n = 40) | LVP (n = 40) | P value | ||
| Age (mean yr) | 59.1 ± 9.4 | 61.8 ± 11.5 | 0.119 | 61.2 ± 9.1 | 61.5 ± 12.2 | 0.885 | |
| Gender | Male | 41 | 46 | 0.275 | 23 | 26 | 0.616 |
| Female | 34 | 29 | 0.275 | 17 | 14 | 0.491 | |
| Aetiology | ArLD | 60 | 47 | 0.174 | 32 | 22 | 0.297 |
| AIH | 1 | 1 | 0 | 1 | |||
| NAFLD | 9 | 9 | 3 | 8 | |||
| Cryptogenic | 2 | 5 | 2 | 4 | |||
| HCV | 1 | 7 | 3 | 2 | |||
| HBV | 0 | 2 | 0 | 1 | |||
| PBC | 0 | 3 | 0 | 1 | |||
| PSC | 0 | 1 | 0 | 1 | |||
| Other | 2 | 0 | 2 | 1 | |||
| History of HCC | 7 | 5 | 0.220 | 3 | 2 | 0.644 | |
| History of spontaneous bacterial peritonitis | 17 | 15 | 0.849 | 13 | 6 | 0.079 | |
| Type of ascites (recurrent/refractory) | 61/14 | 35/40 | < 0.001 | 22/18 | 31/9 | 0.03 | |
| Liver transplantation | 7 | 22 | < 0.001 | 4 | 12 | 0.029 | |
| Varices at baseline | 21 | 26 | 0.304 | 12 | 13 | 0.135 | |
| Use of non-selective beta-blockers | 16 | 20 | 0.484 | 10 | 13 | 0.459 | |
| Mean follow up (mo) | 27.5 ± 29.3 | 16.4 ± 16.4 | 0.005 | 26.3 ± 35.3 | 15.5 ± 14.9 | 0.08 | |
| Median follow up (range, mo) | 16.5 (0.47-179.53) | 10.3 (1.4-73.86) | 0.050 | 8.2 (0.47-179.53) | 10.3 (1.5-73.86) | 0.823 | |
With regards to the severity of liver disease, compared to the TIPSS group, the LVP group had a significantly higher mean UKELD (51.5 ± 4.2 vs 54.6 ± 4.8) and MELD (11.5 ± 3.9 vs 15.9 ± 5.3) (Table 2). There was no difference in the use of NSBB, presence of varices, HCC, or history of spontaneous bacterial peritonitis (SBP) at baseline.
| Whole cohort | Propensity score-matched cohort | |||||||||
| TIPSS (n = 75) | LVP (n = 75) | TIPSS (n = 40) | LVP (n = 40) | |||||||
| mean | SD | mean | SD | P value | mean | SD | mean | SD | P value | |
| UKELD | 51.51 | 4.17 | 54.57 | 4.84 | < 0.001 | 51.55 | 4.08 | 52.45 | 4.07 | 0.326 |
| MELD | 11.47 | 3.86 | 15.93 | 5.31 | < 0.001 | 12.78 | 4.492 | 12.78 | 3.62 | 1 |
| INR | 1.25 | 0.19 | 1.46 | 0.33 | < 0.001 | 1.25 | 0.21 | 1.35 | 0.23 | 0.048 |
| Bilirubin (μmol/l) | 20.28 | 16.77 | 42.13 | 40.49 | < 0.001 | 22.9 | 20.027 | 27.43 | 21.205 | 0.33 |
| Creatinine (μmol/l) | 96.77 | 57.13 | 97.99 | 78.11 | 0.914 | 108.1 | 73.88 | 89.75 | 40.45 | 0.172 |
| Sodium (mmol/L) | 135.40 | 4.84 | 134.79 | 4.67 | 0.431 | 135.98 | 4.092 | 136.07 | 4.548 | 0.918 |
| Platelets (× 109/L) | 167.95 | 73.21 | 141.16 | 75.66 | 0.029 | 160.43 | 73.856 | 154.95 | 90.098 | 0.767 |
Table 1 also shows the characteristics of the propensity score-matched cohort. The TIPSS group and LVP group comprised 40 patients each. The baseline characteristics were well matched although fewer patients underwent liver transplantation and had refractory ascites in the TIPSS group. These differences were also present in the PSM cohort but to a lesser degree. Table 2 shows that laboratory data and clinical scoring systems were similar in both groups.
Effectiveness of TIPSS: The portal pressure gradient (PPG) decreased from 15.7 ± 4.9 mmHg to 6.7 ± 2.7 mmHg following TIPSS implantation with a mean PPG reduction of 54.7% ± 17.6 %. In 59 and 16 patients the stent was dilated to 8 mm and 10 mm respectively. TIPSS resulted in a significant reduction in the requirement for paracentesis per month compared with the LVP group (0.1 ± 0.6 vs 1.2 ± 0.6, P < 0.001). Diuretics were required during the clinical course in all patients in the LVP group, and in only 14.7% of patients in the TIPSS group. Further LVP was not required in 74.7% of patients in the TIPSS group. There was no difference in the baseline aetiology of liver disease (P = 0.44), MELD (P = 0.69), Childs Pugh score (CPS) (P = 0.24) , bilirubin (P = 0.05), platelets (P = 0.53), albumin (P = 0.98), age (P = 0.96), gender (P = 0.39), history of SBP (P = 0.6), presence of varices (P = 0.24), TIPSS diameter (P = 0.30) and PPG % reduction post TIPSS (P = 0.80), PPG post TIPSS (P = 0.58) between those who did or did not require further LVP post TIPSS.
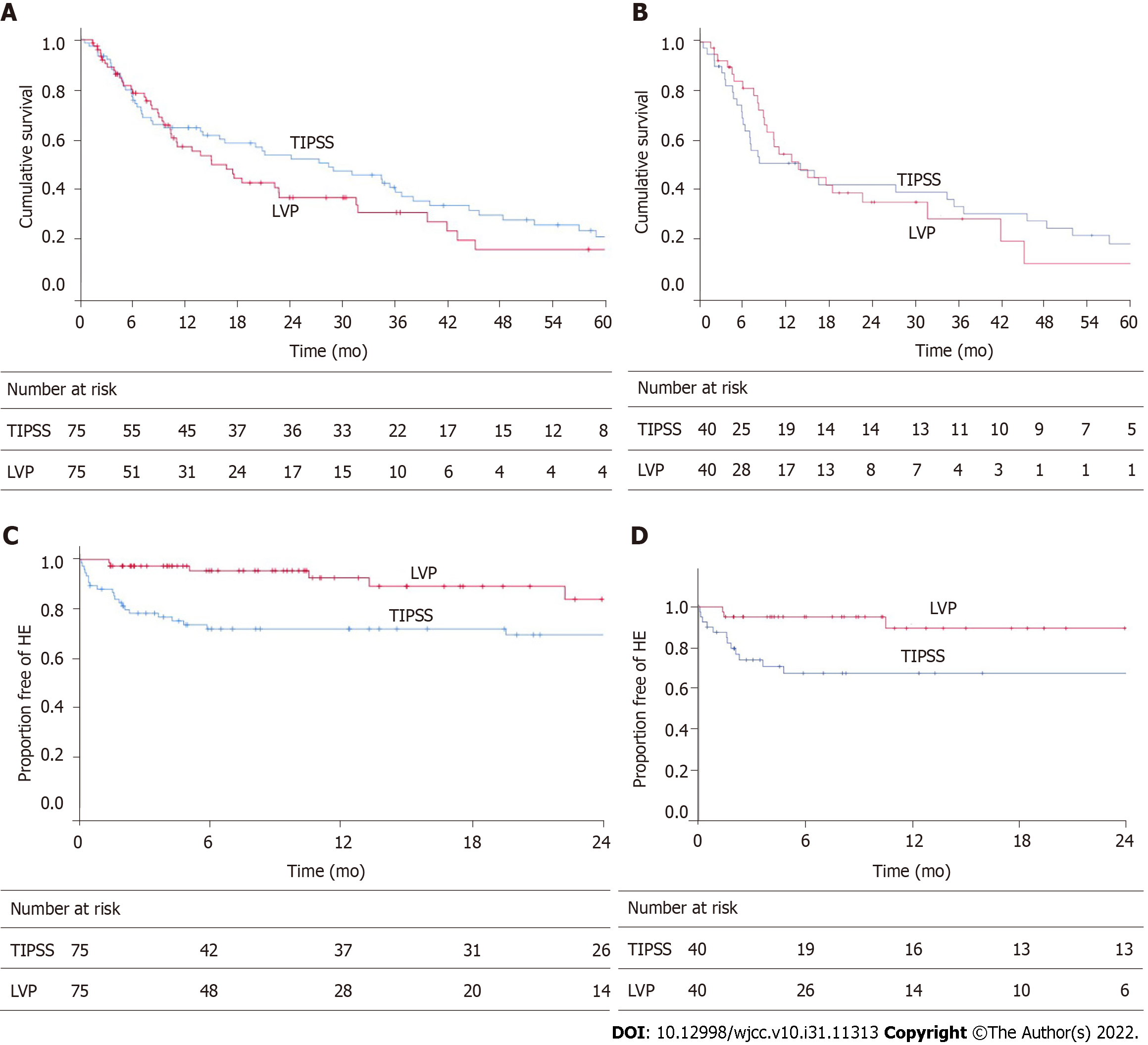
Whole cohort: The actuarial rate of TFS at 6 mo, 12 mo, 24 mo and 60 mo for each group is as follows: TIPSS group 76%, 64%, 53%, 20%; LVP group 78%, 55%, 36%, 15%, respectively. Figure 2A shows the Kaplan Meier curve to represent this. The causes of death are detailed in Table 3. Analysis using log-rank statistics did not reveal any significant difference in TFS (HR: 1.24, 95%CI: 0.83-1.86). In the TIPSS arm, an increased number of paracentesis per month (HR: 1.35, 95%CI: 1.02-1.77) and 8 mm stent diameter (HR: 2.93, 95%CI: 1.31-6.52) was associated with a worse TFS.
| Cause of death | TIPSS (n = 54) | LVP (n = 45) |
| End-stage liver disease | 29 | 31 |
| HCC | 7 | 4 |
| Sepsis | 5 | 7 |
| Cerebrovascular accident | 2 | 1 |
| Other | 11 | 2 |
Univariate analysis demonstrated that albumin, HCC at baseline and CPS predicted TFS (Table 4). Multivariable analyses showed that only HCC at baseline and albumin were significant as predictors of survival (Tables 4 and 5). Further analysis excluding patients with HCC at baseline, which can cause significant confounding, demonstrated that there remained no significant differences in TFS.
| B | SE | Wald | Df | P value | Hazard ratio | 95%CI | |
| Age | 0.011 | 0.010 | 1.259 | 1 | 0.262 | 1.011 | 0.992-1.031 |
| Albumin | -0.086 | 0.021 | 17.127 | 1 | < 0.001 | 0.917 | 0.880-0.955 |
| Recurrent Ascites | -0.033 | 0.211 | .025 | 1 | 0.874 | 0.967 | 0.640-1.462 |
| TIPSS | -0.213 | 0.207 | 1.055 | 1 | 0.304 | 0.808 | 0.539-1.213 |
| HCC at baseline | 1.424 | 0.328 | 18.788 | 1 | < 0.001 | 4.152 | 2.181-7.903 |
| MELD | 0.010 | 0.021 | 0.248 | 1 | 0.619 | 1.010 | 0.970-1.053 |
| Sex (1 = male, 2 = female) | 0.104 | 0.202 | 0.263 | 1 | 0.608 | 1.109 | 0.746-1.649 |
| CPS | 0.169 | 0.065 | 6.716 | 1 | 0.010 | 1.184 | 1.042-1.345 |
| SBP | -0.068 | 0.251 | 0.074 | 1 | 0.786 | 0.934 | 0.571-1.527 |
| B | SE | Wald | Df | P value | Hazard ratio | 95%CI | |
| Age | 0.006 | 0.011 | 0.267 | 1 | 0.605 | 1.006 | 0.984-1.028 |
| Albumin | -0.081 | 0.027 | 9.185 | 1 | 0.002 | .923 | 0.876-0.972 |
| Recurrent ascites | 0.041 | 0.252 | 0.026 | 1 | 0.872 | 1.042 | 0.635-1.708 |
| TIPSS | -0.219 | 0.303 | 0.521 | 1 | 0.471 | 0.804 | 0.444-1.456 |
| HCC at baseline | -1.548 | 0.363 | 18.232 | 1 | < 0.001 | 0.213 | 0.104-0.433 |
| MELD | -0.033 | .035 | 0.900 | 1 | 0.343 | 0.968 | 0.904-1.036 |
| Sex (1 = male, 2 = female) | 0.134 | 0.222 | 0.364 | 1 | 0.546 | 1.144 | 0.739-1.769 |
| CPS | 0.085 | 0.127 | 0.446 | 1 | 0.504 | 1.088 | 0.849-1.395 |
| SBP | -0.054 | 0.264 | 0.042 | 1 | 0.837 | 0.947 | 0.565-1.589 |
Propensity score-matched cohort: The actuarial rate of TFS at 6 mo,12 mo, 24 mo and 60 mo for each group is as follows: TIPSS group 66%, 50%, 41%, and 17%; LVP group 81%, 54%, 34% respectively (Figure 2B). There was no significant difference in TFS (HR: 1.00, 95%CI: 0.58-1.73). A sensitivity analysis using propensity score weighting method confirmed the lack of significance between the cohorts (HR: 0.95, 95%CI: 0.60-1.53).
