Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10655
Peer-review started: April 28, 2022
First decision: July 11, 2022
Revised: July 20, 2022
Accepted: September 7, 2022
Article in press: September 7, 2022
Published online: October 16, 2022
Processing time: 154 Days and 1.8 Hours
BCR-ABL-negative myeloproliferative neoplasms (MPNs) are clonal hemato
We report two patients who were diagnosed with myeloproliferative neoplasms complicated with β-thalassemia. Both patients had abnormal increases in platelet counts. Based on bone marrow pathology and molecular biology assessment, we made the diagnosis of myeloproliferative neoplasms complicated with β-thalassemia. The female patient was given hydroxyurea and interferon, which enabled good control of her blood counts; the male patient was given ruxolitinib tablets, thalidomide tablets, and interferon to control the condition, but the patient poorly responded to drug treatment and died of gastrointestinal bleeding six months later.
Given the findings of our cases and the literature review, we hypothesize that myeloproliferative neoplasms complicated with β-thalassemia can lead to rapid disease progression and a poor prognosis.
Core Tip: The present report describes two cases of Myeloproliferative Neoplasms complicated with β-thalassemia and reviews all similar cases reported in the literature, in terms of indications, diagnosis and treatment. The present two cases highlight the importance of accurate diagnosis of myeloproliferative neoplasms complicated with β-thalassemia. It also could increase awareness among hematologists about the two rare cases. Early identification and appropriate identification of the diagnosis are crucial to start appropriate therapy to improve patient survival.
- Citation: Xu NW, Li LJ. Myeloproliferative neoplasms complicated with β-thalassemia: Two case report. World J Clin Cases 2022; 10(29): 10655-10662
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10655.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10655
BCR-ABL-negative myeloproliferative neoplasms (MPNs) are a group of malignant myeloproliferative diseases originating from pluripotent hematopoietic stem cells that often manifest as an increase in blood cells in one or more myeloid cell lines. This group mainly includes essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF)[1]. Thalassemia is one of the most common monogenic diseases worldwide, which includes alpha and β forms. There are very few case reports of myeloproliferative neoplasms complicated with β-thalassemia, and no cases have been reported from China. Here, we report 2 cases of myeloproliferative neoplasms complicated with β-thalassemia.
Case 1: A 33-year-old female patient was admitted to the hospital for thrombocytosis on January 7, 2020.
Case 2: A 61-year-old male patient complained of worsening cough for 1 mo and was admitted to the hospital on March 10, 2020.
Case 1: The patient complained of thrombocytosis with no symptoms. A routine blood test before admission revealed increased platelets during a routine health examination.
Case 2: The patient had a repeating cough with fever and sputum. In September 2019, routine blood tests showed white blood cells at 6.7 × 109/L, hemoglobin at 100 g/L, mean corpuscular volume (MCV) of 67.8 fl, mean corpuscular hemoglobin (MCH) of 21.5 pg, and platelets at 216 × 109/L, and an abdominal computed tomography showed splenomegaly. A PCR-based reverse dot blot (RDB) showed β-IVS-II-654-thalassemia (C→T). The patient was diagnosed with β-thalassemia.
Case 1: The patient had no other medical history.
Case 2: The patient had no other medical history.
Case 1: The patient had a family history of β-thalassemia.
Case 2: The patient had no remarkable personal or family history.
Case 1: Initial medical examination showed a respiratory rate (RR) of 22 breaths/min, heart rate (HR) of 94 beats/min, temperature of 36.9°C, and blood pressure (BP) of 126/70 mmHg. Splenomegaly was found.
Case 2: Physical examination showed an RR of 19 breaths/min, HR of 87 beats/min, temperature of 38.3°C and BP of 112/64 mmHg. Splenomegaly was found.
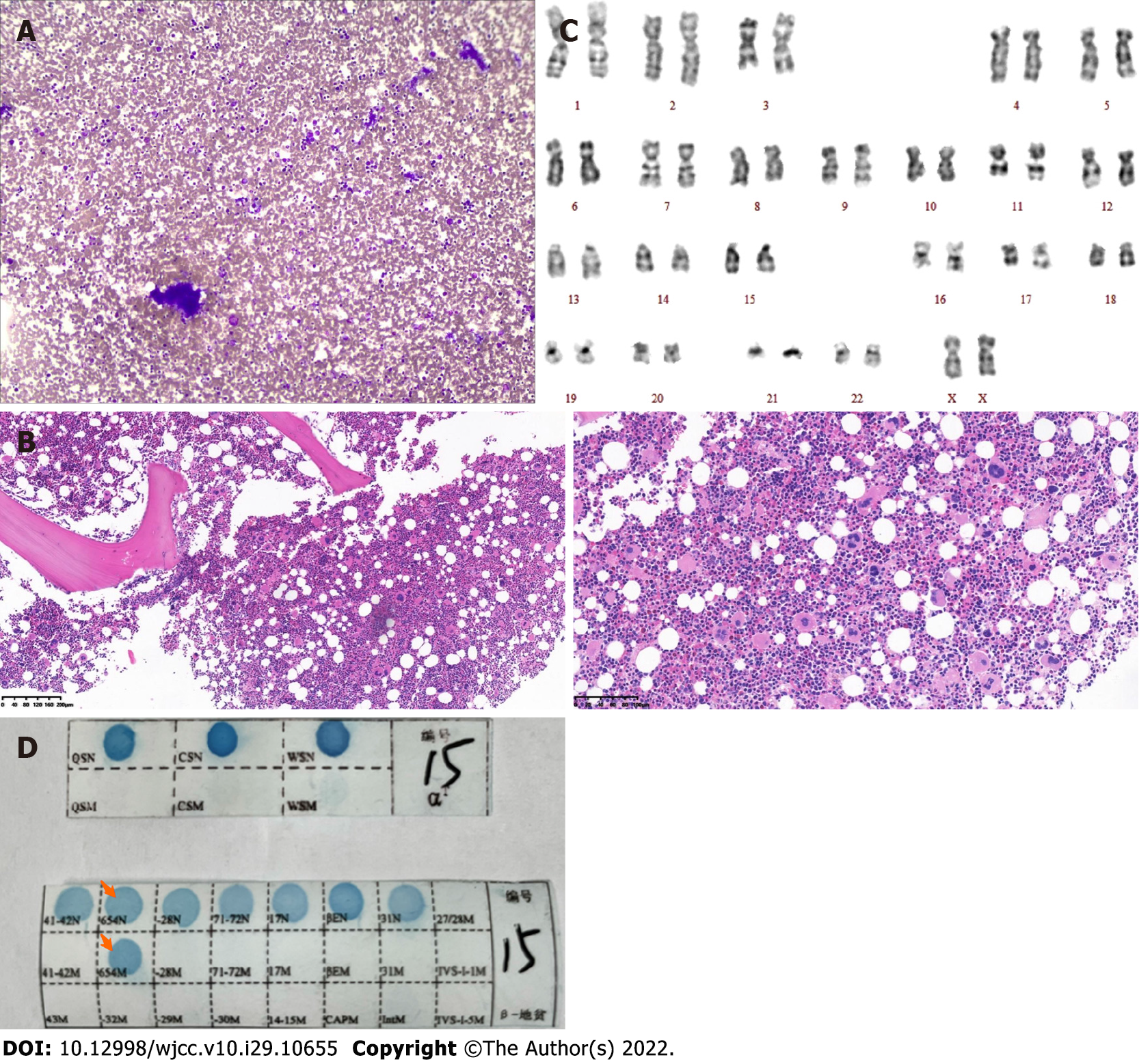
Case 1: Routine blood examination showed white blood cells at 10.9 × 109/L, Hb at 106 g/dL, MCV of 65.3 fl, MCH of 20.2 pg, and platelets at 1396 × 109/L. Total abdomen plain computerized tomography scanning indicated splenomegaly, and bone marrow aspiration showed platelets in clusters (Figure 1A). Bone marrow aspiration and biopsy were performed. Bone marrow pathological sections showed proliferation of abnormal megakaryocytes (Figure 1B). The karyotype showed 46 normal chromosomes, including XX chromosomes, in 20 cells (Figure 1C). There was no BCR-ABL fusion (P190/210/230) detected. JAK2 V617F, CALR and MPL exon 10 mutations were not found upon further molecular testing. The gene mutation in the peripheral blood sample showed that the genotype was the heterozygous mutation of β-IVS-II-654 (Figure 1D).
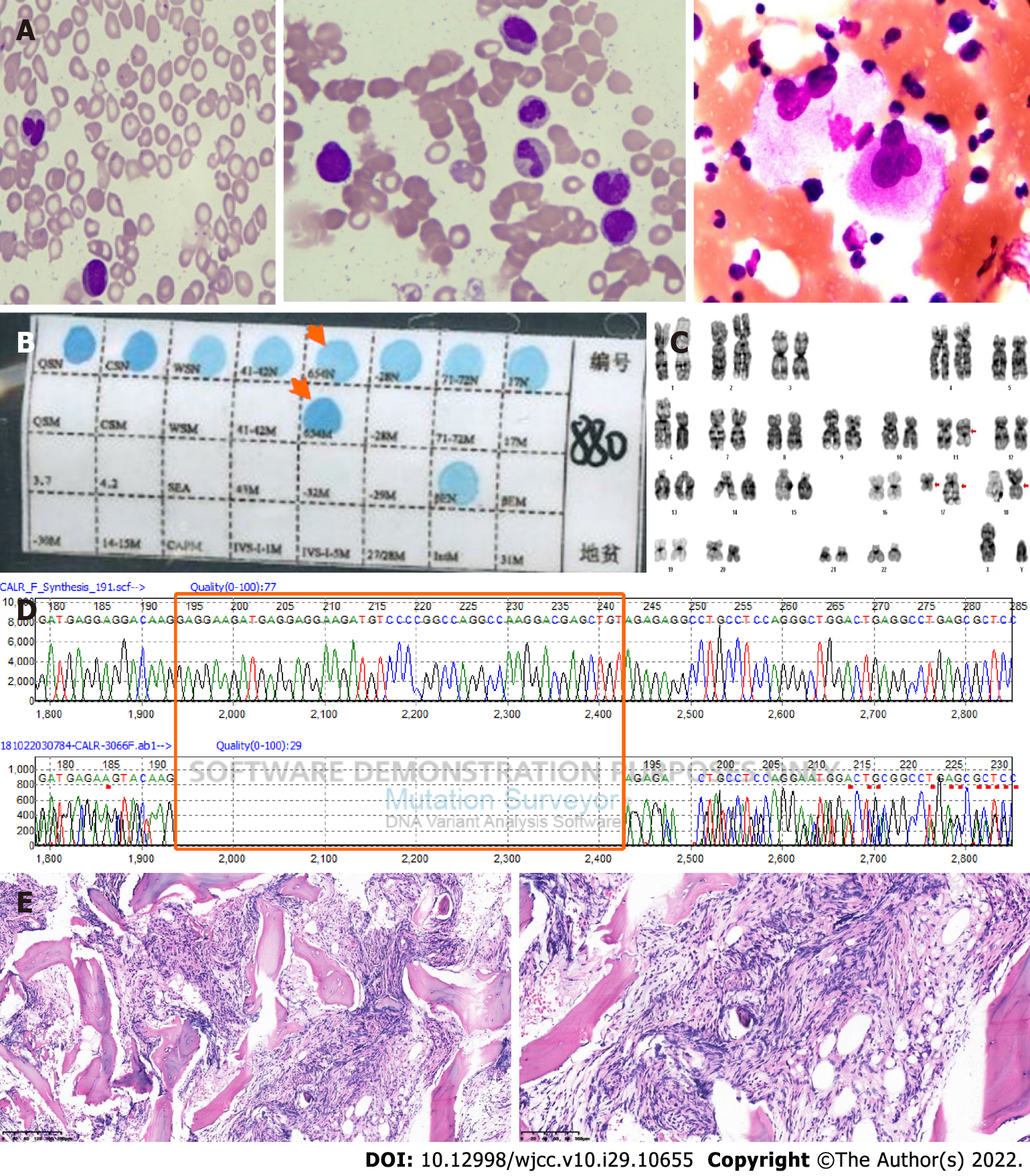
Case 2: Routine blood tests showed white blood cells at 34.7 × 109/L, hemoglobin at 92 g/L, MCV of 71 fl, MCH of 21.9 pg, and platelets at 18 × 109/L. Bone marrow aspiration showed teardrop-shaped, oval, long red blood cells and active hyperplasia of bone marrow granulocytes; erythroblastic cells were hypoplastic, and there was a normal number of megakaryocytes but a decreased platelet production (Figure 2A). PCR-based RDB [A1] showed β-IVS-Ⅱ-654-thalassemia (Figure 2B). Karyotypic analysis detected 46 chromosomes, including XY, t(11;18)(q13;p11.2), and ins(17;17)(q21;q25q12)[20] (Figure 2C); JAK2V617F mutation, MLL exon 10 mutation, and BCR-ABL1 fusion (P190/210/230) were not found upon molecular testing. A mutation was detected in CALR exon 9 (Figure 2D); abdominal B-ultrasound showed hepatomegaly and splenomegaly. Bone marrow pathological sections showed BM reticulin with megakaryocyte hyperplasia and dysplasia (Figure 2E).
No imaging examinations.
The final diagnosis of case 1 was essential thrombocytosis complicated with β-thalassemia. The final diagnosis of case 2 was primary myelofibrosis complicated with β-thalassemia.
Case 1: Administration of hydroxyurea and recombinant human interferon α2b injection (hydroxyurea at 1000 mg once daily and interferon α2b at 500 IU three times a week) was initiated.
Case 2: Administration of ruxolitinib, thalidomide tablets, and recombinant human interferon α2b injection (ruxolitinib at 10 mg once daily, thalidomide at 100 mg once daily and interferon α2b at 500 IU three times a week) was initiated.
Case 1: The blood platelets had decreased to a normal level at the follow-up.
Case 2: The patient responded to drug treatment poorly and finally died of gastrointestinal bleeding six months later due to platelet deficiency.
Thalassemia is a typical single-gene genetic disease that causes globin production disorders due to mutations or deletions in globin genes and can generally be divided into two categories (alpha- and β-thalassemia). The high incidence areas are in the Mediterranean Basin, the Middle East, Southeast Asia and Africa. The southern regions of China, including Guangdong and Guangxi, Guizhou, Yunnan, Hainan, Sichuan and other provinces, are areas with a high incidence of thalassemia. β-Thalassemia is a genetic disease caused by insufficient or absent β globin chain synthesis due to mutations in the β-globin gene[2]. The mutation type with the highest mutation frequency of the β-thalassemia gene is the IVS-Ⅱ-654 heterozygous mutation in Guangdong, Fujian, and Jiangsu Provinces[3]. Related studies have shown that gene abnormalities are closely related to the occurrence, development, diagnosis and prognosis of MPNs. Among BCR-ABL-negative MPNs, the JAK2, MPL and CALR genes have been found to be closely related to the occurrence, development and outcome of MPN. The activation of the JAK-STAT kinase pathway may be the central link in the pathogenesis of MPN[4]. The main mechanism of MPN is that mutated genes disrupt the normal JAK-STAT signaling pathway, modulate the transcription process and cause excessive accumulation of oncoproteins[5]. JAK2V617F can be expressed in patients with PV, ET and PMF. According to statistical data, the JAK2V617F mutation can be found in 95% of PV patients and 55% to 60% of ET and PMF patients[6]. CALR mutations mainly exist in MPNs, which are characteristic mutations of MPN[7]. Studies have shown that CALR mutations can be detected in 60%-80% of JAK2-negative and MPL-negative MPN patients[8-10]. In terms of the cumulative incidence of thrombosis and the incidence of major cardiovascular events, ET patients with CALR gene mutations have a lower incidence than those with JAK2 gene mutations, while the incidence of secondary myelofibrotic transformation is relatively high. Patients with a mutated CALR have a lower risk of thrombosis and longer overall survival than patients with mutated JAK2[11]. Gene mutations can occur at any stage of ontogeny or at any stage of the somatic or reproductive cell cycle. If a genetic mutation occurs in a somatic cell, the mutation can only be transmitted within the somatic cell, and therefore the somatic mutation cannot be directly inherited by the next generation. Germ cells have a higher mutation rate than somatic cells and can be directly inherited by the next generation. Thalassemia is a germline mutation that can be inherited by the next generation, while MPN-related gene mutations are somatic mutations. MPNs are genetically complex and heterogeneous diseases in which the acquisition of somatic driver mutations triggers three main myeloid cytokine receptors and phenotypically manifest as ET, PV and PMF. The course of the disease may be influenced by the sequence of germline susceptibility, modification mutations, acquisition, and environmental factors (such as aging and inflammation). Myeloproliferative neoplasms are derived from single somatic mutant hematopoietic stem cells. In addition to somatic mutations, germline variants can regulate the development of MPNs, which is conducive to the acquisition of somatic mutations and affects the clinical course of the disease[12]. In addition, it is thought that MPNs are driven by somatic mutations. Germline variants can influence the risk of leukemic transformation in MPN. It has been shown that germline factors play an important role in MPN pathogenesis[13]. Case 1 had a triple-negative MPN. The mutations carried by triple-negative patients are not yet known, have yet to be determined and elucidated, or are affected by other factors affecting their HSCs and progenitor cells. In some of these patients, the mutated clone might have been too small to be detected with the current approaches. In fact, somatic cell driver mutations may be a later event in the disease process, and other factors, such as chronic inflammation, may make the patient’s cells prone to transform into a MPN[14-16]. Case 2 had primary myelofibrosis and was positive for a CALR mutation. One research study suggested that CALR-positive myeloproliferative neoplasms have a more benign clinical course than the corresponding disorders associated with JAK2 or MPL mutations[11]. However, this patient had a complex abnormality karyotype, which resulted in a rapid disease progression and an extremely poor prognosis.
The diagnosis of MPN in a patient with β-thalassemia is clinically challenging because of some overlapping clinical features between MPN and β-thalassemia, such as splenomegaly, secondary leukocytosis and thrombocytosis. Splenomegaly is not a definite clinical characteristic of thalassemia minor. The combination of these studies showed that the spleen is enlarged in β-thalassemia minor but usually not to such a degree to be palpable[17]. The final diagnosis of Case 1 and Case 2 was β-thalassemia minor,and enlarged spleen was palpable in the two cases. Thus, we speculated that MPN might result in splenomegaly in the two cases. Only eight cases, including ours[18-20], have been reported thus far in the literature (Table 1). Among these, our cases are the first to establish the diagnosis of PMF by documentation of the CALR mutation and the first to establish the diagnosis of triple-negative MPN complicated with β-thalassemia. A timely diagnosis of an MPN patient with β-thalassemia is important so that the MPN may be managed appropriately.
| Patient characteristics | Case 1 | Case 2 | Lopes da Silva et al[21] | So et al[23] | Ifran et al[19] | Thomas[18] | Castro[20] | Martinez-Lopez et al[22] |
| Age/Sex | 33/F | 61/M | 75/F | 29/M | 68/F | 71/F | 48/F | 19/F |
| Thalassemic disorder | β-thalassemia minor | β-thalassemia minor | β-thalassemia minor | α- thalassemia | β-thalassemia minor | β-thalassemia minor | Sickle cell β-thalassemia minor | Delta-β-thalassemia minor |
| MPN subtypes | Essential thrombocythemia | Primary myelofibrosis | Polycythemia vera | Primary myelofibrosis | Polycythemia vera | Polycythemia vera | Polycythemia vera | Polycythemia vera |
| Splenomegaly | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| Erythrocyte count | 5.26 × 1012/L | 4.21 × 1012/L | 9.0 × 1012/L | NR | 9.3 × 1012/L | 7.4 × 1012/L | 8 × 1012/L | NR |
| Total blood hemoglobin | 106 g/L | 92 g/L | 154 g/L | 103 g/L | 155 g/L | 156 g/L | 144 g/L | 142 g/L |
| White cell count | 10.9 × 109/L | 34.7 × 109/L | 13.5 × 109/L | Normal | 18.5 × 109/L | 14 × 109/L | 8 × 109/L | 22.5 × 109/L |
| Platelet count | 1396× 109/L | 18 × 109/L | 500 × 109/L | Normal | NR | 486 × 109/L | 1233 × 109/L | 193 × 109/L |
| MPN gene mutation | Negative | Type 1 (49-bp) deletion in exon 9 of CALR | JAK2 V617F | Type 1 (52-bp) deletion in exon 9 of CALR | Not assessed | Not assessed | Not assessed | JAK2 V617F |
| Karyotype | 46, XX | 46, XY, t(11;18)(q13;p11.2), ins(17;17)(q21;q25q12) | NR | 46, XY, t(3;12)(p21;q13) | Not assessed | Not assessed | Not assessed | 46, XX |
| Marrow biopsy | Proliferation of giant megakaryocytes with hyperlobulated nuclei | Reticulin graded as 4+ myelofibrosis | Panmyelosis | Stromal fibrosis and clusters of pleomorphic megakaryocytes | Not assessed | Not assessed | Increased megakaryocytes plus fibrosis | Prominent conspicuous megakaryocyte proliferation in clusters and minimal reticulin fibrosis |
| Treatment | Hydroxyurea and interferon | Interferon→ruxolitinib | Hydroxyurea | NR | Phlebotomy | Phlebotomy | Busulfan→Phlebotomy→Mustard | Hydroxyurea and interferon→ruxolitinib |
At present, there are only a few reports and studies on myeloproliferative neoplasms combined with thalassemia. Among the 6 previously reported cases of BCR/ABL-negative chronic myeloproliferative neoplasms with thalassemia, 5 were β-thalassemia[18-22], and the other was a male patient from southern China who was screened for thalassemia after his wife had been diagnosed as an alpha thalassemia carrier during prenatal check-up. The patient was asymptomatic, but prominent splenomegaly 8 cm below the left costal margin was detected on physical examination. A peripheral blood smear demonstrated a myelocyte, a nucleated red blood cell, and many hypochromic microcytic red blood cells with some teardrop forms, and a bone marrow biopsy showed irregular deposition of trabecular bone, stromal fibrosis, dilated sinusoids with intravascular hematopoiesis, and clusters of pleomorphic megakaryocytes. Karyotypic analysis showed the balanced translocation t(3;12)(p21;q13). The partial sequencing electropherogram showed a type 1 (52-bp) deletion in exon 9 of CALR. In further molecular testing, no JAK2 V617F mutation, MPL exon 10 mutation, or BCR-ABL fusion gene was found, and therefore this patient was diagnosed with primary myelofibrosis and osteosclerosis[23]. In 2009, Taher et al[24] studied DNA from 20 patients with β-thalassemia for the JAK2 V617F mutation using the RG-PCR method. None of the patients were positive for this particular mutation. The gene type in alpha-thalassemia is primarily --(SEA)/alpha, and the gene type in β-thalassemia is primarily IVS-654.
Table 1 Clinical and laboratory summary of the reported cases of coexistent β-thalassemia and myeloproliferative neoplasms.
We report two cases of myeloproliferative neoplasms complicated with β-thalassemia. We hope to increase awareness among hematologists about these rare two cases. In conclusion, the etiology of chronic myeloproliferative neoplasms combined with IVS-II-654 heterozygous β-thalassemia is still unclear, and further research is needed to determine the pathogenesis.
We acknowledge the contributions of Mr Ming-shen Lin and Ms Li-Ping Yan for their research assistance.
| 1. | Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5219] [Cited by in RCA: 7014] [Article Influence: 701.4] [Reference Citation Analysis (0)] |
| 2. | Red Blood Cell Diseases (Anemia) Group, Chinese Society of Hematology, Chinese Medical Association. [Consensus of experts on diagnosis and treatment of non-transfusion dependent thalassemia in China (2018 edition)]. Zhonghua Xue Ye Xue Za Zhi. 2018;39:705-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Yang Y, Zhang J. [Research Progress on Thalassemia in Southern China -Review]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH, Bass AJ, Pretz J, Ahn J, Hricik T, Kilpivaara O, Wadleigh M, Busque L, Gilliland DG, Golub TR, Ebert BL, Levine RL. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123:e123-e133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 5. | de Freitas RM, Santos Mde O, Maranduba CM. The JAK2 gene as a protagonist in chronic myeloproliferative neoplasms. Rev Bras Hematol Hemoter. 2013;35:278-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Gäbler K, Behrmann I, Haan C. JAK2 mutants (e.g., JAK2V617F) and their importance as drug targets in myeloproliferative neoplasms. JAKSTAT. 2013;2:e25025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Tefferi A, Pardanani A. Genetics: CALR mutations and a new diagnostic algorithm for MPN. Nat Rev Clin Oncol. 2014;11:125-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L, Fanelli T, Bosi A, Vannucchi AM; Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative Investigators. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123:1552-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 318] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 9. | Andrikovics H, Krahling T, Balassa K, Halm G, Bors A, Koszarska M, Batai A, Dolgos J, Csomor J, Egyed M, Sipos A, Remenyi P, Tordai A, Masszi T. Distinct clinical characteristics of myeloproliferative neoplasms with calreticulin mutations. Haematologica. 2014;99:1184-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, Them NC, Berg T, Elena C, Casetti IC, Milanesi C, Sant'antonio E, Bellini M, Fugazza E, Renna MC, Boveri E, Astori C, Pascutto C, Kralovics R, Cazzola M; Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative Investigators. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123:1544-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 467] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 11. | Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NC, Berg T, Gisslinger B, Pietra D, Chen D, Vladimer GI, Bagienski K, Milanesi C, Casetti IC, Sant'Antonio E, Ferretti V, Elena C, Schischlik F, Cleary C, Six M, Schalling M, Schönegger A, Bock C, Malcovati L, Pascutto C, Superti-Furga G, Cazzola M, Kralovics R. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1420] [Cited by in RCA: 1514] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 12. | Lanikova L, Babosova O, Prchal JT. Experimental Modeling of Myeloproliferative Neoplasms. Genes (Basel). 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Harutyunyan AS, Kralovics R. Role of germline genetic factors in MPN pathogenesis. Hematol Oncol Clin North Am. 2012;26:1037-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kralovics R, Teo SS, Li S, Theocharides A, Buser AS, Tichelli A, Skoda RC. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Hermouet S, Bigot-Corbel E, Gardie B. Pathogenesis of Myeloproliferative Neoplasms: Role and Mechanisms of Chronic Inflammation. Mediators Inflamm. 2015;2015:145293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, Bottomly D, Wilmot B, McWeeney SK, Tognon CE, Pond JB, Collins RH, Goueli B, Oh ST, Deininger MW, Chang BH, Loriaux MM, Druker BJ, Tyner JW. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368:1781-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 455] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 17. | Ntaios G, Chatzinikolaou A. The spleen in beta thalassaemia minor: splenomegaly or just 'scanomegaly'? Br J Haematol. 2008;143:143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Thomas JP. β-thalassemia minor and newly diagnosed polycythemia rubra vera in a 71-year-old woman, Hosp. Physician. 2001;78-83. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Ifran A, Kaptan K, Beyan C. Presence of erythrocytosis in a patient with diagnosis of β thalassemia trait. WJMS. 2007;62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 20. | Castro O. Sickle cell thalassemia, thrombocytosis, and erythrocytosis. South Med J. 1981;74:380-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Lopes da Silva R, Silva M. Coexistence of β-thalassemia and polycythemia vera. Blood Cells Mol Dis. 2011;46:171-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Martinez-Lopez J, Jimenez A, Sanchez-Calero J, Monteagudo D, Urbanowicz M, Cañamares-Orbis I, Cortijo-Cascajares S, Ayala R. Myeloproliferative neoplasm in a thalassaemic patient: response to treatment with a JAK inhibitor. Ann Hematol. 2015;94:1237-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | So CC, Chan AY, Chan JC, Ma ES. Alpha thalassemia trait masquerading as hemoglobin H disease due to co-existing primary myelofibrosis. Ann Hematol. 2015;94:875-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Taher A, Shammaa D, Bazarbachi A, Itani D, Zaatari G, Greige L, Otrock ZK, Mahfouz RA. Absence of JAK2 V617F mutation in thalassemia intermedia patients. Mol Biol Rep. 2009;36:1555-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Papadopoulos VP, Greece; Shahriari M, Iran S-Editor: Liu JH L-Editor: A P-Editor: Liu JH