Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10622
Peer-review started: May 11, 2022
First decision: July 29, 2022
Revised: August 16, 2022
Accepted: September 7, 2022
Article in press: September 7, 2022
Published online: October 16, 2022
Processing time: 141 Days and 6.5 Hours
The occurrence of gastrointestinal stromal tumors (GISTs) in the small intestine is rare, and a case of wandering small intestinal stromal tumor has been rarely reported to date. Dissemination of this case can help inform future diagnosis and effective treatment.
A 68-year-old patient presented to us with tarry stools. Computed tomography showed a mobile tumor moving widely within the abdominal cavity. As the laboratory data showed a low range of red blood cells and an immediate surgery was not indicated, we performed digital subtraction angiography and embo
When a highly vascularized tumor is clinically encountered in the small intestine, the possibility of stromal tumors should be considered. However, when the tumor cannot be visualized at its original location, the possibility of tumor migration is considered.
Core Tip: Stromal tumors of the small intestine are rare. Symptoms range from none to mass effect and hemorrhage. With nonspecific symptoms, computed tomography is helpful in visualizing contrast-enhancing lesions in the small intestine. In even rarer cases, these tumors may mobilize and produce symptoms that mimic conditions, such as intestinal torsion. Recognition of wandering stromal tumors of the small intestine facilitates the timely diagnosis and treatment. Evaluation includes imaging, digital subtraction angiography, and histopathology. Surgery remains the gold standard of treatment.
- Citation: Su JZ, Fan SF, Song X, Cao LJ, Su DY. Wandering small intestinal stromal tumor: A case report. World J Clin Cases 2022; 10(29): 10622-10628
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10622.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10622
Gastrointestinal stromal tumors (GISTs) originate from the interstitial cells of Cajal, which are dependent on stem cell factor receptor interaction, and express both KIT and CD34. Immunohistochemistry plays an important role in differentiating GISTs from leiomyomas and neurogenic tumors[1]. Histopathologic findings are the gold standard for diagnosing GISTs[2]. They become symptomatic if they enlarge, causing ulceration, bleeding (e.g., hematemesis, melena), and iron deficiency anemia. Tumors larger than 2 cm carry an increased risk of malignancy and hence are resected. The role of laparoscopy in the resection of GISTs is constantly being affirmed. Imatinib is a selective KIT inhibitor that achieves sustained clinical benefit and objective efficacy in most patients with GISTs. Tumors of intermediate- and high-risk require imatinib for 3 years after radical resection[3]. GISTs are the most common mesenchymal tumors of the gastrointestinal (GI) tract. However, wandering small intestinal stromal tumors are extremely rare. Herein, we report a case of a 68-year-old man presenting with a small intestinal mass that was diagnosed as a suspected wandering small intestinal stromal tumor. Computed tomography (CT) with contrast and digital subtraction angiography (DSA) of abdomen revealed an aberrant mass that wandered from the right upper quadrant to the left upper quadrant, before returning to the right lumbar region of the abdomen. The location of the mass varied from visit to visit. Surgery was performed, and the diagnosis was confirmed by histopathology. This study aims to enhance our understanding of this rare and intriguing condition.
A 68-year-old man presented to the emergency department with five episodes of tarry stools for 2 d, associated with persistent dull and diffuse abdominal pain.
Six years ago, the patient developed melena without manifesting any obvious symptoms and no abnormalities were observed in the findings of colonoscopy and gastroscopy, which were performed in another hospital. Further, the patient reported that the passage of black stool was repeated 2 to 3 times in the last 6 years, and his condition improved after he was administered Yunnan Baiyao and omeprazole. The specific medication regimen is unknown, and the patient terminated the medication after the bloody stool disappeared.
The patient had no known comorbidities or past illnesses.
The patient had no relevant personal and family medical history.
On physical examination, the abdomen was negative for palpable masses and tenderness.
Laboratory data showed a red blood cell mass of 1.57 × 106/µL (normal range: 4.30–5.80 × 106/µL).
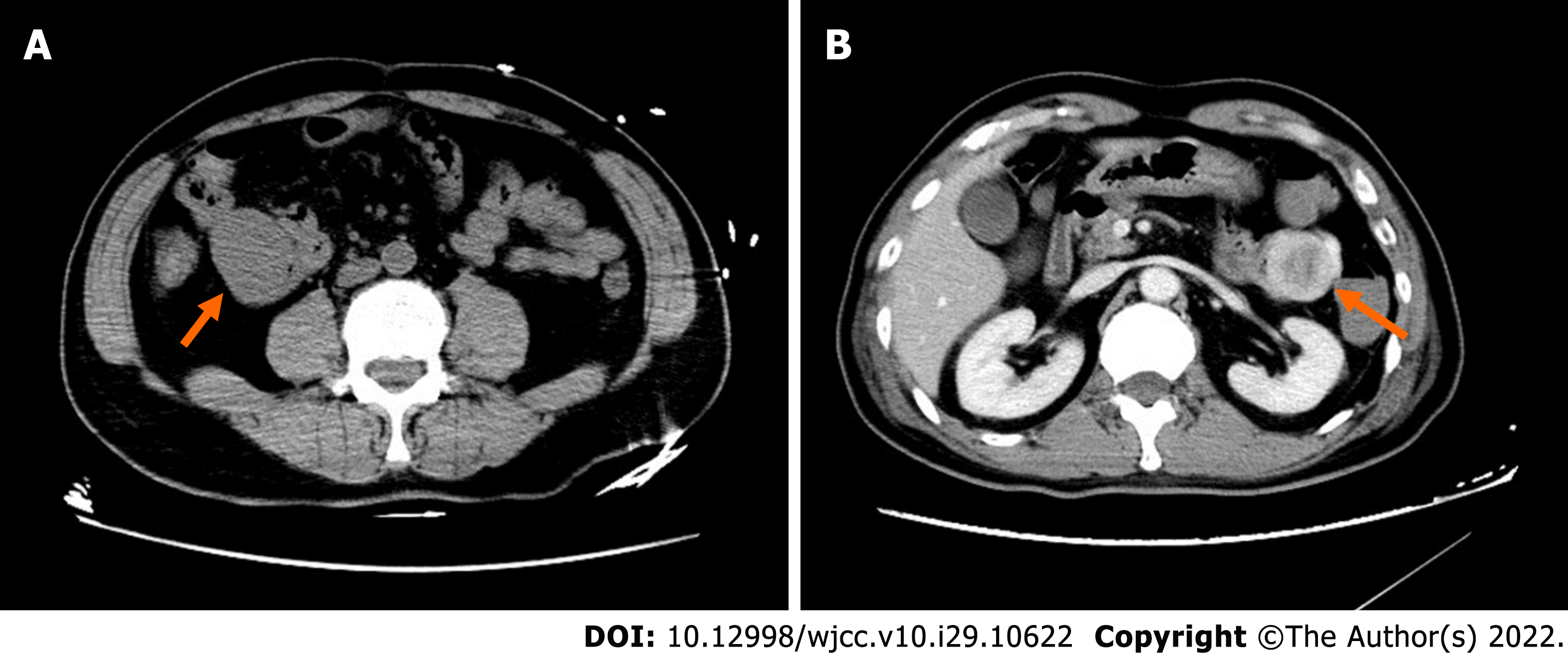
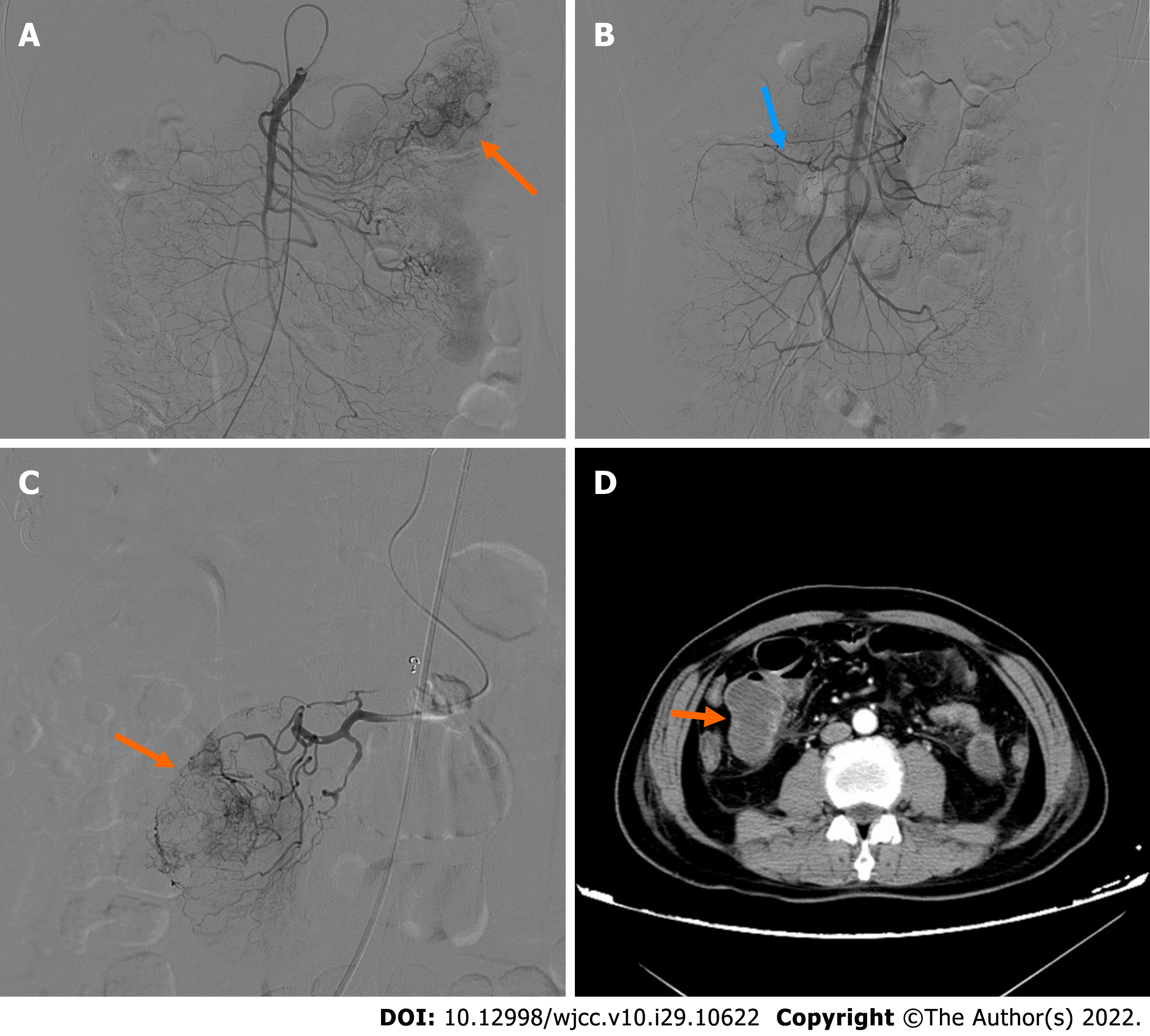
On admission, plain CT of the abdomen revealed an oval, well-circumscribed mass with soft-tissue attenuation, measuring 43 Hounsfield units (HU) on the right upper quadrant of the abdomen (Figure 1A). The mass dimensions were approximately 4.1 cm × 3.3 cm × 5.0 cm in size. Contrast-enhanced abdominal CT scans 2 d later revealed a well-defined, non-homogenous enhanced lesion (density of 78 HU in arterial phase, 90 HU in venous phase), measuring 4.6 cm × 3.4 cm × 5.0 cm in the left upper quadrant (Figure 1B). However, the mass present on the right upper quadrant had not been visualized. Clinical and imaging findings indicated a diagnosis of a highly vascularized, mobile small intestine tumor. We initially considered a GIST in the small intestine. DSA was performed because laboratory data showed a low range of red blood cells and immediate surgery was contraindicated. A selective superior mesenteric artery DSA revealed a mass in the left upper quadrant, with neoplastic vessels from the first branch of the jejunal artery. We observed visible contrast media retention in the intestinal lumen (Figure 2A). For selective embolization, we used a microcatheter (Progreat, TERUMO, Tokyo, Japan). The microcatheters were inserted as near as possible to the lesion, and the embolic materials containing polyvinyl alcohol (COOK Medical, Bloomington, IN, United States) were inserted. A repeat DSA confirmed complete mass staining disappearance. However, melena recurred on the 4th postoperative day. Laboratory data showed a low range of red blood cells (2.16 × 106/µL). On repeat DSA, the first branch of the jejunum appeared to have moved to the right lumbar region, accompanied by vascular detour, thickening, and contrast concentration (Figure 2B). The superselective arteriography and embolization were repeated (Figure 2C). Two days after the second embolization, a contrast-enhanced CT scan revealed an annular irregularly enhancing mass (measuring 5.4 cm × 3.7 cm × 5.5 cm) with a large central area of liquefied necrosis (density of 38-47 HU), which was then located in the right lumbar region (Figure 2D).