Whole cohort: The actuarial rate of de novo HE at 6 mo, 12 mo, and 24 mo for each group are as follows; TIPSS: 28%, 28%, 31%. LVP group 5%, 7%, and 16% (Figure 2C). This was a statistically significant difference in favour of LVP (HR: 0.44, 95%CI: 0.20-0.96). In the TIPSS arm, neither diameter of the stent (P = 0.35), PPG post-TIPSS (P = 0.88), or degree of PPG reduction (0.74) influenced the rate of de novo HE. TIPSS reduction was performed successfully in two patients due to refractory HE.
Propensity score-matched cohort: The actuarial rate of de novo HE at 6 mo, 12 mo, and 24 mo for each group are as follows; TIPSS: 33%, 33%, 33%. LVP group 5%, 11%, and 11% (Figure 2D). This was a statistically significant difference in favour of LVP [Figure 2D; HR: 0.30 95%CI: 0.10-0.94, P = 0.03 (log-rank)]. A sensitivity analysis using a propensity score weighting method confirmed the difference in favour of LVP (HR: 0.38, 95%CI: 0.15-0.95).
Seven patients in the TIPSS group underwent liver transplantation vs twenty-two patients in the LVP group (P = 0.002). The mean time to transplantation during follow up was 13.4 ± 16.5 mo, with no difference between the groups.
We have shown in our retrospective study of 150 patients, which is one of the largest comparative series in the literature, that TFS following covered TIPSS for refractory ascites is similar to LVP, with increased HE with TIPSS. We also found that the control of ascites was significantly better with TIPSS.
We controlled for confounders by using a propensity score matching, which corroborated these findings. We found that the long-term outcomes are indeed poor in both groups, with 5-year TFS in TIPSS and LVP cohorts of 20% and 15% respectively. This compares with 5-year survival post-liver transplantation in Europe of 71%[24]. This supports and reinforces the view that liver transplantation is the best option in eligible patients with end stage liver disease (ESLD).
There is only one randomized controlled trial of covered TIPSS vs LVP which showed improved TFS without increased risk of HE[25]. This trial only included patients with recurrent ascites. We include both recurrent and refractory ascites and did not find the presence of recurrent ascites influenced TFS. The impact on TFS remains uncertain based on a recent network meta-analysis of 287 participants which showed that TIPSS resulted in greater resolution of ascites compared to LVP but no difference in mortality or adverse events[26]. Our real-world cohort reinforces these findings. A recent retrospective study of TIPSS vs LVP showed that TIPSS was not independently associated with TFS.
Hepatic encephalopathy is one of the symptoms that significantly affect those with advanced liver failure. It can manifest both covertly and overtly in patients. Whilst a thorough assessment for the presence of HE is required before consideration for TIPSS, it remains a challenge to manage. We found that de novo HE rates were higher with TIPSS in the whole cohort and the cohort after excluding TIPSS contraindications. Bucsics et al[27] also found in their retrospective study of TIPSS vs LVP similar rates of de novo HE in both LVP and TIPSS cohorts.
A recent study concluded that covered TIPSS resulted in superior control of ascites without increasing the risk for overt HE as compared to LVP[27]. We believe our data reflects the real-world experience, and has the strength of a larger sample size and follow up. There is interest in the role of rifaximin prophylactically before TIPSS in reducing the risk of HE after TIPSS[28]. We did not use rifaximin to prevent HE post TIPSS, as the evidence of benefit was published after the recruitment period for our study. Controlled expansion stents can also reduce the risk of passive dilatation of stents and HE but further controlled data is required[29].
We only had 7 patients (9%) of our TIPSS cohort undergo liver transplantation, which is far less than in the LVP cohort where 22 (29%) patients underwent transplantation. This suggests that patients with TIPSS may have been less likely to be referred for liver transplantation due to control of ascites. Furthermore, the current scoring methods of liver disease are not as helpful in refractory ascites. Our data would strongly suggest that all patients undergoing TIPSS must be considered for transplant at an early stage, in particular, those not responding to TIPSS or where there is deteriorating liver function.
Serum albumin and hepatocellular carcinoma emerged as independent predictors of survival in keeping with recently published data[27]. However, there were differences in the rate of HCC at baseline which were not controlled by propensity score matching, unlike our study. We also performed a separate analysis excluding HCC and found no differences in TFS.
An important finding of a recent study was the superior control of ascites post TIPSS with early TIPSS insertion at lower paracentesis frequencies and creatinine levels. Persistent ascites post-TIPSS was a predictor of liver transplantation and death[30]. We found that lack of effect of TIPSS, as judged by the increased need for LVP post TIPSS was associated with poor TFS. This would suggest that patients with poor efficacy after TIPSS should be considered for liver transplantation at an early stage.
The diameter of the stent is an important consideration. There is conflicting literature on the impact of stent diameter on outcomes, and no recommendation can be made at this time[14]. In most of our patients, the stents were dilated to 8 mm, and interestingly the use of 10mm diameter was associated with better TFS. We would advise caution in the interpretation of this finding due to the small numbers of patients with 10 mm stents. We did not find the post TIPSS PPG or proportional reduction of PPG post TIPSS associated with TIPSS efficacy, and this was also the case in the recent study by Piecha et al[30]. It is also worth noting that passive dilatation of TIPSS stent occurs even with 8 mm dilatation, although recent controlled expansion stents are much less prone to this phenomenon[29,31]. Therefore, the stent diameter and PPG at the time of TIPSS insertion is likely to change significantly over time.
The recognition of sarcopenia is an evolving consideration in those with ESLD. Sarcopenia is associated with increased mortality in those with ESLD and nutritional assessment is now recommended in patients considered for elective TIPSS[32]. However, the data for patients with sarcopenia and advanced cirrhosis and undergoing TIPSS is inconsistent. A recent study found that sarcopenia (defined as muscle mass alone) did not have an impact on survival in a similar cohort of patients with refractory ascites undergoing TIPSS[33]. It is important to recognise the lack of functional elements in this definition of sarcopenia as this may demonstrate different outcomes. Frailty, which incorporates this functional element[34] which should also be a consideration in future work. Whilst we did not comment on the degree of sarcopenia in our cohort, it may have been a contributing factor and should be a future consideration to research.
Our study does have some limitations. The retrospective nature introduces selection bias, but we selected consecutive patients in a real-world setting from a single institution, and the large sample size is a major strength. Moreover, we carefully controlled for key confounders using propensity score matching and still retained a total of 80 patients. The increased rate of transplantation in the LVP group also introduces bias concerning competing risks. However, we selected TFS to minimise this bias. PSM resulted in similar follow up for TIPSS and LVP groups which could help to minimise this confounder.
We found that after controlling for confounding factors, our retrospective real-world data shows that TFS was similar following covered TIPSS for refractory or recurrent ascites compared with LVP. The presence of HCC, low albumin, and poor response to TIPSS are associated with poor survival. The long term outcomes following TIPSS are poor. From this, we can conclude that liver transplantation must be considered for refractory ascites in selected patients. For patients not suitable for liver transplantation, other interventions for refractory ascites could be considered palliative. Further prospective studies are required in multicentre controlled trials to identify prognostic markers to aid patient selection for interventions for refractory ascites.
Refractory ascites has a 1-year survival rate of 50%. In selected patients, treatment options include liver transplantation (LT) or transjugular intrahepatic portosystemic stent shunt (TIPSS).
We aimed to assess the outcomes of patients who underwent a TIPSS compared to large volume paracentesis (LVP).
We devised a retrospective study of patients who underwent a covered TIPSS or LVP for refractory or recurrent ascites over 7 years.
Primary outcome was transplant-free survival (TFS). Further analysis was done with propensity score matching (PSM).
There were 150 patients [TIPSS group (n = 75), LVP group (n = 75)]. Seven patients in the TIPSS group underwent LT vs 22 patients in the LVP group. Overall median follow up, 20 (0.47-179.53) mo. In the whole cohort, there was no difference in TFS [hazard ratio (HR): 0.80, 95% confidence interval (CI): 0.54-1.21], but lower de novo hepatic encephalopathy with LVP (HR: 0.44, 95%CI: 0.20-0.96). These findings were confirmed following PSM analysis. On multivariate analysis albumin and HCC at baseline were associated with TFS.
Covered TIPSS results in similar TFS compared to LVP in cirrhotic patients with advanced liver failure. Liver transplant assessment should be considered in all potential candidates for TIPSS.
Future research should be targeted at controlled studies are recommended to select appropriate patients for TIPSS.
| 1. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 919] [Article Influence: 70.7] [Reference Citation Analysis (2)] |
| 2. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1354] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 3. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1184] [Cited by in RCA: 1121] [Article Influence: 186.8] [Reference Citation Analysis (5)] |
| 4. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2215] [Article Influence: 110.8] [Reference Citation Analysis (3)] |
| 5. | Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, Jimenez W, Planas R, Arroyo V. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 634] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 7. | Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol. 2014;20:6-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (2)] |
| 8. | Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55 Suppl 6:vi1-v12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Bernardi M, Fornalè L, Di Marco C, Trevisani F, Baraldini M, Gasbarrini A, De Collibus C, Zacà F, Ligabue A, Colantoni A. Hyperdynamic circulation of advanced cirrhosis: a re-appraisal based on posture-induced changes in hemodynamics. J Hepatol. 1995;22:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Lang F, Tschernko E, Schulze E, Ottl I, Ritter M, Völkl H, Hallbrucker C, Häussinger D. Hepatorenal reflex regulating kidney function. Hepatology. 1991;14:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 11. | Jalan R, Hayes PC. Sodium handling in patients with well compensated cirrhosis is dependent on the severity of liver disease and portal pressure. Gut. 2000;46:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Haydon GH, Neuberger J. Liver transplantation of patients in end-stage cirrhosis. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:1049-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1986] [Article Influence: 248.3] [Reference Citation Analysis (2)] |
| 14. | Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, Wilkes EA, Moore K, Leithead JA, Hayes PC, O'Brien AJ, Verma S. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 15. | Fagiuoli S, Bruno R, Debernardi Venon W, Schepis F, Vizzutti F, Toniutto P, Senzolo M, Caraceni P, Salerno F, Angeli P, Cioni R, Vitale A, Grosso M, De Gasperi A, D'Amico G, Marzano A; AISF TIPS Special Conference. Consensus conference on TIPS management: Techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 16. | Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, Rowe IA, Roslund N, Ireland H, Lomax M, Leithead JA, Mehrzad H, Aspinall RJ, McDonagh J, Patch D. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 17. | Quiroga J, Sangro B, Núñez M, Bilbao I, Longo J, García-Villarreal L, Zozaya JM, Betés M, Herrero JI, Prieto J. Transjugular intrahepatic portal-systemic shunt in the treatment of refractory ascites: effect on clinical, renal, humoral, and hemodynamic parameters. Hepatology. 1995;21:986-994. [PubMed] |
| 18. | Sanyal AJ, Freedman AM, Luketic VA, Purdum PP 3rd, Shiffman ML, DeMeo J, Cole PE, Tisnado J. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology. 1997;112:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 199] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Ochs A, Rössle M, Haag K, Hauenstein KH, Deibert P, Siegerstetter V, Huonker M, Langer M, Blum HE. The transjugular intrahepatic portosystemic stent-shunt procedure for refractory ascites. N Engl J Med. 1995;332:1192-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 275] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 21. | Maleux G, Perez-Gutierrez NA, Evrard S, Mroue A, Le Moine O, Laleman W, Nevens F. Covered stents are better than uncovered stents for transjugular intrahepatic portosystemic shunts in cirrhotic patients with refractory ascites: a retrospective cohort study. Acta Gastroenterol Belg. 2010;73:336-341. [PubMed] |
| 22. | Qi X, Tian Y, Zhang W, Yang Z, Guo X. Covered versus bare stents for transjugular intrahepatic portosystemic shunt: an updated meta-analysis of randomized controlled trials. Therap Adv Gastroenterol. 2017;10:32-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Ridgeway G, McCaffrey DF, Morral AR, Burgette LF, Griffin BA. Toolkit for Weighting and Analysis of Nonequivalent Groups: A Tutorial for the R TWANG Package. RAND Corporation. 2014;. [DOI] [Full Text] |
