Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10701
Peer-review started: May 10, 2022
First decision: June 27, 2022
Revised: July 16, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: October 16, 2022
Processing time: 141 Days and 20.1 Hours
With the wide application of immune checkpoint inhibitors (ICIs) in cancer treatment, immune-related adverse events occur frequently, involving almost all organs and systems. The incidence of ICI-associated arthritis (IA) is unknown. In most cases, IA is not serious and non-lethal. Higher checkpoint inhibitor arthritis disease activity may be associated with cancer progression. Here, we report a severe case of IA with high arthritis disease activity in advanced pulmonary adenocarcinoma, causing permanent withdrawal of pembrolizumab, but the patient remained in complete remission (CR) 20 mo after the development of IA.
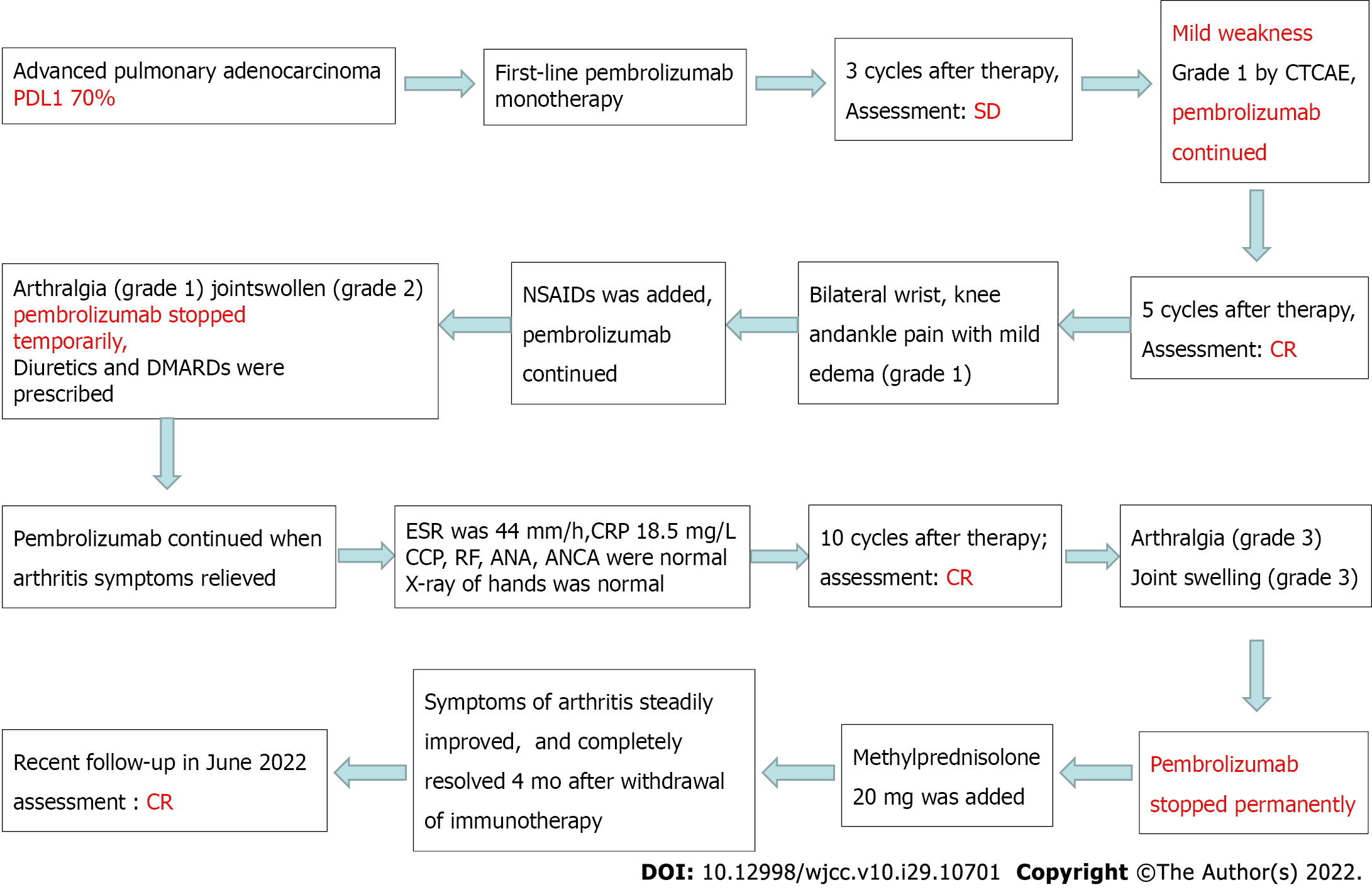
An 81-year-old smoking man was admitted to our hospital because of left chest pain for 9 mo. He was finally diagnosed with advanced pulmonary adenocarcinoma, with programmed cell death 1 ligand 1 expression of 70%. The patient responded to pembrolizumab treatment and achieved CR, but IA occurred after the 5th cycle of pembrolizumab administration. Although non-steroidal anti-inflammatory drugs and disease-modifying anti-rheumatic drugs were pres
This case report highlights that early recognition of IA and appropriate treatment are critical to improving the outcome of both ICI-arthritis and lung cancer.
Core Tip: We report a severe case of immune checkpoint inhibitor-associated arthritis (IA) in advanced pulmonary adenocarcinoma. A recent study suggested that higher checkpoint inhibitor arthritis disease activity may be associated with cancer progression. In our case, Clinical Disease Activity Index (CDAI) score increased to as high as 43, but cancer disease remained in complete remission 20 mo after the development of IA. Whether CDAI can be a predictor of disease progression needs to be confirmed in future studies.
- Citation: Yang Y, Huang XJ. Immune checkpoint inhibitor-associated arthritis in advanced pulmonary adenocarcinoma: A case report. World J Clin Cases 2022; 10(29): 10701-10707
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10701.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10701
Immune checkpoint inhibitors (ICIs) have resulted in a marked change in the therapeutic modality of non-small cell lung cancer (NSCLC). However, due to the widespread application of ICIs, immune-related adverse events (irAEs) involving all organs and systems may occur due to immunological enhancement. ICI-associated arthritis (IA) is rarely reported as case reports or in randomized controlled clinical studies. The reason for this may be that the symptoms such as joint pain are usually not serious and are non-fatal. Therefore, it does not attract enough attention from patients and clinicians, and the diagnosis is easily missed. In addition, some patients have osteoarthiritis, which is easily confused with immune-related arthritis symptoms by patients themselves. With the wide application of immunotherapy in the field of cancer, we have reason to believe that many more cases of IA will be reported. In most cases, IA is mild or moderate and non-fatal. However, it can affect the life quality of patients, reduce their confidence in disease treatment, interrupt the treatment plan, and finally affect the treatment effect or survival. Prompt recognition and appropriate treatment are critical to improving the outcome of IA, which can be realized if physicians are familiar with the clinical manifestations and therapeutic measures. However, in clinical practice, sometimes the diagnosis of IA is difficult or delayed.
Herein, we report a case of IA in a patient with advanced lung adenocarcinoma treated with pembrolizumab. The patient achieved complete remission (CR), but had to permanently discontinue pembrolizumab after 10 treatment cycles due to severe IA. A recent study found that Clinical Disease Activity Index (CDAI) may be a predictor of disease progression[1]. Our patient had a high CDAI score of 43, but remained in CR 20 mo after the development of IA. Thus, whether the CDAI can be used a predictor of disease progression requires confirmation in future studies.
An 81-year-old smoking man complained of bilateral wrist, knee and ankle pain (grade 1) with mild edema (grade 1) after the 5th cycle of pembrolizumab administration for lung cancer.
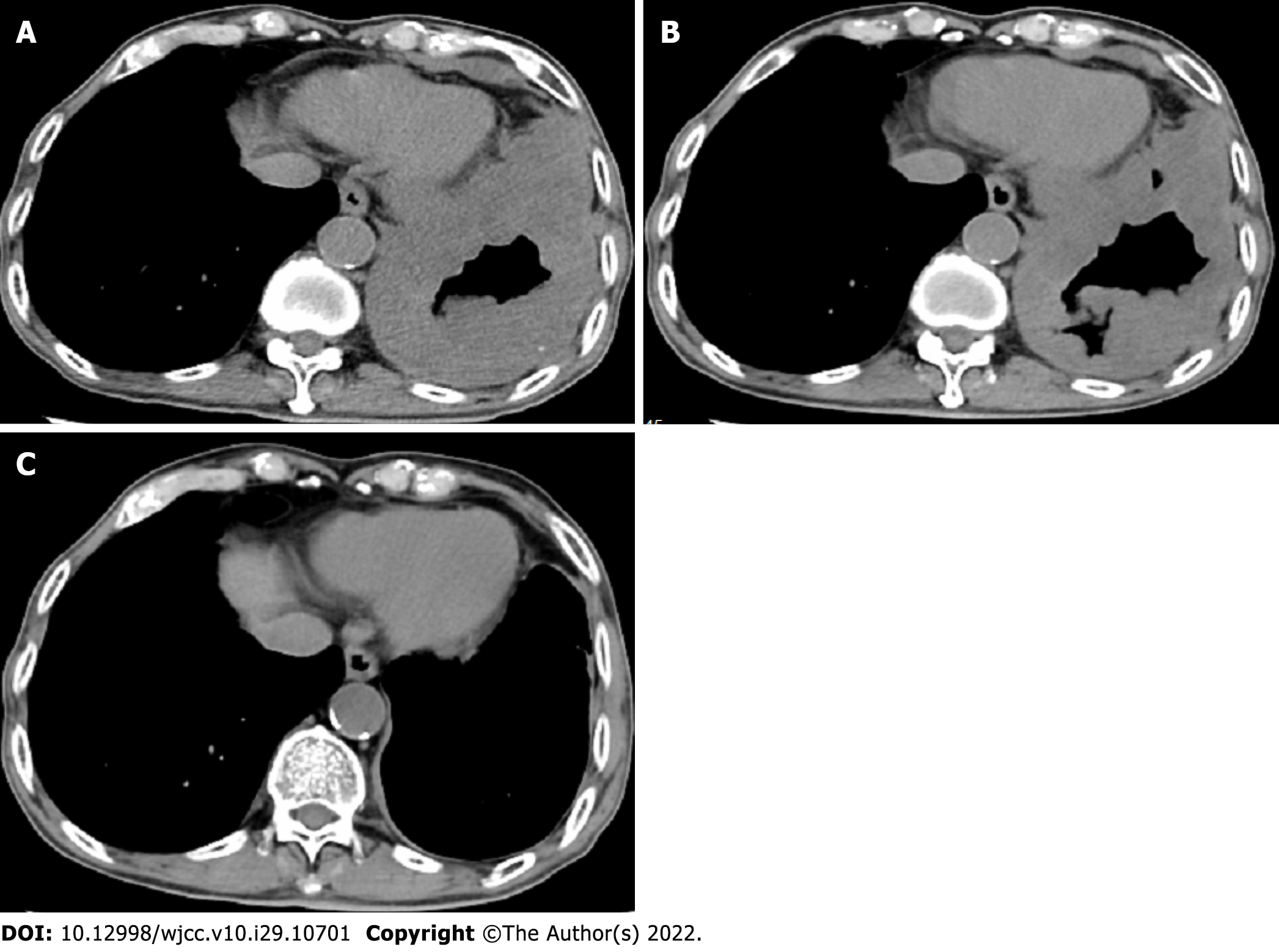
The patient was admitted to the Second Affiliated Hospital, Zhejiang University School of Medicine on May 31, 2020 due to left chest pain for 9 mo. Diffuse thickening of the left pleura and interlobar pleura was found on a chest computed tomography (CT) scan (Figure 1A). CT-guided lung puncture was performed and pathology revealed adenocarcinoma of the lung, with programmed cell death 1 ligand 1 (PD-L1) expression of 70%. He was finally diagnosed with NSCLC (stage IV, adenocarcinoma) at our hospital. Lung cancer related genes including EGFR, ALK, and ROS1 were negative. The patient was treated with first-line pembrolizumab monotherapy at a standard dosage of 2 mg/kg every 3 wk. From June 2020 to August 2020, the patient received three cycles of pembrolizumab. His left chest pain was relieved and chest CT scanning revealed stable disease (Figure 1B). The patient complained of mild weakness, which was grade 1 according to the Common Terminology Criteria for Adverse Events (CTCAE v4.0). Another two cycles of pembrolizumab were administered. Left chest pain disappeared and he achieved CR (Figure 1C).
The patient had a history of lumbar 2 vertebral compression fractures and recovered well after surgical treatment.
The patient had smoked for more than 50 years, 20 cigarettes/d, and stopped smoking following the diagnosis of lung cancer. He had a history of penicillin anaphylaxis. The patient had no previous rheumatoid arthritis or rheumatic immune system diseases.
Bilateral wrists, knees, and ankles showed slightly depressed edema with pain. Functional limitation was reported and symptoms did not abate after mobilization.
Anti-cyclic citrullinated peptide antibody, rheumatoid factor, antinuclear antibody, anti-neutrophil cytoplasmic antibodies, and hepatic and renal function analysis were performed and were found to be normal. C-reactive protein level was 18.5 mg/L, and the erythrocyte sedimentation rate was 44 mm/h. The CDAI score was 43.
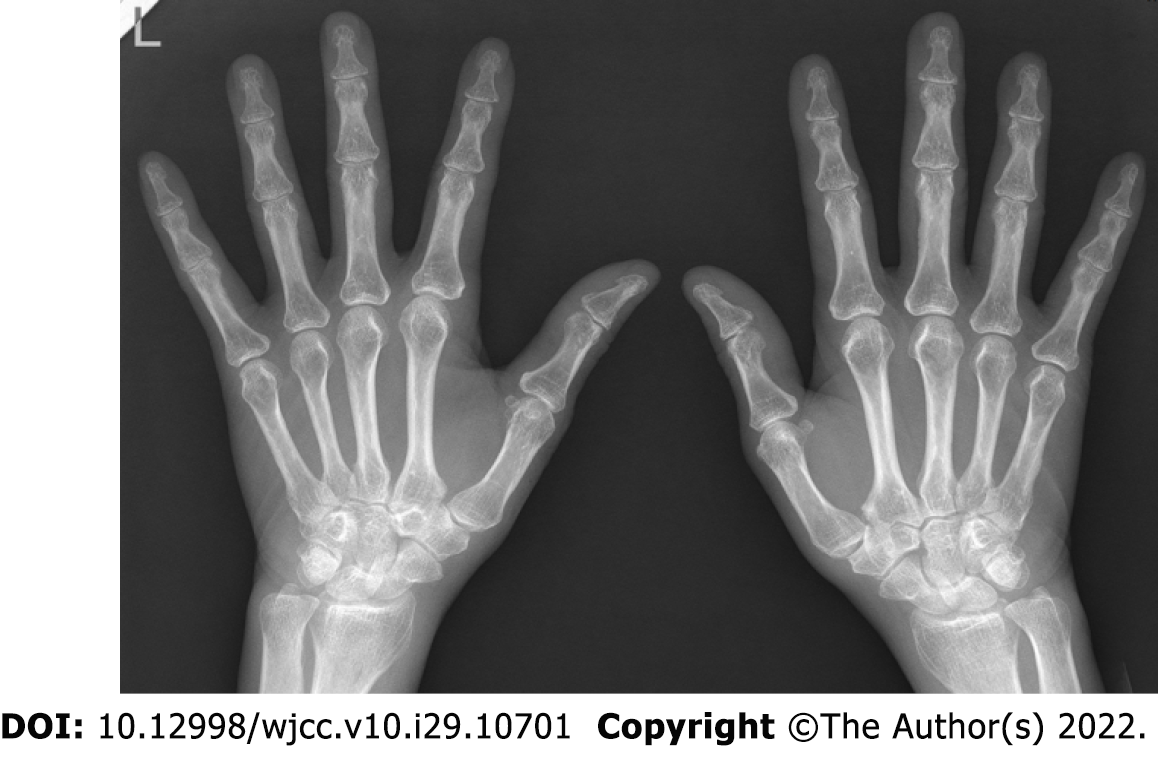
X-ray of the hands was normal (Figure 2).
IA and advanced lung adenocarcinoma.
Non-steroidal anti-inflammatory drugs (NSAIDs) were administered and treatment with pembrolizumab was continued. However, the manifestations of arthritis persisted, defined as arthralgia (grade 1) and swollen joints (grade 2). After consultation with a rheumatic immunologist, diuretics and disease modifying anti-rheumatic drugs (DMARDs) were prescribed. Pembrolizumab was discontinued temporarily. When IA returned to grade 1, pembrolizumab therapy was continued. After the 10th cycle of pembrolizumab administration, pain in the fingers, wrists, and ankles increased (grade 3). The life quality of the patient was seriously affected due to joint swelling and pain. In addition, the patient felt anxious and fearful of lung cancer progression. Intravenous methylprednisolone at 20 mg/d was prescribed and then tapered over the subsequent 4 wk.
The symptoms of arthritis steadily improved after steroid treatment and completely resolved 4 mo after withdrawal of immunotherapy. A recent follow-up in June 2022 revealed satisfactory clinical recovery of arthritis and lung cancer was still in CR as shown by CT scanning.
ICIs have been confirmed to be an effective treatment modality and are widely used for the treatment of NSCLC. With increasing use of ICIs, a large spectrum of immune side effects, described as irAEs, have emerged. Dermatologic, gastrointestinal, pulmonary, cardiac, endocrine, skin, and rheumatologic systems, and almost all organs of the body can be affected[2,3]. Unlike irAEs in other systems, rheu
The most frequent clinical manifestations of IA are similar to those of rheumatoid arthritis[7,8]. As in our case, IA is characterized by small-joint involvement of the metacarpophalangeal joint, proximal interphalangeal joint, the wrist, knee, and feet[4]. Rheumatoid factor and anti-citrullinated peptide antibody levels are usually normal[9]. Another relatively rare phenotype closely resembles that of spinal arthritis, which is characterized by large joint involvement, such as inflammatory back pain, adhesion point inflammation, and phalangitis[10-12]. Unlike spinal arthritis, human leukocyte antigen-B27 was negative in most of these patients[13].
The time to arthritis occurrence after administration of ICIs varies, ranging from 2 mo to 24 mo, and symptoms may persist for more than 1 year[14-18], regardless of whether the ICI is discontinued[1]. The persistence of IA demonstrated in our study lasted 9 mo (4 mo after pembrolizumab discontinuation), which was consistent with other reported cases[19,20].
The treatment principles recommended by the National Comprehensive Cancer Network, European Society for Medical Oncology, and Chinese Society of Clinical Oncology guidelines are basically the same. Therapeutic drugs mainly include NSAIDs, glucocorticoids, and DMARDs. For patients with irAEs above the G2 level, most require systemic corticosteroids in addition to DMARDs. A large single-institution retrospective study found that 37% of patients with musculoskeletal irAEs needed either higher prednisone doses or low-dose maintenance therapy. Several patients even received steroids for more than 1 year[21]. If symptoms do not improve after initial treatment, consultation with rheumatology experts is necessary for disease control. Some patients even require more than one type of immunosuppressive therapy.
IA patients rarely require hospitalization and most cases are defined as having grade 1 or 2 disease. However, some patients reported significant impairment both in function and emotion, even when characterized as low grade[1,21]. Thus, in addition to the CTCAE grade, the CDAI, a traditional measure of arthritis activity, is recommended to assess IA[1]. It is interesting that increased CDAI may be related to cancer progression[1]. However, in our case, the CDAI increased during the treatment of IA, but the patient remained in CR (Figure 3). Whether the CDAI can be used as a predictor of disease progression requires confirmation in future studies.
Immunotherapy has opened a new chapter in the treatment of advanced NSCLC. However, with the wide application of ICIs, more and more irAEs have been reported. Rheumatic arthritis/myalgia has rarely been reported in previous clinical trials. However, the actual incidence in the real world may be much higher than the data shown in current clinical studies. During the course of immunotherapy, clinicians should pay attention to symptoms of arthritis, distinguish IA from traditional rheumatic diseases, and provide appropriate treatment. In addition to the CTCAE grade, the CDAI is recom
We thank the patient for providing consent for the publication of this case report.
| 1. | Chan KK, Tirpack A, Vitone G, Benson C, Nguyen J, Ghosh N, Jannat-Khah D, Bykerk V, Bass AR. Higher Checkpoint Inhibitor Arthritis Disease Activity may be Associated With Cancer Progression: Results From an Observational Registry. ACR Open Rheumatol. 2020;2:595-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Suarez-Almazor ME, Kim ST, Abdel-Wahab N, Diab A. Review: Immune-Related Adverse Events With Use of Checkpoint Inhibitors for Immunotherapy of Cancer. Arthritis Rheumatol. 2017;69:687-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 694] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 4. | Xiao Y, Zeng L, Shen Q, Zhou Z, Mao Z, Wang Q, Zhang X, Li Y, Yao W. Diagnosis and Treatment of Rheumatic Adverse Events Related to Immune Checkpoint Inhibitors. J Immunol Res. 2020;2020:2640273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res (Hoboken). 2017;69:1751-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 6. | Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C, Dousset L, Pham-Ledard A, Prey S, Beylot-Barry M, Daste A, Gross-Goupil M, Lallier J, Ravaud A, Forcade E, Bannwarth B, Truchetet ME, Richez C, Mehsen N, Schaeverbeke T; FHU ACRONIM. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis. 2018;77:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 241] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 7. | Richter MD, Crowson C, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Rheumatic Syndromes Associated With Immune Checkpoint Inhibitors: A Single-Center Cohort of Sixty-One Patients. Arthritis Rheumatol. 2019;71:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 8. | Arnaud L, Lebrun-Vignes B, Salem JE. Checkpoint inhibitor-associated immune arthritis. Ann Rheum Dis. 2019;78:e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Haikal A, Borba E, Khaja T, Doolittle G, Schmidt P. Nivolumab-induced new-onset seronegative rheumatoid arthritis in a patient with advanced metastatic melanoma: A case report and literature review. Avicenna J Med. 2018;8:34-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1337] [Cited by in RCA: 1494] [Article Influence: 166.0] [Reference Citation Analysis (10)] |
| 11. | Tocut M, Brenner R, Zandman-Goddard G. Autoimmune phenomena and disease in cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Ohnuma K, Hatano R, Dang NH, Morimoto C. Rheumatic diseases associated with immune checkpoint inhibitors in cancer immunotherapy. Mod Rheumatol. 2019;29:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Lee KA, Kim HR, Yoon SY. Rheumatic complications in cancer patients treated with immune checkpoint inhibitors. Korean J Intern Med. 2019;34:1197-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Smith MH, Bass AR. Arthritis After Cancer Immunotherapy: Symptom Duration and Treatment Response. Arthritis Care Res (Hoboken). 2019;71:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Mooradian MJ, Nasrallah M, Gainor JF, Reynolds KL, Cohen JV, Lawrence DP, Miloslavsky EM, Kohler MJ, Sullivan RJ, Schoenfeld SR. Musculoskeletal rheumatic complications of immune checkpoint inhibitor therapy: A single center experience. Semin Arthritis Rheum. 2019;48:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Lidar M, Giat E, Garelick D, Horowitz Y, Amital H, Steinberg-Silman Y, Schachter J, Shapira-Frommer R, Markel G. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 17. | Cappelli LC, Gutierrez AK, Baer AN, Albayda J, Manno RL, Haque U, Lipson EJ, Bleich KB, Shah AA, Naidoo J, Brahmer JR, Le D, Bingham CO 3rd. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017;76:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 296] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 18. | Calabrese C, Kirchner E, Kontzias A, Velcheti V, Calabrese LH. Correction: Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open. 2017;3:e000412corr1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, van Riel PL, Tugwell P. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford). 2003;42:244-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 776] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 20. | Braaten TJ, Brahmer JR, Forde PM, Le D, Lipson EJ, Naidoo J, Schollenberger M, Zheng L, Bingham CO, Shah AA, Cappelli LC. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis. 2020;79:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 21. | Cappelli LC, Grieb SM, Shah AA, Bingham CO 3rd, Orbai AM. Immune checkpoint inhibitor-induced inflammatory arthritis: a qualitative study identifying unmet patient needs and care gaps. BMC Rheumatol. 2020;4:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Tovoli F, Italy S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL