Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10435
Peer-review started: March 24, 2022
First decision: June 16, 2022
Revised: June 17, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: October 16, 2022
Processing time: 188 Days and 14.9 Hours
Multisystem inflammatory syndrome in children (MIS-C) has emerged as a new disease associated with COVID-19 that presents in acute critically ill children with acute cardiovascular dysfunction.
To determine whether the age-adjusted N-terminal pro-brain natriuretic peptide (NT-proBNP) value (Z-log-NT-proBNP) is associated with severe MIS-C and myocardial dysfunction.
A retrospective study was conducted which included children with MIS-C managed at our institution between April 1, 2020, and February 28, 2022. We divided the population into groups depending on severity based on pediatric intensive care unit (PICU) admission. We compared Z-log-NT-proBNP values across these groups and analyzed Z-log-NT-proBNP dynamics during the one-month follow-up.
We included 17 participants [median age 3 (2-9) years] and seven (41%) required PICU admission. All (100%) of these cases presented very high (Z-log > 4) levels of NT-proBNP at the time of admission compared to only 5 (50%) patients with non-severe MIS-C (P = 0.025). NT-proBNP was significantly correlated with high-sensitive Troponin I levels (P = 0.045), Ross modified score (P = 0.003) and left ventricle ejection fraction (P = 0.021).
Raised NT-proBNP, specifically very high values (Z-log-NT-proBNP > 4) could help in the early identification of MIS-C patients with myocardial dysfunction requiring inotropic support and PICU admission.
Core Tip: Multisystem inflammatory syndrome in children (MIS-C) is a rare but serious condition associated with coronavirus disease 2019 in which the cardiovascular system is frequently impaired, with more than half of children presenting with heart failure and myocardial dysfunction secondary to the inflammatory response. N-terminal pro-brain natriuretic peptide (NT-proBNP) is a promising biomarker for the detection of cardiac dysfunction in conditions where heart failure and inflammation coexist, but its use in pediatrics is limited by its strong age-dependency. Therefore, we think that the use of age-adjusted NT-proBNP values could help to identify those children with risk for severe MIS-C.
- Citation: Rodriguez-Gonzalez M, Castellano-Martinez A. Age-adjusted NT-proBNP could help in the early identification and follow-up of children at risk for severe multisystem inflammatory syndrome associated with COVID-19 (MIS-C). World J Clin Cases 2022; 10(29): 10435-10450
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10435.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10435
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a stressful and challenging situation globally. Since its onset in early 2020 it has led to widespread morbidity and mortality worldwide, and it is noteworthy that a new disease has emerged: COVID-19-associated multi-systemic inflammatory response syndrome in children (MIS-C). Reports across the world of children presenting with hyperinflammatory shock, myocardial dysfunction, and multiorgan involvement, sharing clinical characteristics with Kawasaki disease and toxic shock syndrome, led the World Health Organization (WHO) to identify these cases as having a novel condition and to define MIS-C criteria for diagnosis[1].
MIS-C is a relatively rare disease affecting 0.6% of children with COVID-19[2]; most were previously healthy children with a mild-symptomatic/asymptomatic COVID-19. Prior to the description of MIS-C, the pediatric population was not considered to be at serious risk of developing severe COVID-19 and complications compared with adult patients[1]. Although MIS-C is characterized by multisystem involvement, acute cardiovascular dysfunction is a prominent and critical manifestation. This significant cardiovascular compromise includes vasoplegic and cardiogenic shock, severe valve regurgitation, ventricular dysfunction, and coronary artery dilatation[2-7]. Remarkably, a high proportion of children with MIS-C present as acute critically ill children, requiring admission to the pediatric intensive care unit (PICU) in more than half of cases, most requiring inotropic support and even extracorporeal membrane oxygenation (ECMO) in a few of them. Furthermore, the mortality rate is estimated at around 1%, higher than the 0.1%-0.6% mortality rate for pediatric COVID-19 reported before MIS-C[8-10].
Despite this critical presentation, the clinical outcomes of MIS-C are favorable. Fortunately, most children recover rapidly from their acute presentation after initiating immunomodulatory therapy, with complete resolution of cardiac alterations. Cardiac sequelae in mild myocardial dysfunction or coronary artery alterations were present in up to 5%-10% of cases at the time of hospital discharge[8-10]. Studies with long-term follow-up periods of 6-9 mo, assessing cardiac function by speckle tracking echocardiography and myocardial fibrosis by cardiac MRI, have shown complete cardiac recovery in most children[11-15].
As the long-term prognosis seems excellent, prompt initiation of anti-inflammatory therapy and cardiovascular support in the acute phase is crucial for a successful, rapid, and complete recovery. Therefore, identifying early markers of cardiac dysfunction would be helpful for both directed therapy and identifying MIS-C patients at the highest risk for deterioration. As echocardiography is a non-widely available technique in all emergency departments and requires training and experience for proper application, it would be interesting to investigate laboratory markers of more widespread use that identify patients with MIS-C in whom there may be echocardiographic alterations. Using cardiac biomarkers such as N-terminal (1–76) pro-brain natriuretic peptide (NT-proBNP) seems to be a promising tool. NT-proBNP secretion from the ventricular myocardium is up-regulated by myocardial stress in situations of myocardial volume/pressure over-load[16]. Thus, it is an excellent biomarker for heart failure and myocardial dysfunction on echocardiography[17-19]. Notably, its secretion is also influenced by the inflammatory response[20,21]. NT-proBNP is increasingly used as a biomarker in pediatric conditions that combine myocardial stress and inflammatory diseases, such as sepsis or Kawasaki disease[22-24]. Therefore, it is not surprising that NT-proBNP levels are markedly raised in almost all patients with MIS-C, where the proposed mechanism for the cardiovascular dysfunction is myocardial inflammation related to systemic inflammation with a cytokine storm[4,8,11,25,26].
An increasing number of studies have reported the characteristics of cardiac markers in MIS-C patients. Some investigations suggested that the patients requiring PICU admission present with the highest peak NT-proBNP values at the time of hospitalization[4]. However, all studies exploring the role of NT-proBNP in MIS-C use heterogeneous and fixed or static cut-off points based on adult reference values for diagnosing congestive heart failure in adults (150-300 pg/mL)[27]. This is a significant limitation, as pediatric NT-proBNP values are strongly age-dependent, with a continuous decrease from birth to adolescence, with a constant decline from infant to adult, being more marked in the neonatal period and the first year of life[28,29]. This age dependence makes it impossible to compare absolute NT-proBNP values in age-heterogeneous populations such as MIS-C[28]. Recently, Palm et al[29] introduced NT-proBNP values adjusted for the age in days, providing continuous reference values across all the pediatric age intervals, which is a more physiological approach. These authors also demonstrated the superiority of the age-adjusted approach compared with the use of absolute values to detect severe myocardial dysfunction in a pediatric population with congenital heart diseases. Therefore, the age-adjusted (Z-log-NT-proBNP) system could provide uniformity to research studies using NT-proBNP.
This study describes the dynamics of NT-proBNP and echocardiographic alterations in our MIS-C case series during the first month of disease. The primary objective is to determine whether the Z-log-NT-proBNP values are associated with severity and echocardiographic alterations during the acute phase of the disease.