| 24. | Adam R, Karam V, Cailliez V, O Grady JG, Mirza D, Cherqui D, Klempnauer J, Salizzoni M, Pratschke J, Jamieson N, Hidalgo E, Paul A, Andujar RL, Lerut J, Fisher L, Boudjema K, Fondevila C, Soubrane O, Bachellier P, Pinna AD, Berlakovich G, Bennet W, Pinzani M, Schemmer P, Zieniewicz K, Romero CJ, De Simone P, Ericzon BG, Schneeberger S, Wigmore SJ, Prous JF, Colledan M, Porte RJ, Yilmaz S, Azoulay D, Pirenne J, Line PD, Trunecka P, Navarro F, Lopez AV, De Carlis L, Pena SR, Kochs E, Duvoux C; all the other 126 contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). 2018 Annual Report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl Int. 2018;31:1293-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 357] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 25. | Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, Mathurin P, Otal P, Cabarrou P, Péron JM, Vinel JP. Transjugular Intrahepatic Portosystemic Shunts With Covered Stents Increase Transplant-Free Survival of Patients With Cirrhosis and Recurrent Ascites. Gastroenterology. 2017;152:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 26. | Benmassaoud A, Freeman SC, Roccarina D, Plaz Torres MC, Sutton AJ, Cooper NJ, Iogna Prat L, Cowlin M, Milne EJ, Hawkins N, Davidson BR, Pavlov CS, Thorburn D, Tsochatzis E, Gurusamy KS. Treatment for ascites in adults with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. 2020;1:CD013123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Bucsics T, Hoffman S, Grünberger J, Schoder M, Matzek W, Stadlmann A, Mandorfer M, Schwabl P, Ferlitsch A, Peck-Radosavljevic M, Trauner M, Karner J, Karnel F, Reiberger T. ePTFE-TIPS vs repetitive LVP plus albumin for the treatment of refractory ascites in patients with cirrhosis. Liver Int. 2018;38:1036-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Bureau C, Thabut D, Jezequel C, Archambeaud I, D'Alteroche L, Dharancy S, Borentain P, Oberti F, Plessier A, De Ledinghen V, Ganne-Carrié N, Carbonell N, Rousseau V, Sommet A, Péron JM, Vinel JP. The Use of Rifaximin in the Prevention of Overt Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt: A Randomized Controlled Trial. Ann Intern Med. 2021;174:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 29. | Coronado WM, Ju C, Bullen J, Kapoor B. Predictors of Occurrence and Risk of Hepatic Encephalopathy After TIPS Creation: A 15-Year Experience. Cardiovasc Intervent Radiol. 2020;43:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Piecha F, Radunski UK, Ozga AK, Steins D, Drolz A, Horvatits T, Spink C, Ittrich H, Benten D, Lohse AW, Sinning C, Kluwe J. Ascites control by TIPS is more successful in patients with a lower paracentesis frequency and is associated with improved survival. JHEP Rep. 2019;1:90-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Schepis F, Vizzutti F, Garcia-Tsao G, Marzocchi G, Rega L, De Maria N, Di Maira T, Gitto S, Caporali C, Colopi S, De Santis M, Arena U, Rampoldi A, Airoldi A, Cannavale A, Fanelli F, Mosconi C, Renzulli M, Agazzi R, Nani R, Quaretti P, Fiorina I, Moramarco L, Miraglia R, Luca A, Bruno R, Fagiuoli S, Golfieri R, Torricelli P, Di Benedetto F, Belli LS, Banchelli F, Laffi G, Marra F, Villa E. Under-dilated TIPS Associate With Efficacy and Reduced Encephalopathy in a Prospective, Non-randomized Study of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2018;16:1153-1162.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 32. | Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 33. | Benmassaoud A, Roccarina D, Arico F, Leandro G, Yu B, Cheng F, Yu D, Patch D, Tsochatzis E. Sarcopenia Does Not Worsen Survival in Patients With Cirrhosis Undergoing Transjugular Intrahepatic Portosystemic Shunt for Refractory Ascites. Am J Gastroenterol. 2020;115:1911-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Lai JC, Sonnenday CJ, Tapper EB, Duarte-Rojo A, Dunn MA, Bernal W, Carey EJ, Dasarathy S, Kamath BM, Kappus MR, Montano-Loza AJ, Nagai S, Tandon P. Frailty in liver transplantation: An expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant. 2019;19:1896-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Perazzo JC, Argentina; Sintusek P, Thailand S-Editor: Wang DM L-Editor: A P-Editor: Wang DM