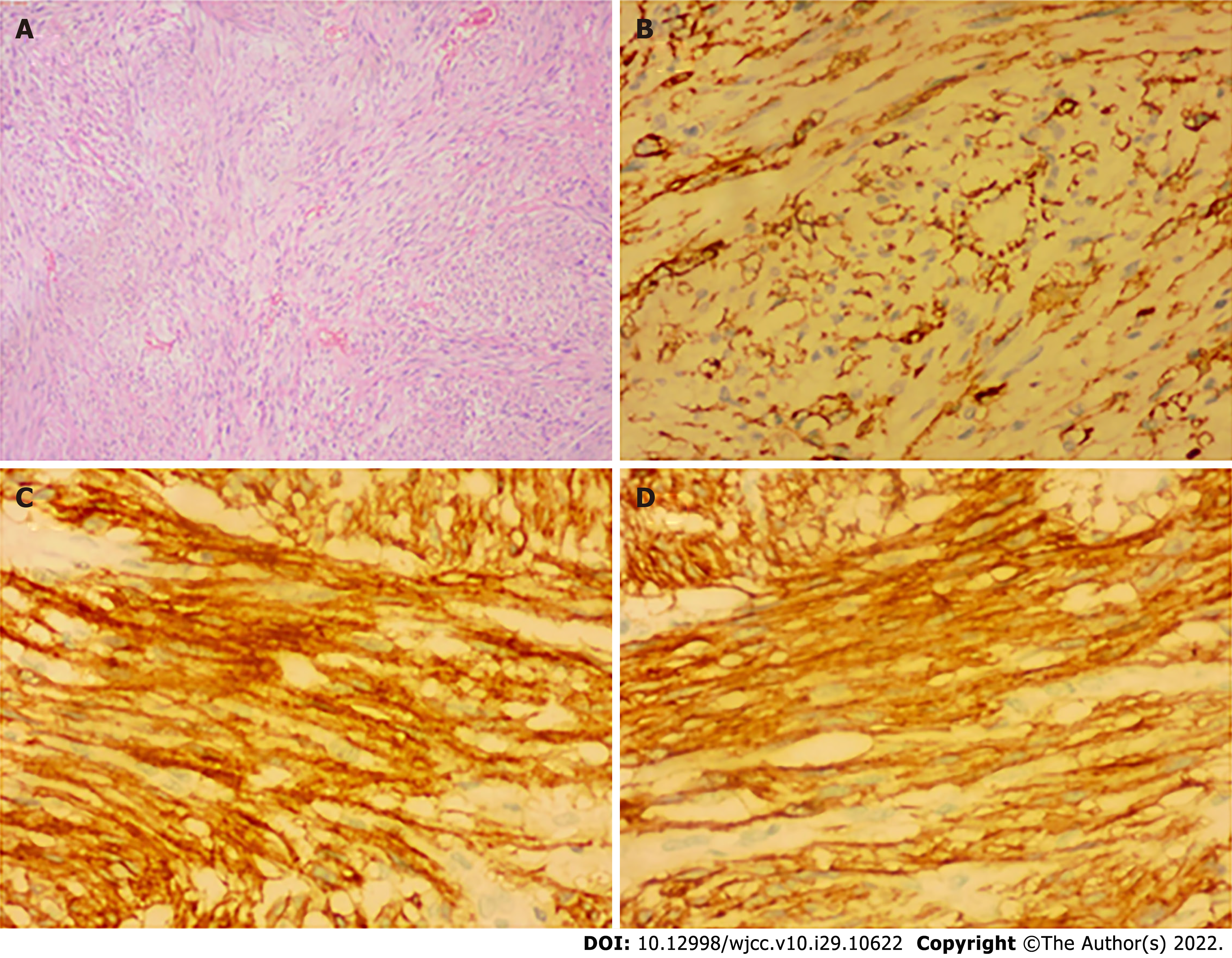
Histopathological examination confirmed the presence of submucosal GIST measuring 5.2 cm × 3.6 cm × 3.4 cm in size with infarction. It was of an intermediate-risk, with mitotic count < 1 per 10 high-power field, spindle unit type, and with an intact coating. Immunohistochemical (IHC) studies revealed the following: CD117+, Dog1+, CD34+, SMA+, S100-, CK-, Des-, SOX-11-, STAT6-, Ki67 Hotspots 10%+. These results indicated that the tumor originated from the jejunal stroma of GIST (Figure 3) and were consistent with the results of the CT examination. The patient was ultimately diagnosed with wandering small intestinal stromal tumor (clinical staging: T3NOMO).
Two weeks later, tumor excision was performed when the patient’s physical condition improved with small bowel resection. Laparoscopically, a 1-cm incision was made layer by layer into the abdomen at the lower edge of the navel. On exploration, the entire abdominal intestine was widely congested and edematous; the intestinal lumen was filled with a large amount of serous bloody fluid; and the local intestinal tube was adherent to the surrounding abdominal wall. Moreover, the tumor had been adherent to the intestinal wall with a longer mesentery. The mass was located approximately 30 cm distal to the ligament of Treitz. We excised the tumor; end-to-end anastomosis of the small intestine was achieved with a straight closed device, and abdominal closure was performed layer by layer.
The patient recovered well after surgery. Imatinib (400 mg daily) was prescribed postoperatively and the patient was followed up. The current basic situation is good.
GISTs are the most common malignant subepithelial lesions of the GI tract [4]. The stomach and small intestine are the most common locations. Small intestinal stromal tumors account for approximately 20%–30% of the incidence of GISTs[5]. GISTs are different from neurogenic tumors and leiomyomas in initial presentation, biological behavior, and immunophenotype[6]. However, IHC is conclusive in determining the histology of the tumor, based on proto-oncogene c-kit (CD117) and CD34 positivity to diagnose GISTs. GISTs measuring > 5 cm in diameter are at a risk of rupture, and most of the GISTs that originate in the small intestine measure > 5 cm in diameter at the time of diagnosis[7]. The most common symptom is gastrointestinal bleeding, which can be life-threatening and requires urgent intervention, such as surgery or catheter embolism[8]. In recent years many studies have reported that GISTs commonly co-exist with other primary tumors, which can involve either the GI tract or other extra-GI sites. It is not yet clear if a causal association exists for the concomitant occurrence of GISTs with other malignancies, or if this is merely coincidence[9].
Tumors measuring larger than 2 cm should be removed because of the increased risk of malignancy. Recurrence, tumor size, mitosis count, site of origin, and tumor rupture are the risk factors for GISTs[10]. Our patient had intermediate-risk of recurrence; therefore, we administered imatinib therapy 1 mo after the surgery. Careful follow-up of our patient is needed due to the risk of recurrence. Although wandering small intestinal stromal tumors are extremely rare, several reports on wandering intestinal tumors[11], mesenteric tumors, uterine fissures, and migraine mesothelioma are currently available in the literature. A wandering mass is usually related to the presence of a wandering spleen, owing to the laxity of its normal ligamentous attachments[12,13]. Similarly, the mesentery of the small intestine is relatively long, which permits mobility to a certain extent. Some of the factors that induce or contribute to a change in the position of the intestinal lumen include distention due to food, gastrointestinal peristalsis, or position changes. Any mass in the lumen is affected by gravity. However, such a spectacular change in location as in our patient, is extremely rare and may suggest a bowel origin. CT examination can accurately show the location, size, growth patterns, presence of necrosis, contrast enhancement, neoplastic angiogenesis, and potential metastasis[14]. Small GISTs (< 5 cm) appear as homogeneous lesions with persistent enhancement in contrast CT. Large tumors (> 5 cm) typically appear heterogeneous, contrast enhanced, and associated with cystic changes, necrosis, and ulceration[15]. Surgical treatment is preferred in the absence of metastasis[16]. DSA has important value in the detection of hemostasis for acute small intestinal bleeding (to distinguish a tumor or vascular origin)[17]. In this case, the tumor traveled widely, accompanied by its vascular pedicle derived from the superior mesenteric artery branch. Patients with these lesions typically present with internal hernia, torsion, and/or acute abdominal pain[18]. However, our patient had no symptoms of obstruction or torsion; similar cases have not been reported in the literature.
In patients with acute or chronic intestinal hemorrhage whose diagnoses are ambiguous, we should consider the possibility of GISTs. CT is a valuable tool to visualize these contrast-enhancing lesions. The wide variations in the embryonic development of the small intestine and its mesentery must be taken into account in anticipating tumor behavior arising from that location. When the tumor cannot be visualized at its original location, we should consider the possibility of tumor migration.
We thank the patient and his family for giving permission for his inclusion in this study. Thank my family for their support.
| 1. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3150] [Article Influence: 112.5] [Reference Citation Analysis (2)] |
| 2. | Rubin BP, Blanke CD, Demetri GD, Dematteo RP, Fletcher CD, Goldblum JR, Lasota J, Lazar A, Maki RG, Miettinen M, Noffsinger A, Washington MK, Krausz T; Cancer Committee, College of American Pathologists. Protocol for the examination of specimens from patients with gastrointestinal stromal tumor. Arch Pathol Lab Med. 2010;134:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Casali PG, Le Cesne A, Velasco AP, Kotasek D, Rutkowski P, Hohenberger P, Fumagalli E, Judson IR, Italiano A, Gelderblom H, Penel N, Hartmann JT, Duffaud F, Goldstein D, Martin-Broto J, Gronchi A, Wardelmann E, Marréaud S, Zalcberg JR, Litière S, Blay JY. Final analysis of the randomized trial on imatinib as an adjuvant in localized gastrointestinal stromal tumors (GIST) from the EORTC Soft Tissue and Bone Sarcoma Group (STBSG), the Australasian Gastro-Intestinal Trials Group (AGITG), UNICANCER, French Sarcoma Group (FSG), Italian Sarcoma Group (ISG), and Spanish Group for Research on Sarcomas (GEIS). Ann Oncol. 2021;32:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 935] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 5. | Cameron S, Beham A, Schildhaus HU. Current standard and future perspectives in the treatment of gastrointestinal stromal tumors. Digestion. 2017;95:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Manxhuka-Kerliu S, Sahatciu-Meka V, Kerliu I, Juniku-Shkololli A, Kerliu L, Kastrati M, Kotorri V. Small intestinal gastrointestinal stromal tumor in a young adult woman: a case report and review of the literature. J Med Case Rep. 2014;8:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 441] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 8. | Tada Y, Yamamoto M, Sawata S, Hara K, Sugesawa K, Ueshima C, Tanio A, Kihara K, Matsunaga T, Tokuyasu N, Takano S, Sakamoto T, Honjo S, Hasegawa T, Fujiwara Y. Ruptured Small Intestinal Stromal Tumor Causing Concurrent Gastrointestinal and Intra-Abdominal Hemorrhage: A Case Report. Yonago Acta Med. 2021;64:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Diamantis A, Samara AA, Symeonidis D, Baloyiannis I, Vasdeki D, Tolia M, Volakakis G, Mavrovounis G, Tepetes K. Gastrointestinal stromal tumors (GISTs) and synchronous intra-abdominal malignancies: case series of a single institution’s experience. Oncotarget. 2020;11:4813-4821.. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 690] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 11. | Qureshi SS, Rekhi B, Kembhavi S. Wandering Pelvic Mass: Rhabdomyosarcoma of the Meckel Diverticulum. J Pediatr. 2017;185:249- 249.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Ganarin A, Fascetti Leon F, La Pergola E, Gamba P. Surgical Approach of Wandering Spleen in Infants and Children: A Systematic Review. J Laparoendosc Adv Surg Tech A. 2021;31:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Matsuyama T, Nakao T, Harada S, Nakamura T, Nobori S, Ushigome H. A Case of Small Intestinal Ileus Due to Wandering Spleen with a Large Cyst. Am J Case Rep. 2019;20:1138-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Wen XL, Wang L, Song XQ, Zhang Z, Zhu XF, Wu SP. Clinical value of multi-slice spiral enhanced CT in diagnosing mild digestive trac the hemorrhage. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | De Cobelli F, Palumbo D, Albarello L, Rosati R, Giganti F. Esophagus and Stomach: Is There a Role for MR imaging? Magn Reson Imaging Clin N Am. 2020;28:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y, He Y, Liang X, Liu X, Zhou Y, Wu X, Zhang X, Wang M, Gao Z, Lin T, Cao H, Shen L; Chinese Society Of Clinical Oncology Csco Expert Committee On Gastrointestinal Stromal Tumor. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. 2017;29:281-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 17. | Gerson LB, Fidler JL, Cave DR, Leighton JA. ACG clinical guideline: diagnosis and management of small bowel bleeding. Am J Gastroenterol. 2015;110:1265-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 469] [Article Influence: 42.6] [Reference Citation Analysis (1)] |
| 18. | Viana C, Cristino H, Veiga C, Leão P. Splenic torsion, a challenging diagnosis: Case report and review of literature. Int J Surg Case Rep. 2018;44:212-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Elkady N, Egypt; Kinami S, Japan; Li WJ, United States; Samara AA, Greece S-Editor: Xing YX L-Editor: A P-Editor: Xing YX