Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11240
Peer-review started: July 30, 2022
First decision: September 2, 2022
Revised: September 7, 2022
Accepted: September 27, 2022
Article in press: September 27, 2022
Published online: November 6, 2022
Processing time: 88 Days and 13.8 Hours
Metabolically associated fatty liver disease (MAFLD) is a common cause of chronic liver disease, the hepatic manifestation of metabolic syndrome. Despite the increasing incidence of MAFLD, no effective treatment is available. Recent research indicates a link between the intestinal microbiota and liver diseases such as MAFLD. The composition and characteristics of the intestinal microbiota and therapeutic perspectives of MAFLD are reviewed in the current study. An imbalance in the intestinal microbiota increases intestinal permeability and exposure of the liver to adipokines. Furthermore, we focused on reviewing the latest "gut-liver axis" targeted therapy.
Core Tip: Recent animal studies and Clinical researches have placed the gut microbiota as a potentially important player in the pathogenesis of metabolically associated fatty liver disease (MAFLD). It is logical to target the gut microbiota to develop new strategies for MAFLD therapy. In addition, we assessed the therapeutic potential of intestinal microbiota manipulation for treating MAFLD and discussed the specific doses and duration of use can provide more help for clinicians in choosing treatment options.
- Citation: Wang JS, Liu JC. Intestinal microbiota in the treatment of metabolically associated fatty liver disease. World J Clin Cases 2022; 10(31): 11240-11251
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11240.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11240
Metabolically associated fatty liver disease (MAFLD) is a spectrum of liver disorders, including metabolically associated steatohepatitis (MASH), and cirrhosis[1]. MAFLD is the most common liver disease worldwide, affecting over half a billion people[2]. This disease can progress from fibrosis to cirrhosis and can be complicated by hepatocellular cancer[3].
Obesity and insulin resistance are two of the many factors that can promote the progression of MAFLD and MASH[4]. The most common manifestation of MAFLD is excessive fat accumulation in hepatocytes[5]. The underlying mechanisms leading to MAFLD are still unknown[6]. Defective lipolysis has been recognized as an important mechanism underlying human MAFLD pathogenesis[7]. Primary and secondary changes in the bile acid pool have also been implicated in MAFLD pathogenesis[8]. Recently, a study reported that Helicobacter pylori (H. pylori) eradication may prevent metabolic syndrome including MAFLD[9]. However, this study found that H. pylori eradication did not affect metabolic indices at all. It was suggested that intestinal microbiota might regulate insulin resistance in MAFLD patients. A potential role for the gut microbiota in the pathogenesis of MAFLD has been found in recent animal studies[10].
In this review, we summarized the role of intestinal microbiota in the treatment of MAFLD. Furthermore, we discussed the specific doses and duration of use of intestinal microbiota manipulation for treating MAFLD and discussed the therapeutic potential.
Microbial communities are widely distributed in the gut, lungs, skin, and other epithelial surfaces[11]. Approximately a thousand different species of bacteria live in the human gut[12]. The human gut microbiota includes bacteria, viruses, and fungi[13]. Bacteroidetes and Firmicutes are the dominant groups of bacteria among the several other groups of bacteria found in the normal intestinal microflora[14]. The non-bacterial intestinal microorganisms are also important for human health as bacteria[15]. Future research should focus more on non-bacterial gut microbes and human diseases.
Bacteria in the gastrointestinal tract produce vitamins, absorb ions, protect the host from pathogens, induce histological development, and enhance immune function, among other benefits[16]. The absence of beneficial microorganisms promoting appropriate immune development results in inflammatory responses underlying various human immune diseases[17]. For example, inflammatory bowel disease pathogenesis is associated with disrupted intestinal barrier function, gut microbiome imbalance, and subsequent dysregulated mucosal immune responses to gut commensal bacteria[18,19]. Their significance for human health has been demonstrated by several studies. The composition and distribution of gut microbiota change under different conditions due to their variation. Dysbiosis of the intestinal microbiota may impact human health and disease[20]. Patients with MAFLD and MASH do not only show compositional changes in the gut microbiota (Table 1), but also have a higher prevalence small intestinal bacterial overgrowth[21-23].
| Bacteria | No MASH (n = 22) | MASH (n = 35) |
| Bifidobacterium | 0.9 | 1.6 |
| Bacteroides | 38.3 | 56.9 |
| Parabacteroides | 2.0 | 1.2 |
| Prevotella | 21.7 | 5.5 |
| Blautia | 1.6 | 1.9 |
| Ruminococcus | 0.8 | 1.4 |
| Megasphaera | 1.5 | 1.5 |
| Sutterella | 1.3 | 0.9 |
Intestinal barriers are comprised of biofilms, mucus layers, and epithelial cells[24]. The intestinal barrier is characterized by physiological and immunological protection and maintains the digestive and absorptive functions of the intestine while restricting pathogen and toxic metabolite invasion into the circulation[25]. The impaired intestinal barrier allows bacteria and their products, including pathogen-associated molecular patterns (PAMPs) into the circulatory system[26]. In addition to the intestinal epithelial barrier, some researchers have discovered that the gut-vascular barrier (GVB) prevents the systemic dissemination of bacteria, bacterial antigens, and other luminal contents through the intestinal epithelial barrier[27]. When the microbiota is dysbiotic, it affects the GVB, leading to an increase in intestinal blood vessel permeability when consumed with a high-fat diet (HFD)[28]. GVB-related research reveals how the gut–liver axis can be regulated to prevent MAFLD, as GVB impairment is a precursor to the disease[28].
The portal vein provides the liver with 70% of its blood supply from the intestine[29]. By combining with the Toll-like receptor-4, intestinal bacterial endotoxins participate in the oxidative stress response and promote the progression of liver disease[30]. Thus, the intestinal microbiota influences MAFLD through the gut-liver axis.
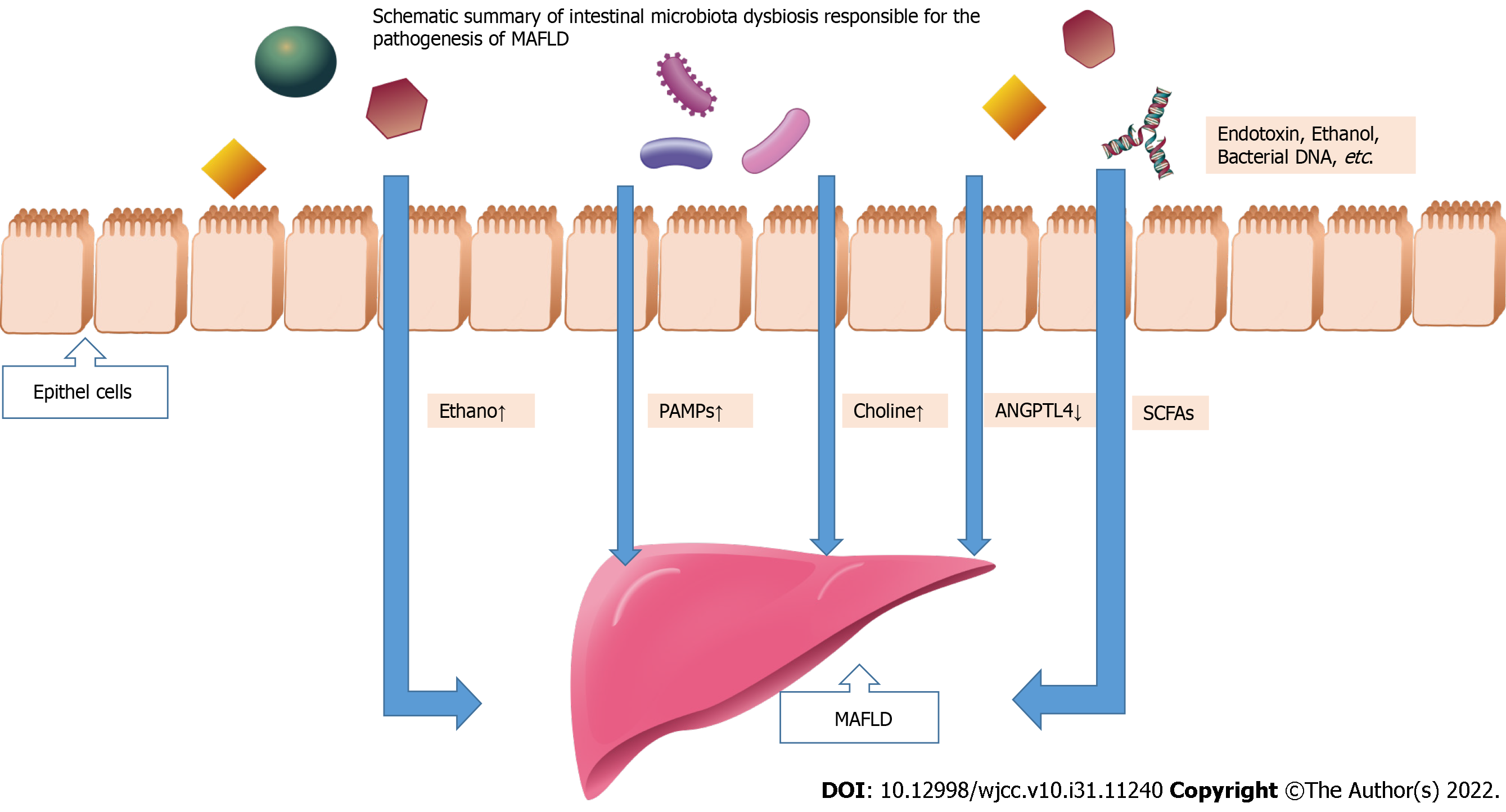
There is an imbalance of microbial populations within the intestinal ecosystem when there is a loss of fragile equilibrium among these entities[31]. Multiple studies have shown that intestinal microflora imbalance is closely related to MAFLD[32,33]. The role of intestinal microbiota in occurrence of MAFLD is as followed (Figure 1): (1) Molecular dysbiosis causes the liver to produce more intestinal ethanol, which damages tight junctions and causes gut permeability problems; (2) Inflammation and fibrosis of the liver can be induced by PAMPs like endotoxin that bind to specific TLRs; (3) Dimethylamine and trimethylamine are formed by gut microbiota hydrolyzing choline[34,35]. Choline deficiency results from increased choline metabolism, which prevents the expulsion of very low-density lipoprotein (VLDL) from the liver and leads to the accumulation of triglycerides; (4) An altered gut microbiota might inhibit the secretion of fasting-induced adipocyte factor (also known as angiopoietin-related protein 4), a specific inhibitor of endothelial lipoprotein lipase, which releases triglycerides from VLDL particles into the liver. Triglyceride storage in the liver is increased as a result of lipid b-oxidation inhibition; and (5) Inhibiting the action of adenosine monophosphate activated protein kinase, excessive short-chain fatty acids (SCFAs) in the liver stimulate free fatty acids synthesis and gluconeogenesis. Next, a summary of the role of intestinal microbiota dysbiosis in the occurrence of MAFLD will be provided.
The intestinal microbiota is known to influence obesity and MAFLD. High sugar and fat diets are sufficient to induce obesity and insulin resistance in germ-free mice[36]. It was observed that fecal microbiota can directly induce MAFLD in mice. This indicates a direct link between gut microbes and MAFLD development[37]. HFD-fed C57BL/6J mice showed obesity-associated MAFLD[38]. Both diets with lower carbohydrate or fat content stabilized weight and reduced adiposity when fed to diet-induced obese animals[38].
Furthermore, MAFLD should be linked to bacteria or microbe-derived products[39]. Endotoxin in the systemic circulation of individuals with MAFLD correlates with the severity of steatohepatitis in animal studies[39,40]. All fatty/fatty rats developed steatohepatitis after endotoxin treatment, with histologic evidence of focal hepatocyte necrosis, hepatic inflammation, and increased serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT)[40]. In the same study, the obese/obese group's serum AST and ALT were ten times higher after endotoxin treatment than their lean littermates[40]. The intestinal microbiota and their harmful metabolites (ethanol, endotoxin) may cause liver injuries. Intestinal microbes and their metabolites may play an important role in the advancement of MAFLD, but more research is needed to identify them.
Like the animal model studies mentioned above, MAFLD patients have endotoxin in their systemic circulation, correlating with the severity of steatohepatitis in clinical research[41,42]. Endotoxin levels were significantly higher in MAFLD patients than in controls[42]. In addition to endotoxin, several studies have compared the intestine bacterial composition of healthy individuals and MAFLD patients. Using healthy controls, MAFLD and MASH patients as comparison subjects, Mouzaki et al[43] characterized the composition of the intestinal microbiota in these three groups. They found that those with MASH had a lower number of Bacteroidetes compared to subjects with simple steatosis or healthy subjects, while the intestinal microbiota did not differ between MAFLD subjects and controls[43]. There has been recent evidence that the composition of bacteria in stool varies with an individual's stage of fibrosis in MAFLD patients based on the sequencing technology[44,45]. Therefore, gastrointestinal microbiota dysbiosis plays an important role in MAFLD development and development into MASH.
The impaired gut barrier may allow endotoxin and other microbial metabolites to enter the portal vein, increasing pro-inflammatory cytokine production and aggravating MAFLD[46]. In addition to using farnesoid X receptor (FXR) agonists to treat MAFLD, there is growing research indicating that gut-liver axis may be a new approach.
Antibiotics are used to reduce the quantity of intestinal microbiota to reduce the effects of the microbiome and their metabolites on host health. Non-absorbable antibiotics such as rifaximin are currently used to treat minimal and overt hepatic encephalopathy[47]. Moreover, the combined use of antibiotics (neomycin and polymyxin B) reduced total hepatic triglycerides[48]. Rifaximin can reduce circulating endotoxins and serum transaminases, resulting in therapeutic effects in MAFLD[46,49]. However, one clinical trial presented the opposite results. Cobbold et al[50] reported that rifaximin showed little therapeutic effects against hepatic lipid content. However, the inconsistency may be due to the small sample size, the relatively low treatment dose, the short duration of the study, and the broad-spectrum activity of rifaximin affecting both the harmful and the beneficial bacteria. Furthermore, antibiotics can induce mutations resulting in antibiotic resistance[51]. Antibiotics appear to alleviate MAFLD, but their clinical use is still questionable (Table 2). To have a beneficial effect on metabolic health and inflammation, future therapies targeting the intestinal microbiota need to be more nuanced.
| Ref. | Clinical Trials ID | Intervention | Agent | Intervention dose | Target population | Results |
| Abdel-Razik et al[49], 2018 | NCT02884037 | Antibiotics | Rifaximin | Rifaximin: 1100 mg/d for 6 mo | MASH, n = 50 | Improved insulin resistance, cytokines, and MAFLD-liver fat score |
| Gangarapu et al[101], 2015 | NCT02009592 | Antibiotics | Rifaximin | Rifaximin: 1320 mg/d, for 4 wk | MAFLD, n = 42 | Reduction in serum AST, ALT, and endotoxin |
| - | NCT01355575 | Antibiotic | Rifaximin | Rifaximin: 800 mg/d for 6 wk | MASH, n = 15 | - |
| - | NCT02510599 | Antibiotic | Solithromycin | Solithromycin: 200 mg/d for 1 wk, followed by 200 mg TIW for 12 wk | MASH, n = 10 | - |
| Vajroet al[58], 2011 | NCT01650025 | Probiotic | VSL#3 | VSL#3: 2 sachets/d for 4 mo | Obese children with MAFLD, n = 48 | Reduce fatty liver, BMI, GLP-1 |
| - | NCT03511365 | Probiotic | VSL#3 | VSL#3: 2 times/d for 8 wk | MAFLD, n = 20 | - |
| Tenorio-Jiménez et al[102], 2018 | NCT02972567 | Probiotic | Lactobacillus strain | Lactobacillus spp: 9 log10 cfu/capsule: 1 capsule/d for 12 wk | Obese subjects with insulin resistance, n = 60 | - |
| Bomhof et al[103], 2019 | NCT03184376 | Prebiotic | Oligofructose | PrebioticPrebiotic oligofructose: 8 g/d for 12 wk followed by 16 g/d for 24 wk. | MASH, n = 14 | Reduced histologically-confirmed steatosis |
| Mofidiet al[104], 2017 | NCT02530138 | Synbiotic | Fructo-oligosaccharide + 7 strains of bacteria | Symbiotic: 2 symbiotics capsules/d for 28 wk | MASH, n = 42 | Reduction in serum cytokines, hepatic steatosis, and fibrosis |
| Wong et al[105], 2013 | NCT00870012 | Synbiotic | Lepicol probiotic and prebiotic formula | Lepicol probiotic and prebiotic formula + simple lifestyle advice | MAFLD, n = 20 | Reduction in liver fat and AST level |
Probiotics are "living microorganisms that confer a health benefit to the host when administered in adequate amounts"[52]. Probiotics appear to alter intestinal microflora and may exert their effects by various mechanisms[53]. Although most probiotics are derived from bacteria, fungi such as Saccharomyces boulardii, originally isolated to combat cholera, have also been proven to be effective probiotics[54].
Gut microbiota modification using probiotics has shown beneficial effects on MAFLD mice[55]. Promising results have been observed in adults and children with MAFLD responding to probiotic treatment[56]. VSL#3 is a mixture of probiotic bacteria, including Lactobacilli, that has been used in many experimental and human MAFLD treatment studies. VSL#3 contains 450 billion bacteria per sachet from eight different bacterial species (Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium breve, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus plantarum, Lactobacillus casei, and Streptococcus thermophilus)[56]. A cornerstone paper published in 2003 reported that VSL#3 treatment significantly reduced hepatic inflammation, ALT levels, and hepatic oleic acid levels in a genetically obese obese/obese mice MAFLD model[57]. Recent studies indicated that the multi-strain probiotic VSL#3 was effective in improving weight loss and liver fibrosis in obese children with MAFLD[58]. It was demonstrated in a double-blind clinical trial that Lactobacillus GG significantly decreased serum ALT in obese children suffering from MAFLD (normalization in 80% of the cases)[59]. Solga et al[60] studied the effect of VSL #3 on four MAFLD adult subjects in an open pilot study over four mo. All four subjects had a significant increase in liver fat and no significant differences in biochemical or clinical parameters[60]. Researchers have identified a small sample size as an important limitation[60]. Another study evaluated the effects of a different probiotic using a randomized, double-blind clinical trial[61]. This study evaluated the effects of Lactobacillus bulgarius and Streptococcus thermophilus (1 tablet/d) in 28 MAFLD patients over three mo[61]. ALT, AST, and gamma-glutamyl transferase levels decreased[61]. VSL#3 or Lactobacilli combined with a prebiotic and vitamin mixture (Bio-Flora) were well tolerated, improved conventional liver function tests, and reduced lipid peroxidation and tumor necrosis factor-α (TNF-α) markers levels[62]. A two-month Bio-Flora supplementation lowered liver enzyme levels in 10 biopsied adults with MASH[62]. Both ALT and gamma-glutamyltransferase levels improved significantly one month after washout. The treatment also reduced oxidative stress markers malondialdehyde and 4-hydroxynonenal[62].
Probiotics may help to improve disease symptoms and limit the damage by promoting the reinforcement and repair of the epithelial barrier. Le Barz et al[63] reported that the probiotics strain Lb102 significantly upregulated the gene expression of two important tight junction proteins, ZO-1 and occludin. Probiotic Clostridium butyricum, known in Asia as MIYAIRI 588, prevented fatty degeneration from progressing to liver cancer in rats with MAFLD[64,65]. These studies demonstrated the therapeutic role of probiotics in MAFLD treatment (Table 2). Furthermore, the probiotic affected treatment of other diseases[66]. The National Health Commission of China recommended using probiotics to maintain intestinal balance and prevent secondary bacterial infections in patients with severe Corona Virus Disease 2019[67]. However, there are still some unanswered questions regarding the role of probiotics against MAFLD. For example, it is unclear how probiotics change the composition of intestinal microflora. In populations from different regions, there is a great deal of variation in the composition of the gut microbiota. Therefore, the beneficial effects of probiotics on MAFLD/MASH must be verified in ethnically diverse populations, and optimal formulations and dosages must be determined to develop commercialized probiotic products.
Prebiotics, a selectively utilized substrate by host microorganisms, confer health benefits[68]. Researchers have shown that prebiotics are effective in the treatment of MAFLD both in vitro and in vivo. Prebiotics are composed of oligosaccharides or short-chain polysaccharides. The best-characterized prebiotics are fructosyl-oligosaccharides (FOS), including inulin (long-chain fructosyl-oligosaccharide), galactosyl-oligosaccharides, and other oligosaccharides present in milk, which are transformed by the gut microbiota into SCFAs and simultaneously promote proliferation of selected commensal bacteria in the colon[69-72]. The de novo lipogenesis was threefold higher in patients with MAFLD, indicating that increased de novo lipogenesis is a defining characteristic of this disease[66]. Animal studies show that prebiotic supplementation may improve MAFLD by inhibiting the fatty acid synthesis pathway[73,74]. Rats fed with 10% oligofructose, a nondigestible but fermentable oligomer of β-D-fructose, showed significantly reduced fructose-induced hepatic triglyceride accumulation[72,75]. Prebiotics also modulate glucose homeostasis and lipid metabolism, thereby modulating MAFLD/MASH progression in clinical trials[76]. Those suffering from obesity benefited from prebiotics by increasing Bifidobacteria growth and lowering plasma endotoxin levels[77]. Lactobacillus, Bifidobacterium, and Gram-positive bacteria grow well on lactulose, while Gram-negative bacteria are inhibited by it[78]. For six weeks, obese mice who were fed HFDs and given lactulose showed less inflammation and liver damage, which was correlated with lowered lipopolysaccharide levels[79]. Additionally, chitin-glucan modulates gut microbiota, thereby limiting weight gain, glucose intolerance, triglyceride accumulation, and fasting hyperglycemia[80].
In conclusion, prebiotics is a potential therapeutic tool against MAFLD (Table 2). However, studies showed that consuming prebiotics over 30 g/d could cause adverse gastrointestinal effects such as flatulence[81]. Clinical trials that demonstrate high-quality results are necessary to generalize prebiotics' use for MAFLD given the limited research in this field.
Gibson and Roberfroid coined the term "synbiotic" in 1995, defining it as "a mixture of probiotics and prebiotics that beneficially affects the host by improving the survival and implantation of live microbial dietary supplements in the GI tract, by selectively stimulating the growth or activating the metabolism of one or a limited number of health-promoting bacteria, thus improving host welfare"[69]. Six months after therapy with Bifidobacterium and FOS, MASH patients had significantly lower serum ALT and AST levels than those who received placebo[82]. A meta-analysis of 15 randomized controlled trials including 782 MAFLD patients revealed that synbiotics significantly reduced liver steatosis, ALT, AST, high-density lipoprotein, low-density lipoprotein, triglyceride and cholesterol levels, TNF-α expression, the degree of liver stiffness, and homeostasis model assessment-insulin resistance[83]. Studies involving animals and clinical trials suggest that synbiotics could potentially treat MAFLD (Table 2).
Liver enzymes synthesize and conjugate bile acid (BA), a cholesterol derivative[76]. Micelles containing conjugated bile acids function to solubilize, digest, and promote the absorption of dietary lipids, cholesterol, and fat-soluble vitamins (A, D, E, and K) in the small intestine[84].
In addition to fat and cholesterol solubilization, BA has bacteriostatic properties inhibiting bacterial growth in the biliary tree[85]. This signaling molecule functions as a link between the liver and intestine. Through a feedback mechanism, BA receptors (also known as FXRs) are abundantly expressed in the liver and intestine[86,87].
The FXR agonists have been proven effective against MAFLD or MASH in many animal experiments and clinical trials (Table 3).
| Ref. | Clinical Trials ID | Intervention | Agent | Intervention dose | Target population | Results |
| Craven et al[106], 2020 | NCT00501592 | FXR agonists | Obeticholic acid | INT-747: 25 mg/d for 1 mo; 50 mg/d for 1 mo | MAFLD, n = 64 | Reduction in body weight, hepatic inflammation andfibrosis, improved insulin sensitivity |
| Neuschwander-Tetri et al[107], 2015 | NCT01265498 | FXR agonists | Obeticholic acid | Obeticholic acid: 25 mg/d for 72 wk | MASH, n = 283 | Reduction in ALT, AST, and γ- Glutamyl transpeptidase, improved histological features of MASH |
| Bailey et al[108], 2014 | NCT02496390 | FMT | - | Fecal Microbial Transplantation: approx 100 mL previously frozen fecal sample obtained from a lean donor prior to colonic preparation | MAFLD, n = 21 | - |
FXR agonists such as obeticholic acid which improve the liver's lipid and glucose metabolism and reduce liver inflammation and fibrosis in MAFLD[88]. Furthermore, FXR agonists reduce pro-inflammatory cytokines expression in macrophages and hepatic inflammation in a mouse model of MAFLD[87]. FXR agonists could also enhance the anti-inflammatory polarization of the macrophages in vitro and in vivo[89]. The first study showing that obeticholic acid improves insulin sensitivity, suppresses hepatic inflammation, and reduces fibrosis has been published by Mudaliar et al[90] in 2013. Younossi et al[91] recently reported the intermediate outcomes (after 18 mo of treatment) of a phase III study evaluating the safety and efficacy of a daily dose of 10 or 25 mg of obeticholic acid in 931 patients (58% females) with F2/3 fibrosis (fibrosis evaluated by liver biopsy). In MASH patients, obeticholic acid (at 25 mg dose) significantly improved liver fibrosis and several MASH disease activity indicators. Despite the encouraging results of this phase III trial, some questions persist (metabolic consequences, management of side effects including pruritus, and elevated LDL cholesterol in patients with elevated risk of cardiovascular disease). Consequently, obeticholic acid therapy should be studied in more detail in order to confirm its safety over the long term.
In addition to obeticholic acid, ursodeoxycholic acid (UDCA) is a frequently used FXR agonist. Bile acids such as UDCA, a naturally occurring hydrophilic bile acid, have been used for treating cholestatic liver disease for decades[92]. Recently, UDCA has been considered a potential therapeutic agent for MAFLD. Patients with MASH showed a significant reduction in liver enzyme levels and steatosis with UDCA treatment in a small pilot trial[93]. It is evident, however, that patients with MASH are ineffective when treated with UDCA[94]. Therefore, the efficacy of UDCA for MAFLD/MASH treatment needs to be further confirmed.
A new approach to clinical treatment is Fecal microbiota transplantation (FMT), in which fecal matter from a healthy donor is transplanted into the patient. Studies have shown that FMT can restore healthy microbiota and normalize blood lipid levels in patients with type 2 diabetes and ulcerative colitis[95-99]. Based on these reports, we can conclude that FMT is a potential therapeutic option for MAFLD and MASH (Table 3). A study involving mice with HFD-induced steatohepatitis showed that FMT was able to restore gut dysbiosis and increase cecal butyrate reduce endotoxin and inflammation factor generation, and ZO-1 concentrations in the small intestine[99]. Researchers have found that after allogeneic FMT, patients with MAFLD experience a significant reduction in abnormal permeability of the small intestine[100]. The efficacy and safety of FMT must be further assessed using high-quality clinical data. Furthermore, standardized protocols for sample preparation, archiving, formulations and dosages should be developed.
MAFLD is a common chronic liver disease progressing from simple steatosis to MASH and potentially to cirrhosis, a risk factor for liver cancer. It is now widely accepted that gut microbiota consisting of bacteria, archaea, fungi, viruses, and non-bacterial gut microorganisms, is closely related to chronic liver diseases. MAFLD is associated with disturbances in the gut-liver axis and intestinal microbiota, as evidenced by several recent studies. Therefore, it is logical to target the microbiota to alleviate MAFLD symptoms. Antibiotics, probiotics, prebiotics, synbiotics, and FXR agonists are safe and effective treatment options for MAFLD. Furthermore, FMT is a promising strategy for reversing the intestinal dysbiosis associated with MAFLD. For these agents to be confirmed as effective in treating MAFLD, more well-designed and mechanism-based laboratory and clinical studies are required. It will also be important to examine the genomes of MAFLD patients to determine whether genetics can be a determinant of therapeutic response.
I would like express my deepest gratitude to my supervisor, Professor Liu, who has done a great favor to my thesis. And I thank encouragement from my girlfriend - Wu TY.
| 1. | Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018;14:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 463] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 2. | Khamphaya T, Chukijrungroat N, Saengsirisuwan V, Mitchell-Richards KA, Robert ME, Mennone A, Ananthanarayanan M, Nathanson MH, Weerachayaphorn J. Nonalcoholic fatty liver disease impairs expression of the type II inositol 1,4,5-trisphosphate receptor. Hepatology. 2018;67:560-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20:137-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1863] [Article Influence: 266.1] [Reference Citation Analysis (0)] |
| 4. | Williams SM, Eleftheriadou A, Alam U, Cuthbertson DJ, Wilding JPH. Cardiac Autonomic Neuropathy in Obesity, the Metabolic Syndrome and Prediabetes: A Narrative Review. Diabetes Ther. 2019;10:1995-2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Rajman L, Chwalek K, Sinclair DA. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018;27:529-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 639] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 6. | Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018;27:22-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 557] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 7. | Li Y, Zalzala M, Jadhav K, Xu Y, Kasumov T, Yin L, Zhang Y. Carboxylesterase 2 prevents liver steatosis by modulating lipolysis, endoplasmic reticulum stress, and lipogenesis and is regulated by hepatocyte nuclear factor 4 alpha in mice. Hepatology. 2016;63:1860-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Zhang S, Wong YT, Tang KY, Kwan HY, Su T. Chinese Medicinal Herbs Targeting the Gut-Liver Axis and Adipose Tissue-Liver Axis for Non-Alcoholic Fatty Liver Disease Treatments: The Ancient Wisdom and Modern Science. Front Endocrinol (Lausanne). 2020;11:572729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Jamali R, Mofid A, Vahedi H, Farzaneh R, Dowlatshahi S. The effect of helicobacter pylori eradication on liver fat content in subjects with non-alcoholic Fatty liver disease: a randomized open-label clinical trial. Hepat Mon. 2013;13:e14679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 419] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 11. | de Jong SE, Olin A, Pulendran B. The Impact of the Microbiome on Immunity to Vaccination in Humans. Cell Host Microbe. 2020;28:169-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 12. | He JW, Zhou XJ, Lv JC, Zhang H. Perspectives on how mucosal immune responses, infections and gut microbiome shape IgA nephropathy and future therapies. Theranostics. 2020;10:11462-11478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Seyed Tabib NS, Madgwick M, Sudhakar P, Verstockt B, Korcsmaros T, Vermeire S. Big data in IBD: big progress for clinical practice. Gut. 2020;69:1520-1532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 14. | Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity. 2020;52:241-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 443] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 15. | Li XV, Leonardi I, Iliev ID. Gut Mycobiota in Immunity and Inflammatory Disease. Immunity. 2019;50:1365-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 16. | Oke S, Martin A. Insights into the role of the intestinal microbiota in colon cancer. Therap Adv Gastroenterol. 2017;10:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. 2020;8:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 18. | Lee Y, Sugihara K, Gillilland MG 3rd, Jon S, Kamada N, Moon JJ. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat Mater. 2020;19:118-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 502] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 19. | Spencer SP, Sonnenburg JL. When Gut Microbiota Creep into Fat, the Fat Creeps Back. Cell. 2020;183:589-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Li L, Butcher J, Stintzi A, Figeys D. Advancing functional and translational microbiome research using meta-omics approaches. Microbiome. 2019;7:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 21. | Brandl K, Schnabl B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2017;33:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 22. | Wijarnpreecha K, Lou S, Watthanasuntorn K, Kroner PT, Cheungpasitporn W, Lukens FJ, Pungpapong S, Keaveny AP, Ungprasert P. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;32:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Mikolasevic I, Delija B, Mijic A, Stevanovic T, Skenderevic N, Sosa I, Krznaric-Zrnic I, Abram M, Krznaric Z, Domislovic V, Filipec Kanizaj T, Radic-Kristo D, Cubranic A, Grubesic A, Nakov R, Skrobonja I, Stimac D, Hauser G. Small intestinal bacterial overgrowth and non-alcoholic fatty liver disease diagnosed by transient elastography and liver biopsy. Int J Clin Pract. 2021;75:e13947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 748] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 25. | Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14:442-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 552] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 26. | Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1710] [Cited by in RCA: 2440] [Article Influence: 305.0] [Reference Citation Analysis (0)] |
| 27. | Noval Rivas M, Wakita D, Franklin MK, Carvalho TT, Abolhesn A, Gomez AC, Fishbein MC, Chen S, Lehman TJ, Sato K, Shibuya A, Fasano A, Kiyono H, Abe M, Tatsumoto N, Yamashita M, Crother TR, Shimada K, Arditi M. Intestinal Permeability and IgA Provoke Immune Vasculitis Linked to Cardiovascular Inflammation. Immunity. 2019;51:508-521.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, Penna G, Rescigno M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71:1216-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 516] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 29. | Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, Zhao Y. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res. 2020;10:2993-3036. [PubMed] |
| 30. | Wei S, Ma X, Zhao Y. Mechanism of Hydrophobic Bile Acid-Induced Hepatocyte Injury and Drug Discovery. Front Pharmacol. 2020;11:1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Li F, Duan K, Wang C, McClain C, Feng W. Probiotics and Alcoholic Liver Disease: Treatment and Potential Mechanisms. Gastroenterol Res Pract. 2016;2016:5491465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Gangi A, Lu SC. Chemotherapy-associated liver injury in colorectal cancer. Therap Adv Gastroenterol. 2020;13:1756284820924194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Kong D, Wang H. Mucosal-Associated Invariant T cell in liver diseases. Int J Biol Sci. 2020;16:460-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 802] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 35. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Cales P, Diehl AM. The Severity of Nonalcoholic Fatty Liver Disease Is Associated With Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatol. 2016;63:764-775. [RCA] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1112] [Article Influence: 111.2] [Reference Citation Analysis (1)] |
| 36. | Pydi SP, Cui Z, He Z, Barella LF, Pham J, Cui Y, Oberlin DJ, Egritag HE, Urs N, Gavrilova O, Schwartz GJ, Buettner C, Williams KW, Wess J. Beneficial metabolic role of β-arrestin-1 expressed by AgRP neurons. Sci Adv. 2020;6:eaaz1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Houttu V, Boulund U, Grefhorst A, Soeters MR, Pinto-Sietsma SJ, Nieuwdorp M, Holleboom AG. The role of the gut microbiome and exercise in non-alcoholic fatty liver disease. Therap Adv Gastroenterol. 2020;13:1756284820941745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Cai Y, Li H, Liu M, Pei Y, Zheng J, Zhou J, Luo X, Huang W, Ma L, Yang Q, Guo S, Xiao X, Li Q, Zeng T, Meng F, Francis H, Glaser S, Chen L, Huo Y, Alpini G, Wu C. Disruption of adenosine 2A receptor exacerbates NAFLD through increasing inflammatory responses and SREBP1c activity. Hepatology. 2018;68:48-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1639] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
| 40. | Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 593] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 41. | Traber MG, Buettner GR, Bruno RS. The relationship between vitamin C status, the gut-liver axis, and metabolic syndrome. Redox Biol. 2019;21:101091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 42. | Nicoletti A, Ponziani FR, Biolato M, Valenza V, Marrone G, Sganga G, Gasbarrini A, Miele L, Grieco A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J Gastroenterol. 2019;25:4814-4834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (4)] |
| 43. | Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 569] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 44. | Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017;25:1054-1062.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 801] [Article Influence: 89.0] [Reference Citation Analysis (1)] |
| 45. | Caussy C, Tripathi A, Humphrey G, Bassirian S, Singh S, Faulkner C, Bettencourt R, Rizo E, Richards L, Xu ZZ, Downes MR, Evans RM, Brenner DA, Sirlin CB, Knight R, Loomba R. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun. 2019;10:1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 261] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 46. | Dai X, Hou H, Zhang W, Liu T, Li Y, Wang S, Wang B, Cao H. Microbial Metabolites: Critical Regulators in NAFLD. Front Microbiol. 2020;11:567654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 47. | Skinner C, Thompson AJ, Thursz MR, Marchesi JR, Vergis N. Intestinal permeability and bacterial translocation in patients with liver disease, focusing on alcoholic aetiology: methods of assessment and therapeutic intervention. Therap Adv Gastroenterol. 2020;13:1756284820942616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, Zeng X, Trefely S, Fernandez S, Carrer A, Miller KD, Schug ZT, Snyder NW, Gade TP, Titchenell PM, Rabinowitz JD, Wellen KE. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579:586-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 415] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 49. | Abdel-Razik A, Mousa N, Shabana W, Refaey M, Elzehery R, Elhelaly R, Zalata K, Abdelsalam M, Eldeeb AA, Awad M, Elgamal A, Attia A, El-Wakeel N, Eldars W. Rifaximin in nonalcoholic fatty liver disease: hit multiple targets with a single shot. Eur J Gastroenterol Hepatol. 2018;30:1237-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 50. | Cobbold JFL, Atkinson S, Marchesi JR, Smith A, Wai SN, Stove J, Shojaee-Moradie F, Jackson N, Umpleby AM, Fitzpatrick J, Thomas EL, Bell JD, Holmes E, Taylor-Robinson SD, Goldin RD, Yee MS, Anstee QM, Thursz MR. Rifaximin in non-alcoholic steatohepatitis: An open-label pilot study. Hepatol Res. 2018;48:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Ba Y, Shi Y, Jiang W, Feng J, Cheng Y, Xiao L, Zhang Q, Qiu W, Xu B, Xu R, Shen B, Luo Z, Xie X, Chang J, Wang M, Li Y, Shuang Y, Niu Z, Liu B, Zhang J, Zhang L, Yao H, Xie C, Huang H, Liao W, Chen G, Zhang X, An H, Deng Y, Gong P, Xiong J, Yao Q, An X, Chen C, Wang J, Wang X, Wang Z, Xing P, Yang S, Zhou C. Current management of chemotherapy-induced neutropenia in adults: key points and new challenges: Committee of Neoplastic Supportive-Care (CONS), China Anti-Cancer Association Committee of Clinical Chemotherapy, China Anti-Cancer Association. Cancer Biol Med. 2020;17:896-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 52. | Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4055] [Cited by in RCA: 5999] [Article Influence: 499.9] [Reference Citation Analysis (4)] |
| 53. | Xiao J, Peng Z, Liao Y, Sun H, Chen W, Chen X, Wei Z, Yang C, Nüssler AK, Liu J, Yang W. Organ transplantation and gut microbiota: current reviews and future challenges. Am J Transl Res. 2018;10:3330-3344. [PubMed] |
| 54. | Gaziano R, Sabbatini S, Roselletti E, Perito S, Monari C. Saccharomyces cerevisiae-Based Probiotics as Novel Antimicrobial Agents to Prevent and Treat Vaginal Infections. Front Microbiol. 2020;11:718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Kim JC, Jeon JY, Yang WS, Kim CH, Eom DW. Combined Amelioration of Ginsenoside (Rg1, Rb1, and Rg3)-enriched Korean Red Ginseng and Probiotic Lactobacillus on Non-alcoholic Fatty Liver Disease. Curr Pharm Biotechnol. 2019;20:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Loguercio C, Federico A, Tuccillo C, Terracciano F, D'Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 345] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 57. | Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 708] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 58. | Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 59. | Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, Caropreso M, Vallone G, Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 60. | Solga SF, Buckley G, Clark JM, Horska A, Diehl AM. The effect of a probiotic on hepatic steatosis. J Clin Gastroenterol. 2008;42:1117-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090-1095. [PubMed] |
| 62. | Loguercio C, De Simone T, Federico A, Terracciano F, Tuccillo C, Di Chicco M, Cartenì M. Gut-liver axis: a new point of attack to treat chronic liver damage? Am J Gastroenterol. 2002;97:2144-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 63. | Le Barz M, Daniel N, Varin TV, Naimi S, Demers-Mathieu V, Pilon G, Audy J, Laurin É, Roy D, Urdaci MC, St-Gelais D, Fliss I, Marette A. In vivo screening of multiple bacterial strains identifies Lactobacillus rhamnosus Lb102 and Bifidobacterium animalis ssp. lactis Bf141 as probiotics that improve metabolic disorders in a mouse model of obesity. FASEB J. 2019;33:4921-4935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 64. | Seo M, Inoue I, Tanaka M, Matsuda N, Nakano T, Awata T, Katayama S, Alpers DH, Komoda T. Clostridium butyricum MIYAIRI 588 improves high-fat diet-induced non-alcoholic fatty liver disease in rats. Dig Dis Sci. 2013;58:3534-3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8:e63388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 66. | Roszczenko-Jasińska P, Wojtyś MI, Jagusztyn-Krynicka EK. Helicobacter pylori treatment in the post-antibiotics era-searching for new drug targets. Appl Microbiol Biotechnol. 2020;104:9891-9905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 67. | Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 68. | Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, Sanders ME, Shamir R, Swann JR, Szajewska H, Vinderola G. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18:649-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 1224] [Article Influence: 244.8] [Reference Citation Analysis (1)] |
| 69. | Daddaoua A, Puerta V, Requena P, Martínez-Férez A, Guadix E, de Medina FS, Zarzuelo A, Suárez MD, Boza JJ, Martínez-Augustin O. Goat milk oligosaccharides are anti-inflammatory in rats with hapten-induced colitis. J Nutr. 2006;136:672-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 70. | van Hoffen E, Ruiter B, Faber J, M'Rabet L, Knol EF, Stahl B, Arslanoglu S, Moro G, Boehm G, Garssen J. A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Allergy. 2009;64:484-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 71. | Delzenne NM, Kok N. Effects of fructans-type prebiotics on lipid metabolism. Am J Clin Nutr. 2001;73:456S-458S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes. 2006;55:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 299] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 73. | Pachikian BD, Essaghir A, Demoulin JB, Catry E, Neyrinck AM, Dewulf EM, Sohet FM, Portois L, Clerbaux LA, Carpentier YA, Possemiers S, Bommer GT, Cani PD, Delzenne NM. Prebiotic approach alleviates hepatic steatosis: implication of fatty acid oxidative and cholesterol synthesis pathways. Mol Nutr Food Res. 2013;57:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Parnell JA, Raman M, Rioux KP, Reimer RA. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012;32:701-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 75. | Kim JY, Kwon YM, Kim IS, Kim JA, Yu DY, Adhikari B, Lee SS, Choi IS, Cho KK. Effects of the Brown Seaweed Laminaria japonica Supplementation on Serum Concentrations of IgG, Triglycerides, and Cholesterol, and Intestinal Microbiota Composition in Rats. Front Nutr. 2018;5:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Chen HT, Huang HL, Li YQ, Xu HM, Zhou YJ. Therapeutic advances in non-alcoholic fatty liver disease: A microbiota-centered view. World J Gastroenterol. 2020;26:1901-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Chu JR, Kang SY, Kim SE, Lee SJ, Lee YC, Sung MK. Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: A randomized placebo-controlled intervention study. World J Gastroenterol. 2019;25:6129-6144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (3)] |
| 78. | Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 79. | Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 604] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 80. | Neyrinck AM, Possemiers S, Verstraete W, De Backer F, Cani PD, Delzenne NM. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem. 2012;23:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 81. | Martins GN, Ureta MM, Tymczyszyn EE, Castilho PC, Gomez-Zavaglia A. Technological Aspects of the Production of Fructo and Galacto-Oligosaccharides. Enzymatic Synthesis and Hydrolysis. Front Nutr. 2019;6:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 82. | Kriss M, Verna EC, Rosen HR, Lozupone CA. Functional Microbiomics in Liver Transplantation: Identifying Novel Targets for Improving Allograft Outcomes. Transplantation. 2019;103:668-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Liu L, Li P, Liu Y, Zhang Y. Efficacy of Probiotics and Synbiotics in Patients with Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Dig Dis Sci. 2019;64:3402-3412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 84. | Hoogerland JA, Lei Y, Wolters JC, de Boer JF, Bos T, Bleeker A, Mulder NL, van Dijk TH, Kuivenhoven JA, Rajas F, Mithieux G, Haeusler RA, Verkade HJ, Bloks VW, Kuipers F, Oosterveer MH. Glucose-6-Phosphate Regulates Hepatic Bile Acid Synthesis in Mice. Hepatology. 2019;70:2171-2184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Nagahashi M, Yuza K, Hirose Y, Nakajima M, Ramanathan R, Hait NC, Hylemon PB, Zhou H, Takabe K, Wakai T. The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases. J Lipid Res. 2016;57:1636-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 86. | Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1717] [Cited by in RCA: 1790] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 87. | Dufour JF, Caussy C, Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut. 2020;69:1877-1884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 88. | Souza PCT, Thallmair S, Conflitti P, Ramírez-Palacios C, Alessandri R, Raniolo S, Limongelli V, Marrink SJ. Protein-ligand binding with the coarse-grained Martini model. Nat Commun. 2020;11:3714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 89. | Luci C, Bourinet M, Leclère PS, Anty R, Gual P. Chronic Inflammation in Non-Alcoholic Steatohepatitis: Molecular Mechanisms and Therapeutic Strategies. Front Endocrinol (Lausanne). 2020;11:597648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 90. | Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574-82.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 751] [Article Influence: 57.8] [Reference Citation Analysis (4)] |
| 91. | Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 984] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 92. | Gao RY, Shearn CT, Orlicky DJ, Battista KD, Alexeev EE, Cartwright IM, Lanis JM, Kostelecky RE, Ju C, Colgan SP, Fennimore BP. Bile acids modulate colonic MAdCAM-1 expression in a murine model of combined cholestasis and colitis. Mucosal Immunol. 2021;14:479-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 93. | Kim KS, Lee BW, Kim YJ, Lee DH, Cha BS, Park CY. Nonalcoholic Fatty Liver Disease and Diabetes: Part II: Treatment. Diabetes Metab J. 2019;43:127-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 94. | Wijarnpreecha K, Aby ES, Ahmed A, Kim D. Evaluation and management of extrahepatic manifestations of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2021;27:221-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 95. | Wang H, Lu Y, Yan Y, Tian S, Zheng D, Leng D, Wang C, Jiao J, Wang Z, Bai Y. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Front Cell Infect Microbiol. 2019;9:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 96. | Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, Ducarmon QR, Keller JJ, Kuijper EJ, Contarino MF. Fecal Microbiota Transplantation in Neurological Disorders. Front Cell Infect Microbiol. 2020;10:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (2)] |
| 97. | Napolitano M, Covasa M. Microbiota Transplant in the Treatment of Obesity and Diabetes: Current and Future Perspectives. Front Microbiol. 2020;11:590370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 98. | Taddese R, Garza DR, Ruiter LN, de Jonge MI, Belzer C, Aalvink S, Nagtegaal ID, Dutilh BE, Boleij A. Growth rate alterations of human colorectal cancer cells by 157 gut bacteria. Gut Microbes. 2020;12:1-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 99. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1929] [Cited by in RCA: 1718] [Article Influence: 245.4] [Reference Citation Analysis (0)] |
| 100. | Wang H, Zhou C, Huang J, Kuai X, Shao X. The potential therapeutic role of Lactobacillus reuteri for treatment of inflammatory bowel disease. Am J Transl Res. 2020;12:1569-1583. [PubMed] |
| 101. | Gangarapu V, Ince AT, Baysal B, Kayar Y, Kılıç U, Gök Ö, Uysal Ö, Şenturk H. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 102. | Tenorio-Jiménez C, Martínez-Ramírez MJ, Tercero-Lozano M, Arraiza-Irigoyen C, Del Castillo-Codes I, Olza J, Plaza-Díaz J, Fontana L, Migueles JH, Olivares M, Gil Á, Gomez-Llorente C. Evaluation of the effect of Lactobacillus reuteri V3401 on biomarkers of inflammation, cardiovascular risk and liver steatosis in obese adults with metabolic syndrome: a randomized clinical trial (PROSIR). BMC Complement Altern Med. 2018;18:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 103. | Bomhof MR, Parnell JA, Ramay HR, Crotty P, Rioux KP, Probert CS, Jayakumar S, Raman M, Reimer RA. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: a pilot clinical trial. Eur J Nutr. 2019;58:1735-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 104. | Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. 2017;117:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 105. | Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, Chan HL. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 106. | Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K, Hramiak I, Hegele R, Joy T, Meddings J, Urquhart B, Harvie R, McKenzie C, Summers K, Reid G, Burton JP, Silverman M. Allogenic Fecal Microbiota Transplantation in Patients With Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am J Gastroenterol. 2020;115:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 256] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 107. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1840] [Article Influence: 167.3] [Reference Citation Analysis (3)] |
| 108. | Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 351] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jamali R, Iran; Maslennikov R, Russia; Song J, China S-Editor: Wang LL L-Editor: A P-Editor: Wang LL