This is a retrospective case series study conducted in the Pediatric Cardiology Division of our institution, a tertiary-level university hospital in Cadiz, Spain. We included children aged less than 16 years meeting classification criteria for MIS-C according to the World Health Organization definition (Table 1)[1] between April 1, 2020, and February 28, 2022. All patients were managed following current international recommendations[30] at the discretion of the attending pediatrician. We excluded patients with a known underlying or new diagnosis of heart disease during hospitalization and patients lost to follow-up, or those with incomplete data. As retrospective data were collected from clinical reviews only, consent from patients, parents, or guardians was not obtained. As the data analysis was retrospective and no additional data were collected beyond those required for standard medical care, a full ethics review was not required.
| All criteria must be met |
| Age 0 to 19 yr |
| Fever ≥ 3 d |
| Clinical signs of multisystem involvement (at least 2 of the following): |
| Rash, bilateral non purulent conjunctivitis, or mucocutaneous inflammation (oral, hands, or feet) |
| Hypotension or shock |
| Cardiac dysfunction, pericarditis, valvulitis, or coronary abnormalities (including echocardiographic findings or elevated troponin/BNP) |
| Evidence of coagulopathy (prolonged PT or PTT; elevated D-dimer) |
| Acute gastrointestinal symptoms (diarrhea, vomiting, or abdominal pain) |
| Elevated markers of inflammation (e.g., ESR, CRP, or procalcitonin) |
| No other obvious microbial cause of inflammation, including bacterial sepsis and staphylococcal/streptococcal toxic shock syndromes |
| Evidence of SARS-CoV-2 infection with any of the following: |
| Positive SARS-CoV-2 RT-PCR |
| Positive serology |
| Positive antigen test |
| Contact with an individual with COVID-19 either laboratory confirmation of SARS-CoV-2 infection by RT-PCR, serology, or antigen test, or known COVID-19 exposure within 4 weeks before symptom onset |
All MIS-C patients managed in our institution were evaluated by the Pediatric Cardiology Division through a physical exam, ECG, cardiac biomarkers (High Sensitivity-Troponin I (hs-TnI) and NT-proBNP), and 2D-Doppler echocardiography at least at four different time points: (1) First 24 h of admission; (2) 24 h after administering immunomodulatory therapy; (3) Hospital discharge; and (4) 1-mo post-admission. The start and discontinuation of any cardiac medication were carried out at the discretion of the attending pediatric cardiologist, following local protocols and international guidelines for cardiogenic shock and heart failure[19]. Further cardiac controls were performed for each patient during admission and post-discharge follow-up based on their clinical state and evolution. These data were not collected for the present study. Cardiac MRI was not performed on any patient during the acute or subacute phase.
Our institution's electronic clinical records were reviewed by one investigator (ACM), who abstracted data for each time point described previously. Information collected included patient demographics and preexisting comorbidities, clinical presentation, laboratory findings, imaging findings, microbiological investigations, treatment, and outcomes.
Heart failure: Clinical heart failure status was defined based on the age-based Ross modified score[31]. In this score, each age range (0–3 mo, 4–12 mo, 1–3 years, 4–8 years, and 9–18 years) has ten variables with scores of 0, 1, or 2 possible for a range of 0 to 20. The scoring system can be used as a continuous data set for comparison with outcomes, or it can be categorized by points assessed as Ross classes I (0–5; no heart failure), II (6–10; mild heart failure), III (11–15; moderate heart failure) and IV (16–20; severe heart failure).
Raised hs-TnI: hs-TnI was the biomarker used in our institution to assess myocardial injury or ischemia. hs-cTnI levels were measured in ng/L using the Architect i1000SR platform (Abbott Diagnostics®, Spain) with 1.1–1.9 ng/L of a lower detection limit, 2.5% intra-run variation, and < 4% inter-run variation). In the absence of clear pediatric reference values for hs-cTnI, we defined myocardial injury as the presence of serum levels plus 50 ng/L, the reported 75th percentile for this assay in infants and children[32].
Raised NT-proBNP: NT-proBNP was the biomarker used in our institution to assess myocardial strain. NT-proBNP levels were measured in pg/mL using the Alere NT-proBNP for Alinity I assay (Abbott Diagnostics®, Spain). The intra-assay and inter-assay coefficients of variation were 1.9% to 2.9% and 2.6% to 5.4%, respectively, with an analytical range of 8.3 to 35 000 pg/mL. As reference values for children are markedly age-dependent, we calculated Z-log-NT-proBNP values adjusted for age as recommended by Palm et al[29], and Z-log > 1.96 was considered high NT-proBNP. We anticipated that all patients in our case series had Z-log-NT-proBNP > 1.96. For the statistical analysis, we defined very high NT-proBNP as Z-log-NT-proBNP > 4 (double of average values for age).
Abnormal echocardiographic findings: Standard techniques to obtain M-mode, two-dimensional, and Doppler echocardiograms were performed in all patients by the senior pediatric cardiologist as recommended in the guidelines for pediatric echocardiography[33]. Images were obtained using IE33 (Phillips®) or Aplio i-series (Canon Medical Systems®) machines with a 5, 8, or 12-MHz sectorial transducer. This study focused on left and right ventricular function and coronary artery dimensions, as these are the main cardiac alterations previously described in MIS-C patients. Left ventricular (LV) dysfunction was defined as an LV ejection fraction (LVEF) < 55%, and graded as mild (LVEF 45% to 54%), moderate (LVEF 35% to 44%), or severe (LVEF < 35%). Right ventricular (RV) dysfunction was defined as tricuspid annular plane systolic excursion (TAPSE) < 2 Z-score for body surface area[34]. In cases where LVEF or TAPSE were unavailable, ventricular dysfunction definition was based on the qualitative grade of hypokinesis. Coronary artery dilation was defined as diameter > 2 Z-scores for body surface area (BSA) by the published reference standard in the affected segment[35], and graded as dilation 2.0–2.49, small aneurysm > 2.5 to < 5, medium aneurysm > 5 to < 10, and large aneurysm > 10.
Cardiac sequelae: All children with MIS-C who had any abnormal cardiac measurement of those described above at the time of discharge or after one month were defined as cardiac sequelae.
Research endpoints: The research endpoints in this study were: (1) Development of severe MIS-C during hospitalization was defined as the need for PICU admission; (2) The presence of abnormal echocardiography at admission; and (3) The presence of cardiac sequelae. The study population was divided into two groups based on the predefined research endpoints. The analysis focused on comparing cardiac biomarkers values across these groups and the cardiac dynamics throughout the follow-up.
Descriptive statistics were used to summarize our population's baseline key features and outcomes. The hs-TnI values were log-transformed and standardized to account for widely varying ranges, and Z-log-NT-proBNP values were calculated as previously described. These values of cardiac biomarkers were those used in statistical analysis and graphics. Continuous data were expressed as median and interquartile range values. Categorical data were expressed as frequencies and proportions (%). Continuous variables were analyzed using the nonparametric U-Mann Whitney test as normality was not assumed with the sample size of our case series. Categorical variables were analyzed using the chi-square and Fisher's exact test. Spearman rank correlation coefficients were used to identify relationships between continuous variables. Differences in repeated cardiac measurements between the four-time points used in this study were assessed using the Wilcoxon signed rank-sum test. Due to the limited sample size of this study, we were unpowered to establish cut-off points to identify outcomes. For all analyses, P values of < 0.05 were considered significant. Due to the potential for type I error because of multiple comparisons, our findings should be interpreted as exploratory. All statistical analyses were performed with Stata v.16 software (StataCorp, College Station, TX, United States).
This case series included 17 MIS-C participants [9 (53%) males; 14 (82%) white race] with a median age of 3 (2-9) years. Tables 2 and 3 summarize baseline clinical, laboratory, and echocardiographic data at admission. Sixteen (94%) cases were previously healthy children. Four cases (24%) were diagnosed with COVID in the previous 4-8 wk, and in 15 (88%), we documented IgG antibodies to SARS-CoV-2, and 7 (41%) had positive RT-PCR tests. No other microbial cause of the illness was identified in these patients.
| N = 17 | Results |
| Age (yr)2 | 3 (2-9) |
| Weight (kg)2 | 17 (12-34) |
| Gender (male)1 | 9 (53) |
| Ethnicity (white)1 | 14 (82) |
| Comorbidity1 | 1 (6) |
| Known previous COVID-19 disease (4-8 wk before)1 | 4 (24) |
| Contact with known COVID-19 case1 | 7 (41) |
| IgG antibodies to SARS-CoV-21 | 15 (88) |
| SARS-CoV-2 RT-PCR positive test1 | 7 (41) |
| Fever1 | 17 (100) |
| Days of fever2 | 4 (3-4) |
| Cutaneous rash1 | 7 (41) |
| Conjunctivitis1 | 7 (41) |
| Lymphadenopathy1 | 5 (29) |
| Palmar or plantar erythema1 | 3 (17) |
| Changes in oral mucosa1 | 6 (35) |
| Respiratory symptoms1 | 3 (17) |
| Hypoxemia1 | 2 (12) |
| SpO2 (%)2 | 98 (97-99) |
| Gastrointestinal symptoms1 | 10 (58) |
| Neurological symptoms1 | 3 (17) |
| Heart failure (age-based Ross score > 5)1 | 11 (65) |
| Cardiogenic/Vasoplegic shock1 | 3 (17) |
| Tachycardia1 | 13 (76) |
| Heart rate (bpm)2 | 150 (120-160) |
| Hypotension1 | 4 (23) |
| Systolic arterial pressure (mmHg)2 | 97 (75-106) |
| Diastolic arterial pressure (mmHg)2 | 60 (40-68) |
| N = 17 | Results |
| Leukocytes (/µL)2 | 9930 (83490-12250) |
| Leukocytosis1 | 4 (23) |
| Lymphocytes (/µL)2 | 1480 (680-3100) |
| Lymphopenia1 | 9 (53) |
| Hemoglobin (g/dL)2 | 10.7 (8-12.2) |
| Anemia1 | 8 (47) |
| Thrombocytes (/µL)2 | 161000 (120000-238000) |
| Thrombocytopenia1 | 5 (29) |
| Dimer D (ng/mL)2 | 3504 (3284-5290) |
| Coagulopathy1 | 7 (41) |
| CRP (mg/L)2 | 171 (121-201) |
| Procalcitonin (ng/mL)2 | 3.2 (1.4-10.2) |
| Ferritin (ng/mL)2 | 789 (552-978) |
| Creatinine (mg/dL)2 | 0.5 (0.4-0.57) |
| Urea (mg/dL)2 | 23 (19-31) |
| AKI1 | 2 (11) |
| Sodium (mEq/L)2 | 134 (131-137) |
| Hyponatremia1 | 8 (47) |
| GPT (U/L)2 | 40 (25-66) |
| Hypertransaminemia1 | 6 (35) |
| pH2 | 7.37 (7.34-7.4) |
| pCO2 (mmHg)2 | 37 (33-39) |
| HCO3 (mmol/L)2 | 23 (21-24) |
| Acidosis1 | 4 (23) |
| NT-proBNP (pg/mL)2 | 5221 (2638-10020) |
| NT-proBNP (Z-log value adjusted for age)2 | 4.62 (4.46-5.23) |
| High NT-proBNP (Z-log for age > 2)1 | 17 (100) |
| Very high NT-proBNP (Z-log for age > 4)1 | 12 (71) |
| hs-TnI (ng/L)2 | 35 (10-116) |
| High hs-TnI (> 50 ng/L)1 | 5 (29) |
| LVEF (%)2 | |
| LV dysfunction, n (%)1 | |
| Maximal CA diameter (Z-score)2 | |
| CA dilation1 | |
All patients presented with fever with a median duration of 4 (3-4) days before hospitalization, with mucocutaneous inflammatory (41%) and gastrointestinal symptoms (58%) as the common non-cardiac manifestations. Eleven (65%) cases were diagnosed with heart failure by the age-based Ross modified score. Of these, 5 (29%) had severe heart failure, and 3 (17%) were in cardiogenic shock at presentation.
At the time of hospitalization, 8 (47%) patients had anemia, 5 (29%) thrombocytopenia, and 9 (53%) lymphopenia, and all laboratory markers of an inflammatory response (C-reactive protein (CRP), procalcitonin, ferritin, and Dimer-D) were markedly elevated (Table 2). With regard to cardiac biomarkers, both NT-proBNP (median values of 5221 (2638-10020) pg/mL) and hs-TnI (35 (10-116) ng/L) were markedly raised. All patients presented with high (Z-log > 2) NT-proBNP, most of them (71%) with very-high (Z-log > 4) plasma levels, whereas 5 (29%) participants showed hs-TnI concentrations indicative of myocardial injury.
Abnormal echocardiographic findings were found in 10 (58%) cases; 9 (53%) patients had LV dysfunction, 2 (11%) of them with concomitant RV dysfunction. On admission, the medial LVEF was 58 (48-65)%; in 2 (11%) patients, the myocardial dysfunction was graded as severe and in the other 2 (11%) as moderate. There was mild coronary artery dilation (Zscore 2-2.5) in 3 (17%) patients. ECG was performed in all patients, with sinus tachycardia in 14 (82%) and T-wave inversion at left precordial leads in 2 (11%) cases). Chest X-ray was performed in 12 (70%) children and was considered abnormal in 3 (17%), 2 cases with cardiomegaly, and 1 (6%) case with lung edema. Abdominal ultrasound was carried out in 8 (47%) participants with unspecific lymphadenopathy and ileocolitis as the main findings.
Treatment was provided as recommended in the standard treatment guidelines for MIS-C. All our patients showed an excellent short-term clinical course in this study and were discharged after 7 (5-10) days of hospitalization. Table 4 summarizes the treatment and outcomes of the study population. Immunomodulatory therapy was administered to all patients. Steroids plus intravenous immunoglobulin (IVIG) were used in 14 (82%), and only steroids were used in 2 (11 %) cases. In 4 (23%) children, the inflammatory response was not controlled with first-line therapy and required a biological medication; anakinra in 3 (17%) cases and tocilizumab in 1 (6%) case leading to rapid control of the hyperinflammatory state. All patients continued steroids until fever disappeared and all inflammatory markers were in the normal ranges. Aspirin was used in most (88%) cases, whereas anticoagulation with low molecular weight heparin was administered only in 3 (17%) critically ill patients presenting with shock and myocardial dysfunction. Empirical broad-spectrum antibiotics were started in many patients (88%) at the time of hospitalization and were discontinued after the blood and urine cultures were noted to be sterile. Ten (53%) children required diuretics to relieve congestive heart failure, and in 6 (35%), therapy against myocardial remodeling (enalapril + carvedilol) was started. Of these patients, 8 (59%) continued on cardiac medications at discharge, and all were tapered off after one month of hospitalization in the outpatient clinic. There were no arrhythmic events, and no anti-arrhythmic medication was needed.
| N = 17 | Results |
| IVIG1 | 14 (82) |
| Steroids1 | 16 (94) |
| Aspirin1 | 15 (88) |
| LWH1 | 3 (17) |
| Anakinra1 | 3 (17) |
| Tocilizumab1 | 1 (6) |
| Antibiotics1 | 15 (88) |
| Diuretics1 | 10 (53) |
| Beta blockers1 | 6 (35) |
| ACEIs1 | 6 (35) |
| Antiarrhythmics1 | 0 (0) |
| Oxygen (nasal cannula)1 | 2 (11) |
| CPAP1 | 1 (6) |
| Mechanical ventilation1 | 1 (6) |
| Inotropics1 | 7 (41) |
| ECMO1 | 0 (0) |
| Resistance to immunomodulatory therapy1 | 4 (23) |
| PICU admission1 | 7 (41) |
| PICU stay (days)2 | 3.5 (3-4.5) |
| LOS hospitalization (days)2 | 7 (5-10) |
| Heart failure at discharge1 | 1 (6) |
| Myocardial dysfunction at discharge1 | 1 (6) |
| Coronary artery dilation at discharge1 | 2 (11) |
| Raised hs-TnI or NT-proBNP at discharge1 | 9 (53) |
| Cardiac medications at discharge1 | 8 (47) |
| Any cardiac sequelae or medications at 1 month follow-up1 | 0 (0) |
| Death1 | 0 (0) |
Seven (41%) participants required PICU admission (median PICU stay of 3.5 (3-4.5 days) and administration of inotropic support in the severe MIS-C group. Milrinone was used alone in 3 (17%) cases, milrinone in combination with norepinephrine in 3 (17%) cases, and levosimendan combined with norepinephrine in 1 (6%) case. No patients required ECMO support for the management of cardiogenic shock. Respiratory therapy included invasive ventilation in 1 patient (6%), continuous positive airway pressure in 1 patient (6%), and conventional oxygen therapy in 2 patients (11%). Two (11%) patients presented acute kidney injury without requiring renal replacement therapy. There were no deaths.
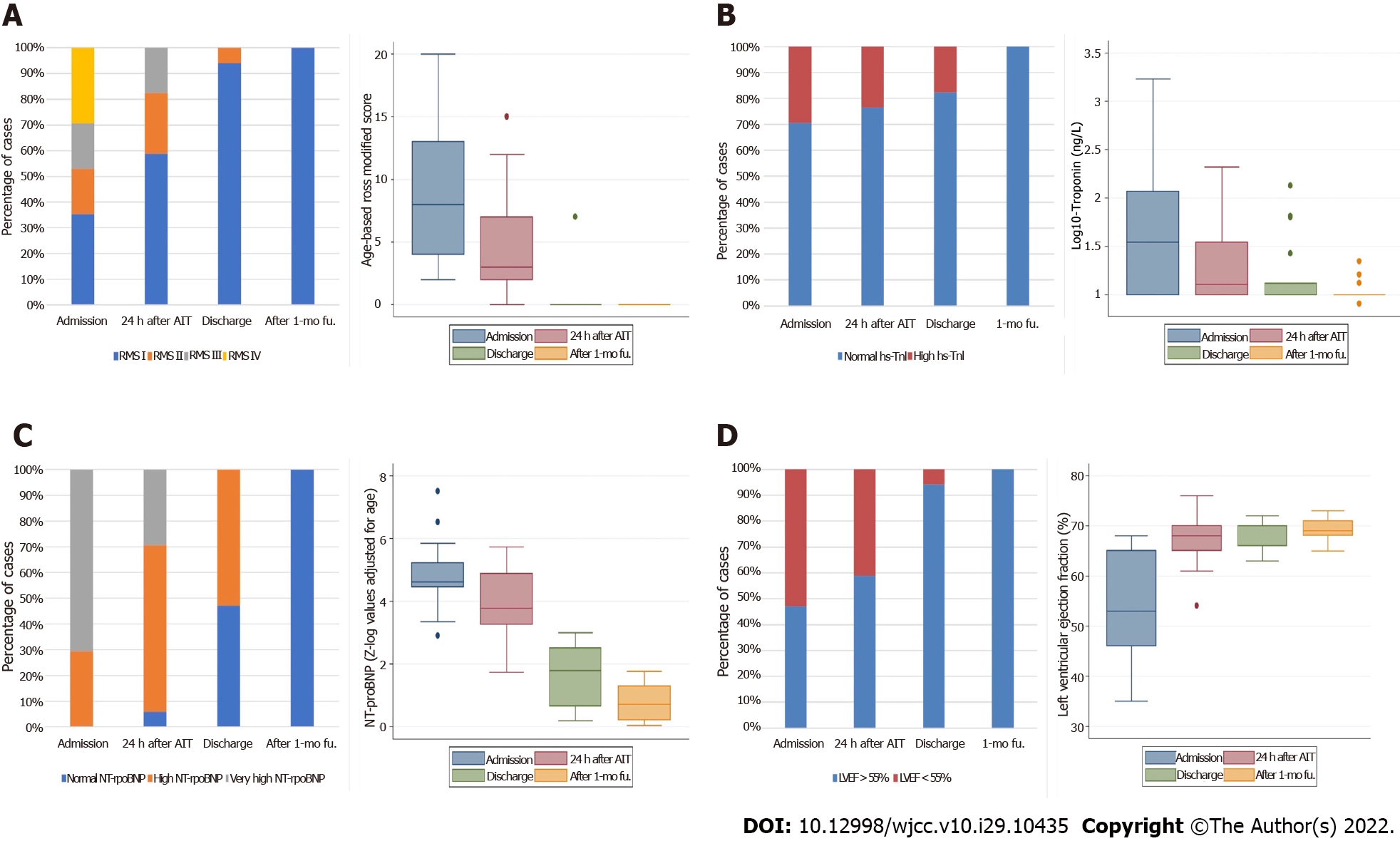
Regarding the dynamic changes of cardiac abnormalities, we observed a significant rapid, gradual, and continuous clinical, laboratory, and echocardiographic improvement in our patients following immunomodulatory therapy administration. This improvement was coupled with the disappearance of fever and a marked decrease of the remaining laboratory inflammatory markers (data not shown). Cardiac abnormalities observed on the four-time points assessed in this study are detailed in Table 5 and Figure 1.
| Cardiac parameter | Admission | 24 h after AIT | Discharge | After 1-mo fu. |
| NT-proBNP (Z-log value adjusted for age)2 | 4.62 (4.46-5.23) | 3.78 (3.26-4.87)1 | 1.79 (0.66-2.5)1 | 0.72 (0.21-1.29)1 |
| Log (10)-Hs-TnI (ng/L)2 | 35 (10-116) | 13 (5-35)1 | 10 (10-13)1 | 10 (10-10) |
| LVEF (%)2 | 58 (48-65) | 68 (65-70)1 | 70 (66-72) | 70 (68-71) |
| Coronary arteries maximal dimension (Z-score for BSA)2 | 1.39 (0.56-2.66) | 1.2 (0.77-1.5) | 1 (0.5-1.2)1 | 0.8 (0.51-1.2) |
| Age-based Ross classification for heart failure in children2 | 8 (4-12) | 3 (2-7)1 | 0 (0-0)1 | 0 (0-0) |
After 24 h of treatment, heart failure persisted in 7 (63%) children, but with no severe cases and a significant decrease in the age-based Ross modified score from 8 (4-12) points to 3 (2-7) points (P < 0.001) in parallel with significant enhancement of LVEF from 58 (48-65)% to 68 (65-70)% (P < 0.001), with 6 (35%) cases of mild and 1 (6%) case of moderate LV dysfunction, and complete resolution of RV dysfunction. There was also a significant decrease in the plasma levels of both cardiac biomarkers. At this time point, NT-proBNP decreased from Z-log of 4.62 (4.46-5.23) to Z-log of 3.78 (3.26-4.87) (P = 0.001), with very high levels still observed in 5 (29%) cases; and hs-TnI decreased from 35 (10-116) ng/L to 13 (5-35) ng/L (P = 0.008), with raised levels in 4 (23%) cases.
At discharge, we documented a continuous significant (P < 0.001) improvement in all these cardiac measurements and coronary artery dimensions concerning the previous time points assessed. However, some cardiac abnormalities remained: 1 (6%) case of mild heart failure with mild LV dysfunction, the patient presenting with the most severe disease and decreased LVEF; 2 (11%) cases of mild coronary artery dilation; 3 (17%) cases of raised hs-TnI in 3 (17%) levels and 9 (53%) cases of high (Z-log>2) NT-proBNP levels.
In the follow-up visit at the pediatric cardiologist outpatient clinic one month after hospitalization, we documented complete normalization of the cardiac state, without cardiac sequelae in any of the patients.
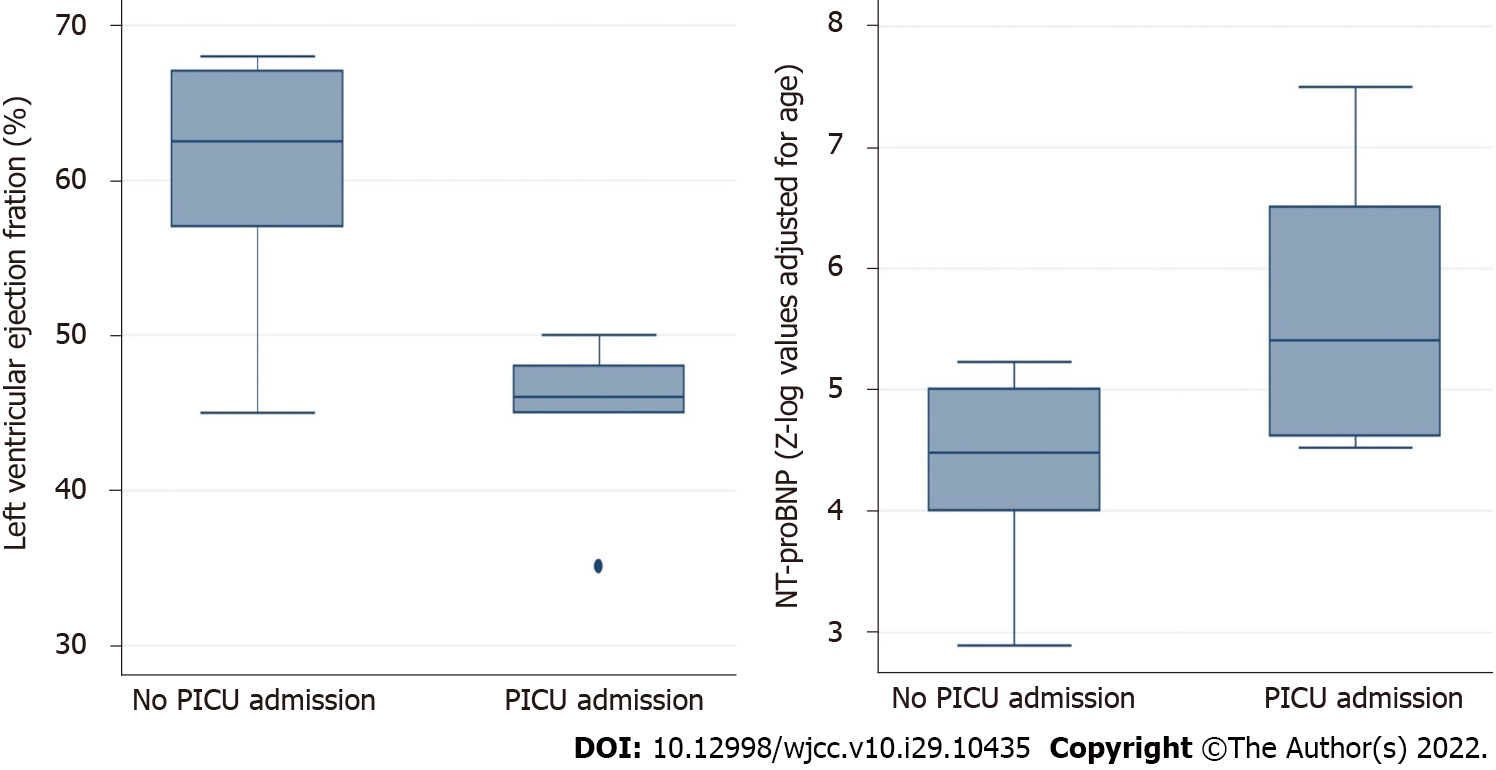
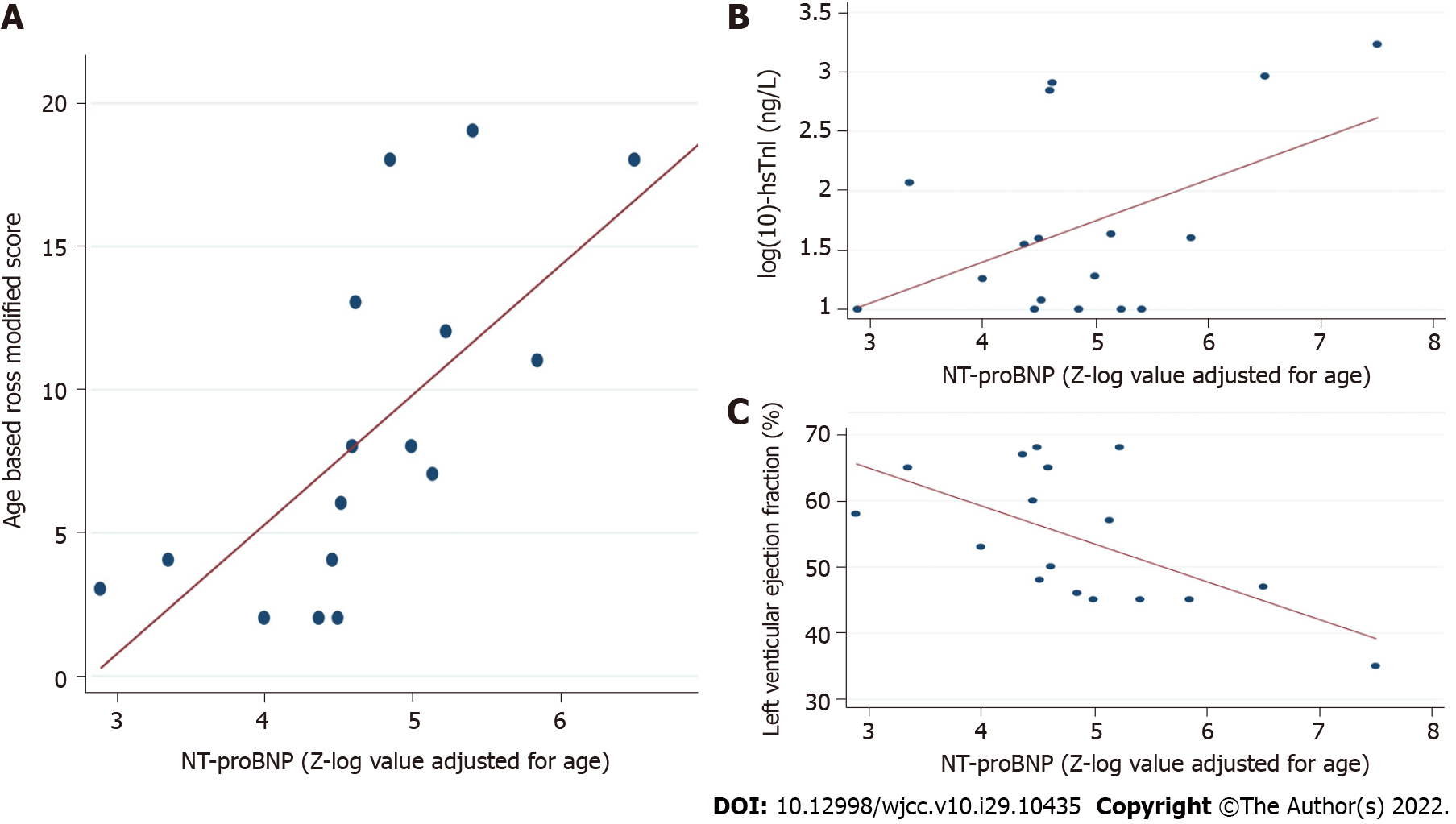
The group with severe MIS-C was composed of the 7 (41%) patients that required PICU admission and inotropic support. Compared with those cases with non-severe MIS-C, these patients presented with a higher Ross modified score (P = 0.003), lower LVEF (P = 0.034), and higher Z-log-NT-proBNP (P = 0.016) (Table 6 and Figure 2). There were no differences regarding hs-TnI levels, coronary dimensions, demographics, days of fever, and any laboratory marker of inflammation (all P > 0.05). Focusing on NT-proBNP, we were not powered to assess sensitive laboratory cutoffs of NT-proBNP predictive of severe MIS-C. Therefore, we assessed the association of high NT-proBNP with severe MIS-C as a dichotomous variable. We observed that 7/7 (100%) patients with severe MIS-C had very high (Z-log > 4) levels of NT-proBNP at the time of admission compared to only 5 (50%) patients with non-severe MIS-C (P = 0.025). We also found that our patients' NT-proBNP levels were associated with other cardiac abnormalities. Specifically, NT-proBNP was significantly correlated with hs-TnI levels (P = 0.045), Ross modified score (P = 0.003), and LVEF (P = 0.021), but not with the maximal coronary artery diameter (Table 7 and Figure 3).
| Variable | PICU admission (n = 7; 41%) | No PICU admission (n = 10; 59%) | P value |
| Age (mo)2 | 6 (1-12) | 2.5 (2-8) | NS. |
| Male sex1 | 8 (53) | 14 (56) | NS. |
| Weight (kg)3 | 5 (71) | 4 (40) | NS. |
| Days of fever2 | 4 (2-5) | 4 (3-4) | NS. |
| Age-based Ross score2 | 16 (11-19) | 4 (2-8) | 0.003 |
| Lymphocytes (/µL)2 | 1240 (720-5940) | 1552 (1260-3100) | NS. |
| CRP (mg/L)2 | 171 (120-237) | 167 (131-201) | NS. |
| Procalcitonin (ng/mL)2 | 3.6 (1.3-21.6) | 2.5 (1.4-8.8) | NS. |
| Ferritin (ng/mL)2 | 814 (552-2789) | 750 (517-878) | NS. |
| Dimer D (ng/mL)2 | 3436 (2461-9434) | 4728 (3284-5290) | NS. |
| NT-proBNP (Z-log for age)2 | 5.41 (4.62-6.51) | 4.48 (4-5) | 0.016 |
| Troponin I (ng/L)2 | 40 (10-909) | 27 (10-43) | NS. |
| LVEF (%)2 | 48 (45-65) | 62.5 (57-67) | 0.034 |
| Maximal CA diameter (Z-score)2 | 1.2 (0.55-1.4) | 1.5 (0.5-3) | NS. |
| NT-proBNP (Z-log value adjusted for age) | Correlation coefficient | P value |
| hs-TnI (ng/L) | 0.47 | 0.045 |
| Age-based Ross score for heart failure | 0.76 | 0.003 |
| LVEF (%) | -0.55 | 0.021 |
| Maximal CA diameter | -0.21 | NS. |
In this article, we describe the evolution of cardiac biomarker elevation and echocardiographic findings in 17 MIS-C cases focusing on NT-proBNP dynamics during both the acute phase and recovery. Our cohort's spectrum of cardiac involvement is similar to the larger MIS-C case series. We observed a high rate of echocardiographic abnormalities in the acute phase in our patients, with 53% of cases presenting with myocardial dysfunction. These alterations improved rapidly after immunomodulatory treatment, with 94% of cases asymptomatic and normal LV function at hospital discharge and recovered completely after one month of follow-up. In one of the first multicenter studies focusing on cardio
In addition to being very frequent, myocardial dysfunction was associated with severe MIS-C disease in our patients and had prognostic implications. All patients who required PICU had myocardial dysfunction on admission echocardiography. Valverde et al[4] described the children requiring intensive care unit admission as having significantly reduced LV systolic ventricular function. Sanil et al[37], in a longitudinal observational study of 54 patients with MIS-C, reported that impaired LV function at initial presentation indicates a higher risk of an adverse acute clinical course and persistent subclinical left ventricular dysfunction at the 10-wk follow-up could be applied to identify higher-risk children with MIS-C.
The previously mentioned observations highlight the relevance of identifying myocardial dysfunction in MIS-C patients, and based on our findings, NT-proBNP could be an adequate tool for this purpose. The association of higher levels of NT-proBNP with myocardial dysfunction and severe MIS-C requiring PICU and inotropic support has been previously described in larger studies. Abrams et al[38] analyzed 1080 cases of MIS-C. They determined that PICU admission, LV dysfunction, and the need for inotropic support were associated with increased concentrations of C-reactive protein, troponin, ferritin, D-dimer, NT-proBNP, or interleukin-6, or reduced platelet or lymphocyte counts. A recent meta-analysis on the role of cardiac biomarkers in MIS-C that included 1613 patients from 24 studies determined that NT-proBNP was the only cardiac biomarker able to differentiate between patients with severe/non-severe MIS-C[39]. However, there is no evidence on the best cut-off point to use in this context.
Our study is novel in using Z-log-NT-proBNP for the first time to assess the severity and echocardiographic abnormalities in MIS-C, overcoming the main limitation of using this biomarker in children, its strong age dependence. Using this approach, we observed that all our patients presented with raised levels of NT-proBNP (Z-log-NT-proBNP > 2) and that 71% were twice the average for their age (Z-log-NT-proBNP > 4). These data point to the importance of NT-proBNP as a biomarker in the differential diagnosis of MIS-C with other conditions with lesser potential severity with which it shares clinical and laboratory findings (acute appendicitis, Kawasaki disease, exanthematous fevers...). Notably, Z-log-NT-proBNP showed a moderate to strong correlation with all the cardiac alterations measured in this study (except for coronary artery dilation): LVEF, the Ross modified score and hs-TnI levels. Furthermore, it is also noteworthy that all patients admitted to the PICU and required inotropic support had Z-log-NT-proBNP > 4 on admission. Z-log-NT-proBNP is increasingly used in congenital heart disease, where it is a suitable marker of severity[40]. Specifically, Palm et al[29], in a recent study that included 138 children with a wide age range (1 day-7.5 years), concluded that patients with very high NT-proBNP values (Z-log-NT-proBNP > 3) were at higher risk of developing major adverse events during follow-up, highlighting a negative predictive value of 96% for a cut-off point Z-log-NT-proBNP < 1.96 (average for the age). The small sample size limits our ability to calculate the diagnostic accuracy of NT-proBNP for these outcomes. However, based on our observations, Z-log-NT-proBNP > 4 may be more indicative of concerning echocardiographic findings associated with illness severity, including reduced LVEF and the need for PICU admission for inotropic support. Therefore, it could be an appropriate starting point to explore in future prospective studies with larger sample sizes.
Finally, another interesting finding in our case series is the delayed improvement of NT-proBNP levels regarding myocardial function normalization. We observed that 94% of our patients were free of symptoms of congestive heart failure and with normal LVEF at discharge, whereas 53% still presented biochemical signs of myocardial stress with a Z-log-NT-proBNP > 1.96. NT-proBNP secretion is stimulated by myocardial stress and the systemic inflammatory response in MIS-C[20,25,26]. Several reasons could explain the persistence of elevated NT-proBNP levels in patients with normal myocardial function. Subtle systemic inflammatory responses may persist, although this is unlikely as all patients had normal inflammatory marker levels at discharge. More likely, this is due to the persistence of subclinical myocardial dysfunction. Although myocardial function recovered when measured by standard methods such as LVEF, recent studies using speckle tracking imaging demonstrated that subclinical LV dysfunction persists in these patients for at least 1-3 mo after hospitalization[13,15,36]. Therefore, NT-proBNP could be used as a laboratory marker of this subclinical dysfunction, aiding in the appropriate monitoring of cardiovascular complications in these patients during their post-hospitalization follow-up, especially in centers where speckle tracking echocardiography is not available.
The main limitations of this study include the single-center nature, retrospective design, and small sample size. In addition, laboratory values of NT-proBNP are assay-dependent and cannot be compared between centers using different laboratory methods for its determination. Finally, the follow-up period was limited to 1 mo, and we did not perform advanced imaging techniques (speckle tracking echocardiography or cardiac MRI) to determine subclinical myocardial impairment. This approach prevents us from establishing a long-term prognosis and ensuring that there are no mid-term myocardial alterations in MIS-C patients.
Our experience supports previous findings that MIS-C presents a high rate of myocardial involvement, impacting the severity of the disease. This myocardial involvement appears to recover quickly and with near-complete normalization of cardiac function a few days after immunomodulatory therapy and administration of cardiovascular support. Therefore, the early identification of cardiac dysfunction is crucial to start prompt treatment modalities and prevent cardiovascular complications. Based on our observations, NT-proBNP seems to be a promising biomarker for the initial screening and monitoring of myocardial dysfunction during the acute phase, where Z-log-NT-proBNP > 4 may be more indicative of concerning echocardiographic findings associated with illness severity; also, it could have a role in the post-hospitalization follow-up of these patients. Using Z-log-NT-proBNP values would provide constant reference values of NT-proBNP in children with MIS-C and would lead to consistency in data analysis across centers worldwide.
Multisystem inflammatory syndrome in children (MIS-C) emerged as a severe new disease associated with coronavirus disease 2019. One of the most critical issues is the high prevalence of cardiovascular involvement in these children, leading to a high percentage of cardiogenic shock, myocardial dysfunction secondary to the inflammatory response, and the need for inotropic support and extracorporeal membrane oxygenation (ECMO).
MIS-C is a severe new entity, and we still know very little about it. Therefore, it is necessary to communicate the experience in the management of these patients as well as to generate scientific evidence on all aspects of MIS-C that allow the best management of these patients.
This study was designed to identify clinical and laboratory markers of severity in this new disease. MIS-C is a condition with cardiac involvement in almost all cases. Therefore, we decided to analyze whether NT-proBNP, one of the most widely used cardiac biomarkers in routine clinical practice, was capable of identifying the most severe cases that required admission to the pediatric intensive care unit (PICU) and administration of inotropic support. We also aimed to determine whether NT-proBNP was an adequate follow-up parameter in this setting.
A retrospective study was conducted which included children with MIS-C managed at our institution between April 1, 2020, and February 28, 2022. We divided the population into groups of severity based on PICU admission. We compared Z-log-NT-proBNP values adjusted for age in days across these groups and analyzed Z-log-NT-proBNP dynamics throughout the one-month follow-up.
We included 17 participants (median age 3 (2-9) years) and seven (41%) required PICU admission. All (100%) of these cases presented very high (Z-log > 4) levels of NT-proBNP at the time of admission compared to only 5 (50%) patients with non-severe MIS-C (P = 0.025). NT-proBNP was significantly correlated with high-sensitivity Troponin I levels (P = 0.045), Ross modified score (P = 0.003) and left ventricle ejection fraction (P = 0.021). NT-proBNP was raised in all of our patients at admission, and we observed a significant rapid, gradual, and continuous decrease in our patients following immunomodulatory therapy administration. In the follow-up visit at the pediatric cardiologist outpatient clinic one month after of the hospitalization, we documented complete normalization of the cardiac state, without cardiac sequelae in any of the patients.
Raised NT-proBNP, specifically very high values (Z-log-NT-proBNP > 4), could help identify MIS-C patients with myocardial dysfunction requiring inotropic support and PICU admission. NT-proBNP could also have a role in the post-hospitalization follow-up of these patients to monitor their cardiovascular recovery.
NT-proBNP is a promising biomarker for the initial screening and monitoring of myocardial dysfunction during the acute phase of MIS-C. However, its use in pediatrics is limited by its strong age dependency. Using Z-log-NT-proBNP values could provide constant reference values of NT-proBNP in children with MIS-C and would lead to consistency in data analysis across centers worldwide. NT-proBNP could also be used as a laboratory marker of subclinical myocardial dysfunction, aiding in the appropriate monitoring of cardiovascular complications in these patients during their post-hospitalization follow-up, especially in centers where speckle tracking echocardiography is not available. Therefore, this study could be an appropriate starting point to explore in future prospective studies with larger sample sizes and to confirm our results.
| 1. | World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19: Scientific Brief. 2020. (Accessed on Jan 17, 2022). Available from: https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19(https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19). |
| 2. | Health department-reported cases of multisystem inflammatory syndrome in children (MIS-C) in the United States | CDC. Accessed June 1, 2021. Available from: https://www.cdc.gov/mis-c/cases/index.html. |
| 3. | Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. 2020;39:355-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 714] [Cited by in RCA: 707] [Article Influence: 117.8] [Reference Citation Analysis (7)] |
| 4. | Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, Kuciñska B, Mannarino S, Tamariz-Martel A, Gutierrez-Larraya F, Soda G, Vandekerckhove K, Gonzalez-Barlatay F, McMahon CJ, Marcora S, Napoleone CP, Duong P, Tuo G, Deri A, Nepali G, Ilina M, Ciliberti P, Miller O; AEPC COVID-19 Rapid Response Team*. Acute Cardiovascular Manifestations in 286 Children With Multisystem Inflammatory Syndrome Associated With COVID-19 Infection in Europe. Circulation. 2021;143:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 238] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 5. | McArdle AJ, Vito O, Patel H, Seaby EG, Shah P, Wilson C, Broderick C, Nijman R, Tremoulet AH, Munblit D, Ulloa-Gutierrez R, Carter MJ, De T, Hoggart C, Whittaker E, Herberg JA, Kaforou M, Cunnington AJ, Levin M; BATS Consortium. Treatment of Multisystem Inflammatory Syndrome in Children. N Engl J Med. 2021;385:11-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 6. | Capone CA, Misra N, Ganigara M, Epstein S, Rajan S, Acharya SS, Hayes DA, Kearney MB, Romano A, Friedman RA, Blaufox AD, Cooper R, Schleien C, Mitchell E. Six Month Follow-up of Patients With Multi-System Inflammatory Syndrome in Children. Pediatrics. 2021;148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, Soma VL, Maddux AB, Mourani PM, Bowens C, Maamari M, Hall MW, Riggs BJ, Giuliano JS Jr, Singh AR, Li S, Kong M, Schuster JE, McLaughlin GE, Schwartz SP, Walker TC, Loftis LL, Hobbs CV, Halasa NB, Doymaz S, Babbitt CJ, Hume JR, Gertz SJ, Irby K, Clouser KN, Cvijanovich NZ, Bradford TT, Smith LS, Heidemann SM, Zackai SP, Wellnitz K, Nofziger RA, Horwitz SM, Carroll RW, Rowan CM, Tarquinio KM, Mack EH, Fitzgerald JC, Coates BM, Jackson AM, Young CC, Son MBF, Patel MM, Newburger JW, Randolph AG; Overcoming COVID-19 Investigators. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA. 2021;325:1074-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 642] [Article Influence: 128.4] [Reference Citation Analysis (0)] |
| 8. | Rodriguez-Gonzalez M, Castellano-Martinez A, Cascales-Poyatos HM, Perez-Reviriego AA. Cardiovascular impact of COVID-19 with a focus on children: A systematic review. World J Clin Cases. 2020;8:5250-5283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Reiff DD, Cron RQ. Who Would Have Predicted Multisystem Inflammatory Syndrome in Children? Curr Rheumatol Rep. 2022;24:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180:307-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 264] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 11. | Farooqi KM, Chan A, Weller RJ, Mi J, Jiang P, Abrahams E, Ferris A, Krishnan US, Pasumarti N, Suh S, Shah AM, DiLorenzo MP, Zachariah P, Milner JD, Rosenzweig EB, Gorelik M, Anderson BR; Columbia University Interdisciplinary MIS-C Follow-up Program and the CUIMC Pediatric/Adult Congenital Heart Research Collaborative. Longitudinal Outcomes for Multisystem Inflammatory Syndrome in Children. Pediatrics. 2021;148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 12. | Dove ML, Oster ME, Hashemi S, Slesnick TC. Cardiac Magnetic Resonance Findings after Multisystem Inflammatory Syndrome in Children. J Pediatr. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Matsubara D, Chang J, Kauffman HL, Wang Y, Nadaraj S, Patel C, Paridon SM, Fogel MA, Quartermain MD, Banerjee A. Longitudinal Assessment of Cardiac Outcomes of Multisystem Inflammatory Syndrome in Children Associated With COVID-19 Infections. J Am Heart Assoc. 2022;11:e023251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 14. | Aeschlimann FA, Misra N, Hussein T, Panaioli E, Soslow JH, Crum K, Steele JM, Huber S, Marcora S, Brambilla P, Jain S, Navallas M, Giuli V, Rücker B, Angst F, Patel MD, Azarine A, Caro-Domínguez P, Cavaliere A, Di Salvo G, Ferroni F, Agnoletti G, Bonnemains L, Martins D, Boddaert N, Wong J, Pushparajah K, Raimondi F. Myocardial involvement in children with post-COVID multisystem inflammatory syndrome: a cardiovascular magnetic resonance based multicenter international study-the CARDOVID registry. J Cardiovasc Magn Reson. 2021;23:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Kobayashi R, Dionne A, Ferraro A, Harrild D, Newburger J, VanderPluym C, Gauvreau K, Son MB, Lee P, Baker A, de Ferranti S, Friedman KG. Detailed Assessment of Left Ventricular Function in Multisystem Inflammatory Syndrome in Children, Using Strain Analysis. CJC Open. 2021;3:880-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Goetze JP, Bruneau BG, Ramos HR, Ogawa T, de Bold MK, de Bold AJ. Cardiac natriuretic peptides. Nat Rev Cardiol. 2020;17:698-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 370] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 17. | Klimczak-Tomaniak D, van den Berg VJ, Strachinaru M, Akkerhuis KM, Baart S, Caliskan K, Manintveld OC, Umans V, Geleijnse M, Boersma E, van Dalen BM, Kardys I. Longitudinal patterns of N-terminal pro B-type natriuretic peptide, troponin T, and C-reactive protein in relation to the dynamics of echocardiographic parameters in heart failure patients. Eur Heart J Cardiovasc Imaging. 2020;21:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Hauser JA, Demyanets S, Rusai K, Goritschan C, Weber M, Panesar D, Rindler L, Taylor AM, Marculescu R, Burch M, Wojta J, Michel-Behnke I. Diagnostic performance and reference values of novel biomarkers of paediatric heart failure. Heart. 2016;102:1633-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Kantor PF, Lougheed J, Dancea A, McGillion M, Barbosa N, Chan C, Dillenburg R, Atallah J, Buchholz H, Chant-Gambacort C, Conway J, Gardin L, George K, Greenway S, Human DG, Jeewa A, Price JF, Ross RD, Roche SL, Ryerson L, Soni R, Wilson J, Wong K; Children's Heart Failure Study Group. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013;29:1535-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 20. | Ogawa T, de Bold AJ. Brain natriuretic Peptide production and secretion in inflammation. J Transplant. 2012;2012:962347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Fish-Trotter H, Ferguson JF, Patel N, Arora P, Allen NB, Bachmann KN, Daniels LB, Reilly MP, Lima JAC, Wang TJ, Gupta DK. Inflammation and Circulating Natriuretic Peptide Levels. Circ Heart Fail. 2020;13:e006570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 22. | Alataby H, Nfonoyim J, Diaz K, Al-Tkrit A, Akhter S, David S, Leelaruban V, Gay-Simon KS, Maharaj V, Colet B, Hanna C, Gomez CA. The Levels of Lactate, Troponin, and N-Terminal Pro-B-Type Natriuretic Peptide Are Predictors of Mortality in Patients with Sepsis and Septic Shock: A Retrospective Cohort Study. Med Sci Monit Basic Res. 2021;27:e927834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Lee SH, Song ES, Yoon S, Hong S, Cho HJ, Yang EM, Eom GH, Kang G, Cho YK. Usefulness of Age-Stratified N-Terminal Prohormone of Brain Natriuretic Peptide for Diagnosing Kawasaki Disease. Dis Markers. 2017;2017:6263121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Rodríguez-González M, Castellano-Martínez A, Alonso-Ojembarrena A. Usefulness of age-adjusted N-terminal prohormone of brain natriuretic peptide level as a diagnostic marker for incomplete Kawasaki disease in children. Emergencias. 2019;31:111-114. [PubMed] |
| 25. | Sharma C, Ganigara M, Galeotti C, Burns J, Berganza FM, Hayes DA, Singh-Grewal D, Bharath S, Sajjan S, Bayry J. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat Rev Rheumatol. 2021;17:731-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 26. | Chang JC, Matsubara D, Morgan RW, Diorio C, Nadaraj S, Teachey DT, Bassiri H, Behrens EM, Banerjee A. Skewed Cytokine Responses Rather Than the Magnitude of the Cytokine Storm May Drive Cardiac Dysfunction in Multisystem Inflammatory Syndrome in Children. J Am Heart Assoc. 2021;10:e021428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Taylor KS, Verbakel JY, Feakins BG, Price CP, Perera R, Bankhead C, Plüddemann A. Diagnostic accuracy of point-of-care natriuretic peptide testing for chronic heart failure in ambulatory care: systematic review and meta-analysis. BMJ. 2018;361:k1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Kiess A, Green J, Willenberg A, Ceglarek U, Dähnert I, Jurkutat A, Körner A, Hiemisch A, Kiess W, Vogel M. Age-Dependent Reference Values for hs-Troponin T and NT-proBNP and Determining Factors in a Cohort of Healthy Children (The LIFE Child Study). Pediatr Cardiol. 2022;43:1071-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Palm J, Hoffmann G, Klawonn F, Tutarel O, Palm H, Holdenrieder S, Ewert P. Continuous, complete and comparable NT-proBNP reference ranges in healthy children. Clin Chem Lab Med. 2020;58:1509-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, Behrens EM, Kernan KF, Schulert GS, Seo P, Son MBF, Tremoulet AH, VanderPluym C, Yeung RSM, Mudano AS, Turner AS, Karp DR, Mehta JJ. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3. Arthritis Rheumatol. 2022;74:e1-e20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 31. | Ross RD. The Ross classification for heart failure in children after 25 years: a review and an age-stratified revision. Pediatr Cardiol. 2012;33:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Caselli C, Ragusa R, Prontera C, Cabiati M, Cantinotti M, Federico G, Del Ry S, Trivella MG, Clerico A. Distribution of circulating cardiac biomarkers in healthy children: from birth through adulthood. Biomark Med. 2016;10:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465-95; quiz 576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 1177] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 34. | Koestenberger M, Ravekes W, Everett AD, Stueger HP, Heinzl B, Gamillscheg A, Cvirn G, Boysen A, Fandl A, Nagel B. Right ventricular function in infants, children and adolescents: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr. 2009;22:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 316] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 35. | Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008;21:922-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 619] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 36. | Penner J, Abdel-Mannan O, Grant K, Maillard S, Kucera F, Hassell J, Eyre M, Berger Z, Hacohen Y, Moshal K; GOSH PIMS-TS MDT Group. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health. 2021;5:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 37. | Sanil Y, Misra A, Safa R, Blake JM, Eddine AC, Balakrishnan P, Garcia RU, Taylor R, Dentel JN, Ang J, Cashen K, Heidemann SM, Bauerfield C, Sethuraman U, Farooqi A, Aggarwal S, Singh G. Echocardiographic Indicators Associated with Adverse Clinical Course and Cardiac Sequelae in Multisystem Inflammatory Syndrome in Children with Coronavirus Disease 2019. J Am Soc Echocardiogr. 2021;34:862-876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Abrams JY, Oster ME, Godfred-Cato SE, Bryant B, Datta SD, Campbell AP, Leung JW, Tsang CA, Pierce TJ, Kennedy JL, Hammett TA, Belay ED. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5:323-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 39. | Zhao Y, Patel J, Huang Y, Yin L, Tang L. Cardiac markers of multisystem inflammatory syndrome in children (MIS-C) in COVID-19 patients: A meta-analysis. Am J Emerg Med. 2021;49:62-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Müller N, Rothkegel ST, Boerter N, Sumaria K, Breuer J, Freudenthal NJ. Perioperative urinary NT-ProBNP values and their usefulness as diagnostic and prognostic markers in children with congenital heart disease. Clin Chim Acta. 2021;518:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta MK, Germany; Sitkin S, Russia S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH