Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.1041
Peer-review started: June 16, 2021
First decision: June 25, 2021
Revised: July 5, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: January 21, 2022
Processing time: 212 Days and 21.8 Hours
Lymphocytic hypophysitis (LYH) is an important condition to consider in the differential diagnosis of patients with a pituitary mass. The main clinical manifestations of LYH include headache, symptoms related to sellar compression, hypo
Here, we report a patient with LYH whose initial symptom was headache and whose pituitary function assessment showed the presence of secondary hypoadrenalism, central hypothyroidism and hypogonadotropic hypogonadism. Pi
This rare headache regression suggests that patients with chronic headaches should also be alerted to the possibility of LYH.
Core Tip: Lymphocytic hypophysitis (LYH) is an important condition to consider in the differential diagnosis of patients with a pituitary mass, and headache is a frequent complaint of patients with LYH. We present a patient with LYH whose initial symptom was headache and who presented with repeatedly worsening and prolonged headaches three times even though the hypopituitarism was fully resolved after glucocorticoid treatment. This rare headache regression suggests that the cause of headaches in patients with LYH may not be exclusively due to the pituitary mass effect and that patients with chronic headaches should also be alerted to the possibility of LYH.
- Citation: Yang MG, Cai HQ, Wang SS, Liu L, Wang CM. Full recovery from chronic headache and hypopituitarism caused by lymphocytic hypophysitis: A case report. World J Clin Cases 2022; 10(3): 1041-1049
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/1041.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.1041
Histologically, lymphocytic hypophysitis (LYH) is the most common type of primary autoimmune hypophysitis (PAH), accounting for approximately 70% of all causes of PAH, and LYH is characterized by extensive lymphocytic and plasma cell infiltration in the pituitary gland with varying degrees of pituitary dysfunction[1-3]. LYH is a rare disease with an annual incidence of only 1 case per 9 million[1,4]. However, the pre
A 56-year-old female patient presented with an intermittent throbbing headache located in the left temporal region. She was admitted to our hospital for neurosurgery.
Two months before hospitalization, the patient did not complain about headache. One month before hospitalization, the headache worsened and became more pronounced at night, and she experienced vision loss with bilateral temporal visual field defects.
The patient had a history of hypertension for more than 20 years. There was no family history of autoimmune disease.
There was no personal or family history.
The patient presented with a height of 160 cm, a weight of 65 kg, a temperature of 36.5 °C, and a blood pressure of 140/108 mmHg. The clinical neurological examination showed no abnormalities. Our initial clinical diagnosis was cellar area occupancy.
The laboratory data showed a potassium level of 3.2 mmol/L, uroprotein+-, and a urinary specific gravity of 1.025. The patient was negative for antinuclear antibodies, immunoglobulin G (IgG), IgM, IgA, and IgG4, which did not support IgG4-related hypophysitis, and no antithyroid antibodies were detected. The remaining biochemical and coagulation test results were unremarkable. The cortisol, adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), growth hormone (GH), thyroid-stimulating hormone (TSH), free triiodothyronine 3 (FT3), and free triiodothyronine (FT4) levels were measured (Table 1). A water deprivation test was not performed because the patient did not have polyhydramnios or polyuria.
| Hormones | ACTH | Cortisol nmol/L | TSH μIU/mL | FT3 pmol/L | FT4 pmol/L | LH mIU/mL | FSH mIU/mL | PRL mIU/L | GH ng/mL |
| Normal value | 1.6-13.9 | 240-619 | 0.27-4.2 | 3.1-6.8 | 12.0-22.0 | 10.87-58.64 | 16.74-113.6 | 58-416.4 | 0.010-3.607 |
| Onset | - | 124.59 | 0.242 | 3.56 | 6.05 | < 0.2 | 4.31 | 75.8 | 0.142 |
| 1 mo | 0.89 | 176.99 | 0.073 | 3.09 | 13.18 | 0.590 | 5.12 | 82.83 | - |
| 3 mo | - | - | 2.29 | 4.08 | 16.05 | 18.38 | 48.89 | 183.19 | - |
| 6 mo | - | - | 0.115 | 3.42 | 21.05 | 26.85 | 56.11 | 397.19 | - |
| 8 mo | - | - | 2.11 | 3.89 | 20.84 | 32.64 | 65.75 | 400.78 | - |
| 11 mo | - | - | 0.634 | 3.77 | 13.82 | 21.49 | 57.79 | 280.57 | - |
| 12 mo | 6.97 | 293.63 | - | - | - | 15.73 | 46.18 | 415.4 | - |
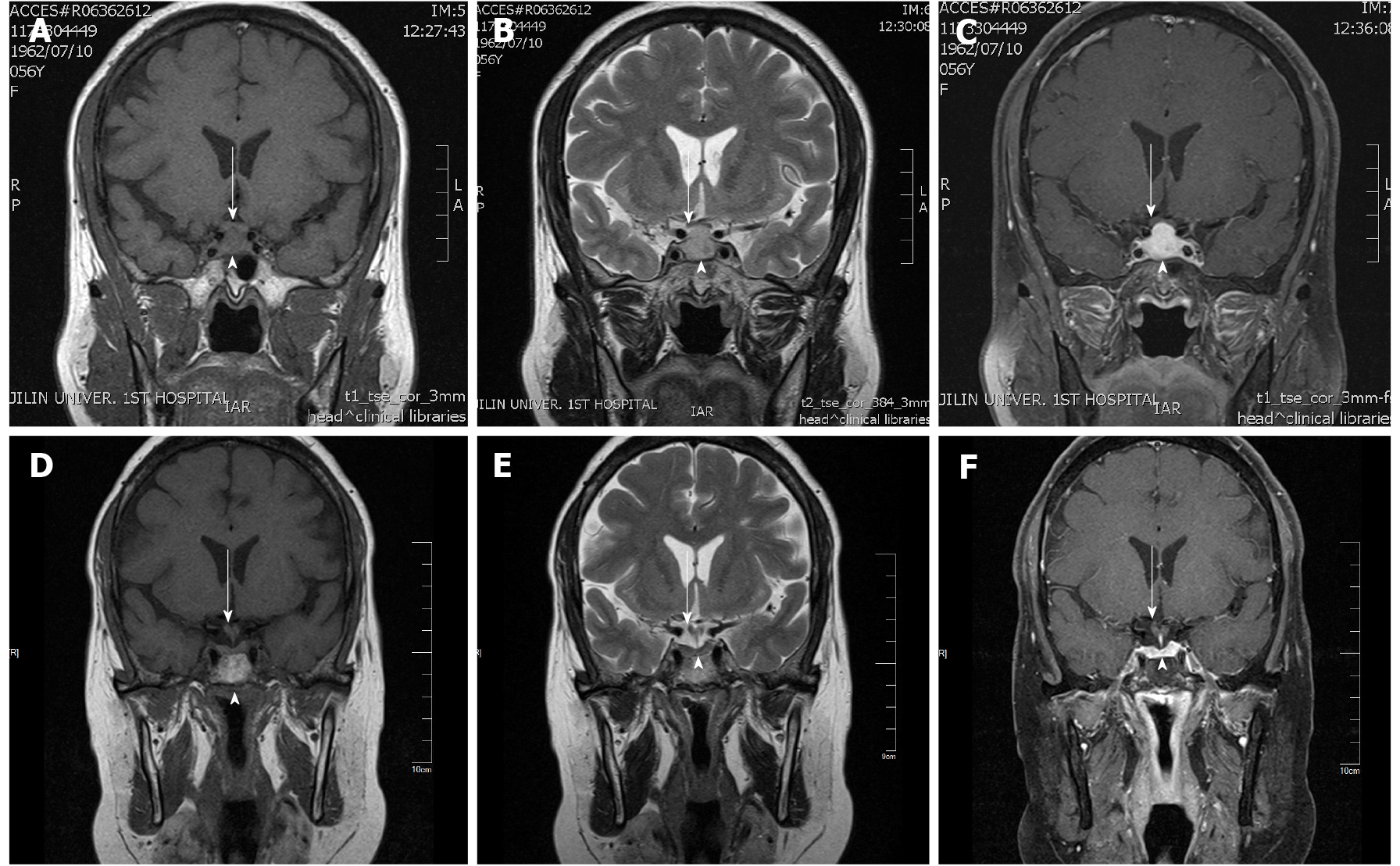
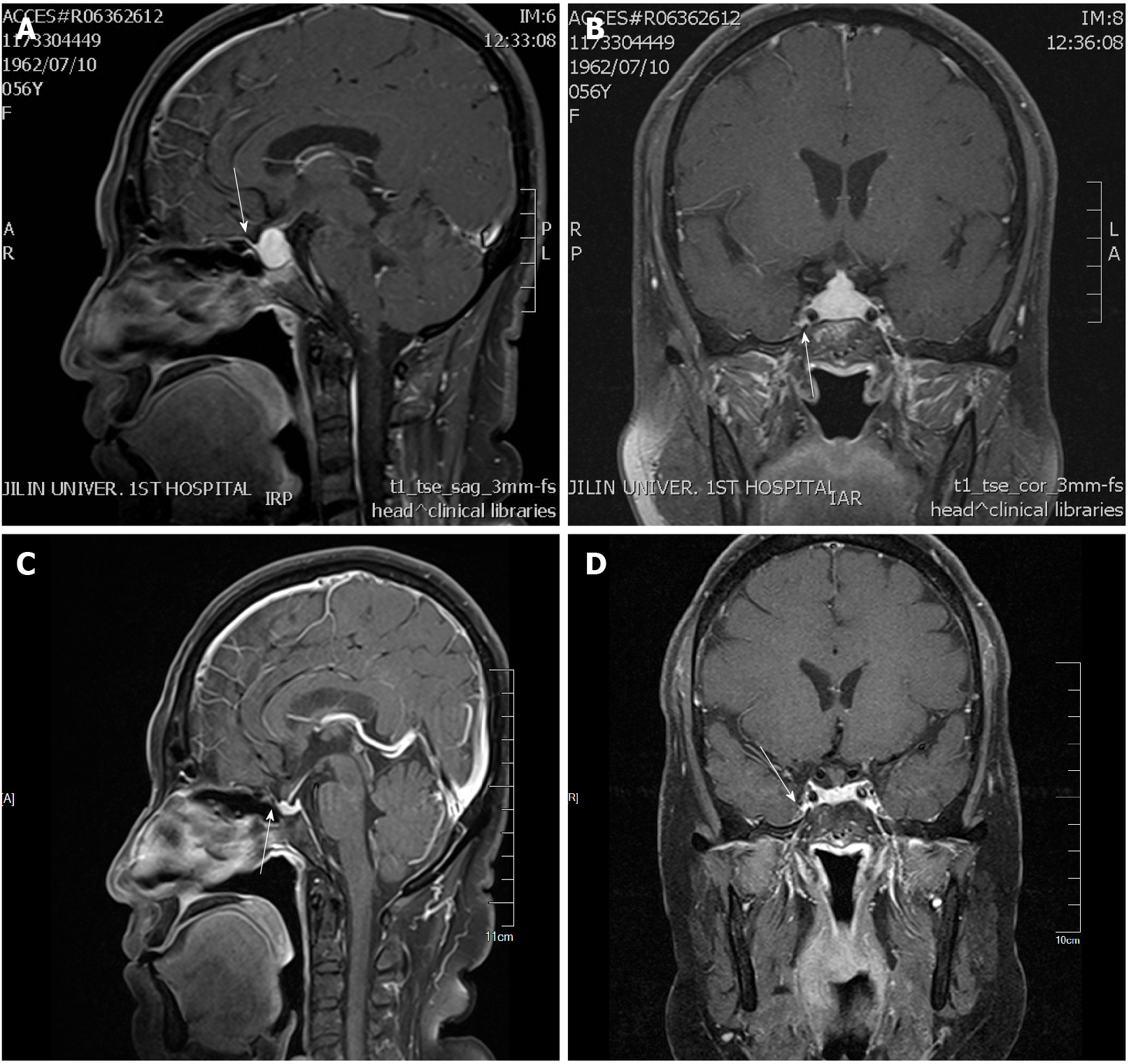
The chest computerized tomography was normal. The electrocardiography was characterized by a flat t wave. The MRI of the pituitary gland showed symmetrical enlargement with suprasellar extension in a dumbbell shape with significant homogeneous enhancement after gadolinium enhancement (Figure 1), high signal in the posterior pituitary lobe in the T1 sequence, low signal in the anterior pituitary gland in the T1 sequence and high signal in the T2 sequence. The pituitary stalk was thickened, but not deviated, approximately 3.1 mm at the optic cross and approximately 3.1 mm at the pituitary insertion with an elevation of the optimal crossing (Figure 1). The lesion grew bilaterally toward the cavernous sinuses and encircled the bilateral internal carotid arteries, and the parasternal dural caudal sign was visible (Figure 2), all of which are specific MRI manifestations consistent with LYH. According to the scoring system by Gutenberg et al[7], our patient was -8, strongly suggesting the diagnosis of LH.
Ophthalmologic assistance was requested for the examination, and the assessment suggested temporal visual field defects in both eyes, a corrected visual acuity of 0.5 in the left eye and a visual acuity of 0.8 in the right eye.
Considering the rapid progression of hypopituitarism and the combination of the MRI features, laboratory tests, clinical manifestations and epidemiological features, LYH was strongly considered.
Glucocorticoid therapy was administered in conjunction with an endocrinology consultation, starting with a daily intravenous infusion of 50 mg hydrocortisone. This treatment was changed to 30 mg/d combined with 25 μg/d levothyroxine tablets after 3 d and 20 mg/d after 4 d. The patient's symptoms significantly improved, but the pituitary function did not significantly improve upon reassessment at 1 wk of the steroid treatment. The pituitary-adrenal axis and pituitary-thyroid axis were impro
Repeat pituitary MRI showed a decrease in the size of the suprasellar mass with homogeneous enhancement, thinning and no deviation of the pituitary stalk, no elevation of the chiasm, and no abnormal signal in the cavernous sinus. However, the patient was found to have concomitant glucocorticoid-related diabetes mellitus, was treated with insulin and tested negative for diabetic autoimmune antibodies. Methylprednisolone was continued at 60 mg/d. The dose was reduced to 40 mg/d after 1 wk, and a regimen of 4 mg reduction every 2 wk and discontinuation of levothyroxine tablets was employed. Unfortunately, 6 mo after the diagnosis of LYH, the patient again presented with headaches of the same nature that were worse than before. Ophthalmologic assistance was requested for the examination, and the assessment suggested bilateral refractive error, no abnormalities in the bilateral visual fields and symptomatic treatment. Repeat MRI showed no significant change from the previous MRI. The pituitary function assessment suggested complete recovery of the thyroid and gonadal axes (Table 1), the patient was considered to have no recurrence of LYH, and methylprednisolone was continued with a regimen of 4 mg reduction every 3 wk. The patient's headache worsened for the third time two months later, and she presented with features similar to those previously noted. On examination, she had a full-moon face, centripetal obesity, weight gain and hirsutism. However, repeated pituitary MRI and endocrine function assessment did not show any deterioration. The patient was discharged from the hospital on 6 mg/d methylprednisolone and was instructed to adjust her glucocorticoid dose to 4 mg/d after 1 mo. However, 3 mo later, the patient's headache worsened for the fourth time, with the same features. Repeated pituitary MRI and assessment of the pituitary function showed no significant changes; therefore, LYH was considered stable. Thus, glucocorticoid therapy was stopped, but other treatments, such as glucose-lowering treatments, were continued. Finally, 1 year after the diagnosis, the patient's pituitary function was evaluated to have no abnor
LYH is the most common subtype of PAH and is characterized by diffuse lymphocytic and plasma cell infiltration with fibrosis in the pituitary gland[13], and the path
However, the most common complaint in 60% of patients with LYH is headache[15], followed by amenorrhea/erectile dysfunction (59%) and diplopia (27%)[9]. Headache is also the most common complaint in the first neurosurgical consultation, with an incidence of 89%[15]. LYH has been reported to present as frontal, temporal, or occipital headache[16-18], severe dull or progressive headache[18], or even trigemino-autonomic cephalalgia[17]. Our patient initially presented with intermittent headaches and later with persistent frontal pain that fluctuated in nature that was characterized by a long duration. The diagnosis of chronic postintracranial disorder headache (CPIDH) is reasonable when the etiologic disease is effectively treated or resolves on its own, but the headache does not resolve or significantly improve after 3 mo[19]. Our patient's headache persisted and repeatedly worsened for more than 8 mo and could be considered CPIDH. It has been suggested that headache is associated with cerebrospinal fluid lymphocytosis, but Honegger et al[20] did not identify a clear correlation between the degree of headache and the cerebrospinal fluid leukocyte count. It has been suggested that pituitary masses causing cavernous sinus invo
ACTH deficiency is the most common endocrine disorder in LYH (60%), followed by TSH deficiency, gonadotropin deficiency and hyperprolactinemia[23]; thus, the pattern of ACTH > TSH > LH/FSH > GH axis deficiency and the specific vulnerability of ACTH secretion to LYH have been suggested in several reports[5,24]. Our patient exhibited ACTH, TSH, and LH/FSH deficiency, which is consistent with the above reports. Another feature of LYH is that the degree of hypopituitarism is disproportionate to the size of the mass, which is also supported by findings in some cases[25]. One case report describes pituitary inflammation with pituitary enlargement exhi
In recent years, the application of MRI in the sellar region has contributed to the feasibility of clinical diagnosis[8] and has become the preferred modality for the study of pituitary lesions. Typical MRI of LYH shows symmetrical enlargement of the pituitary gland with suprasellar extension with marked homogeneous enhancement, thickening of the pituitary stalk without deviation, disappearance of the bright spot of the pituitary gland in the T1 sequence, and the dural tail sign[20,27-30]. A lingual suprasellar and retrosellar extension of the saddle mass in contact with the basal hypothalamus and even infiltration of the basal hypothalamus is a relatively typical finding in granulomatous pituitary inflammation[20], but this feature was not present in our patient. These features have been confirmed in a larger number of cases, ren
The objective of LYH treatment is to rectify the hormone deficiency and relieve the symptoms associated with the effects of the mass. Although glucocorticoids are the preferred pharmacological treatment for LYH, surgery may be considered in the presence of severe neurological or ophthalmic manifestations or the absence of a response to pharmacological treatment[39]. In 2015, Khare et al[24] described 15 patients from western India with pituitary masses that regressed with conservative treatment. Therefore, unless the symptoms are severe or progressively worsen, conservative treatment may be considered[40]. In addition, postoperative hypopituitarism may occur, and deterioration caused by surgery or biopsy may be avoided[9]. Surgical treatment may contribute to the permanent relief of headache, and headache and visual field defects usually improve shortly after surgery[12]. However, considering the risk of hypopituitarism associated with surgery[41], we did not perform surgery but adhered to long-term glucocorticoid treatment and follow-up, and the outcome was good[42]. Although some patients with LYH may show spontaneous recovery, it is also too late to initiate glucocorticoid therapy 3 mo after symptom onset[7,24]. Our time window for initiating glucocorticoid therapy was 2 mo. Thus, the pituitary function completely recovered, and the headaches, despite the longer duration, were eventually relieved. One study indicates that the first pulse of methylprednisolone was the most effective at less than 6 mo of onset[42], and Wang et al[23] reported a lower relapse rate associated with longer steroid administration because these authors found a significant difference in relapse rates with steroid drug dose administration times of 6 and < 6 mo. Fortunately, in the absence of significant efficacy with short-term hydrocortisone pulse therapy combined with continuous oral hydrocortisone treatment, the endocrinologist administered long-term methylprednisolone pulse therapy shortly after the onset of the disease to the patient in this case study, and the pituitary function significantly improved until it completely recovered. However, the headache recurred before eventually disappearing completely due to early detection and timely management, and the patient was satisfied with the outcome[5].
This report presents a rare case of LYH in combination with chronic headache despite complete resolution of hypopituitarism. Although the patient experienced long-term recurrent exacerbation of chronic headache, all symptoms eventually resolved in the patient after adequate evaluations of the clinical and MRI features due to early diagnosis and long-term high-dose glucocorticoid therapy.
We thank the patient and appreciate the help of all family members who participated in this study.
| 1. | Fukuoka H. Hypophysitis. Endocrinol Metab Clin North Am. 2015;44:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Glezer A, Bronstein MD. Pituitary autoimmune disease: nuances in clinical presentation. Endocrine. 2012;42:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Zhu Q, Qian K, Jia G, Lv G, Wang J, Zhong L, Yu S. Clinical Features, Magnetic Resonance Imaging, and Treatment Experience of 20 Patients with Lymphocytic Hypophysitis in a Single Center. World Neurosurg. 2019;127:e22-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Oguz SH, Soylemezoglu F, Sendur SN, Mut M, Oguz KK, Dagdelen S, Erbas T. Clinical Characteristics, Management, and Treatment Outcomes of Primary Hypophysitis: A Monocentric Cohort. Horm Metab Res. 2020;52:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev. 2005;26:599-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 442] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 6. | Hashimoto K, Takao T, Makino S. Lymphocytic adenohypophysitis and lymphocytic infundibuloneurohypophysitis. Endocr J. 1997;44:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Gutenberg A, Larsen J, Lupi I, Rohde V, Caturegli P. A radiologic score to distinguish autoimmune hypophysitis from nonsecreting pituitary adenoma preoperatively. AJNR Am J Neuroradiol. 2009;30:1766-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 8. | Imber BS, Lee HS, Kunwar S, Blevins LS, Aghi MK. Hypophysitis: a single-center case series. Pituitary. 2015;18:630-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Angelousi A, Cohen C, Sosa S, Danilowicz K, Papanastasiou L, Tsoli M, Pal A, Piaditis G, Grossman A, Kaltsas G. Clinical, Endocrine and Imaging Characteristics of Patients with Primary Hypophysitis. Horm Metab Res. 2018;50:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 10. | Thodou E, Asa SL, Kontogeorgos G, Kovacs K, Horvath E, Ezzat S. Clinical case seminar: lymphocytic hypophysitis: clinicopathological findings. J Clin Endocrinol Metab. 1995;80:2302-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Buture A, Gooriah R, Nimeri R, Ahmed F. Current Understanding on Pain Mechanism in Migraine and Cluster Headache. Anesth Pain Med. 2016;6:e35190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Tuğcu B, Gunaldi O, Postalci L, Tanriverdi O, Ofluoglu E, Sever N. Lymphocytic hypophysitis: an underestimated disease affecting the sellar region. Neurol Neurochir Pol. 2011;45:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Mittal R, Kalra P, Dharmalingam M, Verma RG, Kulkarni S, Shetty P. Lymphocytic hypophysitis masquerading as pituitary adenoma. Indian J Endocrinol Metab. 2012;16:S304-S306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Molitch ME, Gillam MP. Lymphocytic hypophysitis. Horm Res. 2007;68 Suppl 5:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Kyriacou A, Gnanalingham K, Kearney T. Lymphocytic hypophysitis: modern day management with limited role for surgery. Pituitary. 2017;20:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Joshi H, Hikmat M, Devadass AP, Oyibo SO, Sagi SV. Anterior hypopituitarism secondary to biopsy-proven IgG4-related hypophysitis in a young man. Endocrinol Diabetes Metab Case Rep. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Motte J, Kreitschmann-Andermahr I, Fisse AL, Börnke C, Schroeder C, Pitarokoili K, Müller O, Lukas C, van de Nes J, Buslei R, Gold R, Ayzenberg I. Trigemino-autonomic headache and Horner syndrome as a first sign of granulomatous hypophysitis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Tao T, Zhang Z, Li H. Lymphocytic hypophysitis associated with Behcet's disease: A case report and review of the literature. Neuro Endocrinol Lett. 2018;39:43-48. [PubMed] |
| 19. | Diener HC, Johansson U, Dodick DW. Headache attributed to non-vascular intracranial disorder. Handb Clin Neurol. 2010;97:547-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Honegger J, Fahlbusch R, Bornemann A, Hensen J, Buchfelder M, Müller M, Nomikos P. Lymphocytic and granulomatous hypophysitis: experience with nine cases. Neurosurgery. 1997;40:713-22; discussion 722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 113] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Asa SL, Mete O. What's new in pituitary pathology? Histopathology. 2018;72:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Falorni A, Minarelli V, Bartoloni E, Alunno A, Gerli R. Diagnosis and classification of autoimmune hypophysitis. Autoimmun Rev. 2014;13:412-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Wang S, Wang L, Yao Y, Feng F, Yang H, Liang Z, Deng K, You H, Sun J, Xing B, Jin Z, Wang R, Pan H, Zhu H. Primary lymphocytic hypophysitis: Clinical characteristics and treatment of 50 cases in a single centre in China over 18 years. Clin Endocrinol (Oxf). 2017;87:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Khare S, Jagtap VS, Budyal SR, Kasaliwal R, Kakade HR, Bukan A, Sankhe S, Lila AR, Bandgar T, Menon PS, Shah NS. Primary (autoimmune) hypophysitis: a single centre experience. Pituitary. 2015;18:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Howlett TA, Levy MJ, Robertson IJ. How reliably can autoimmune hypophysitis be diagnosed without pituitary biopsy. Clin Endocrinol (Oxf). 2010;73:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Weimann E, Mölenkamp G, Böhles HJ. Diabetes insipidus due to hypophysitis. Horm Res. 1997;47:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Lee S, Choi JH, Kim CJ, Kim JH. Clinical Interrogation for Unveiling an Isolated Hypophysitis Mimicking Pituitary Adenoma. World Neurosurg. 2017;99:735-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Chrisoulidou A, Boudina M, Karavitaki N, Bili E, Wass J. Pituitary disorders in pregnancy. Hormones (Athens). 2015;14:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Funazaki S, Yamada H, Hara K, Ishikawa SE. Spontaneous pregnancy after full recovery from hypopituitarism caused by lymphocytic hypophysitis. Endocrinol Diabetes Metab Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Katsiveli P, Sfakiotaki M, Voulgaris N, Papanastasiou L, Kounadi T, Lymperopoulos K, Piaditis G. A complicated case of primary hypophysitis with bilateral intracavernous carotid artery occlusion. Hormones (Athens). 2016;15:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Lupi I, Cosottini M, Caturegli P, Manetti L, Urbani C, Cappellani D, Scattina I, Martino E, Marcocci C, Bogazzi F. Diabetes insipidus is an unfavorable prognostic factor for response to glucocorticoids in patients with autoimmune hypophysitis. Eur J Endocrinol. 2017;177:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Takahashi Y. Autoimmune hypophysitis: new developments. Handb Clin Neurol. 2014;124:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Cheung CC, Ezzat S, Smyth HS, Asa SL. The spectrum and significance of primary hypophysitis. J Clin Endocrinol Metab. 2001;86:1048-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Bando H, Iguchi G, Fukuoka H, Taniguchi M, Yamamoto M, Matsumoto R, Suda K, Nishizawa H, Takahashi M, Kohmura E, Takahashi Y. The prevalence of IgG4-related hypophysitis in 170 consecutive patients with hypopituitarism and/or central diabetes insipidus and review of the literature. Eur J Endocrinol. 2014;170:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Ćaćić M, Marinković J, Kruljac I, Perić B, Čerina V, Stipić D, Pažanin L, Pećina HI, Vrkljan M. ISCHEMIC PITUITARY APOPLEXY, HYPOPITUITARISM AND DIABETES INSIPIDUS: A TRIAD UNIQUE TO NECROTIZING HYPOPHYSITIS. Acta Clin Croat. 2018;57:768-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Hanna B, Li YM, Beutler T, Goyal P, Hall WA. Xanthomatous hypophysitis. J Clin Neurosci. 2015;22:1091-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Lin W, Gao L, Guo X, Wang W, Xing B. Xanthomatous Hypophysitis Presenting with Diabetes Insipidus Completely Cured Through Transsphenoidal Surgery: Case Report and Literature Review. World Neurosurg. 2017;104:1051.e7-1051.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Joung JY, Jeong H, Cho YY, Huh K, Suh YL, Kim KW, Bae JC. Steroid responsive xanthomatous hypophysitis associated with autoimmune thyroiditis: a case report. Endocrinol Metab (Seoul). 2013;28:65-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Gubbi S, Hannah-Shmouni F, Stratakis CA, Koch CA. Primary hypophysitis and other autoimmune disorders of the sellar and suprasellar regions. Rev Endocr Metab Disord. 2018;19:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Honegger J, Buchfelder M, Schlaffer S, Droste M, Werner S, Strasburger C, Störmann S, Schopohl J, Kacheva S, Deutschbein T, Stalla G, Flitsch J, Milian M, Petersenn S, Elbelt U; Pituitary Working Group of the German Society of Endocrinology. Treatment of Primary Hypophysitis in Germany. J Clin Endocrinol Metab. 2015;100:3460-3469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Tzou SC, Lupi I, Landek M, Gutenberg A, Tzou YM, Kimura H, Pinna G, Rose NR, Caturegli P. Autoimmune hypophysitis of SJL mice: clinical insights from a new animal model. Endocrinology. 2008;149:3461-3469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Kristof RA, Van Roost D, Klingmüller D, Springer W, Schramm J. Lymphocytic hypophysitis: non-invasive diagnosis and treatment by high dose methylprednisolone pulse therapy? J Neurol Neurosurg Psychiatry. 1999;67:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Kurokawa R, Ulasoglu C S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