Published online Sep 26, 2022. doi: 10.12998/wjcc.v10.i27.9776
Peer-review started: March 10, 2022
First decision: April 13, 2022
Revised: April 22, 2022
Accepted: July 25, 2022
Article in press: July 25, 2022
Published online: September 26, 2022
Processing time: 190 Days and 5.5 Hours
Corneal keloid is a rare clinical disease with an unknown etiology, which is easily misdiagnosed. Surgery is the most effective treatment but is rarely reported in the literature. Herein, we report the clinical features, histopathology, and surgical outcome of a giant corneal keloid with trophoblastic vessels and discuss the genesis of the mass.
A 36-year-old young man was admitted to the hospital because of a large mass on the surface of the left cornea. The patient had suffered an injury to his left eye at the age of 6-years-old; however, as the injury did not cause cornea perforation, he did not undergo treatment. Slit lamp exam showed a large, elevated, opaque lesion that covered the entire cornea and protruded from the surface of the eyeball. Anterior segment optical coherence tomography (AS-OCT) revealed a lesion of irregular density involving the anterior stroma. We suspected a secondary corneal fibroproliferative mass based on the clinical history, and slit lamp and AS-OCT findings. The patient subsequently underwent a superficial keratectomy and keratoplasty, and the final diagnosis of corneal keloid was confirmed by intraoperative histopathological examination.
Non-penetrating corneal trauma damages corneal epithelium basement membrane, initiating stromal fibrosis and causing corneal keloids. AS-OCT and biopsy confirm diagnosis.
Core Tip: Corneal keloid is a benign proliferation of fibrous or fibrovascular tissue in the corneal stroma, and is rarely encountered in the clinic. The onset of secondary corneal keloids can occur months to years after the surgery or trauma, and the depth of corneal infiltration varies. Histopathology is the gold standard for diagnosis, but the cause of the disease is not yet clear. Here, we report a case of a giant corneal keloid that occurred 26 years after trauma and discuss the cause in detail.
- Citation: Li S, Lei J, Wang YH, Xu XL, Yang K, Jie Y. Rare giant corneal keloid presenting 26 years after trauma: A case report . World J Clin Cases 2022; 10(27): 9776-9782
- URL: https://www.wjgnet.com/2307-8960/full/v10/i27/9776.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i27.9776
Corneal keloid is a rare type of ophthalmic disease that presents as a white, painless, enlarged vascular or avascular solitary nodule. It can also involve the entire corneal stroma, with a smooth surface[1]. The diagnosis of corneal keloid can be challenging. It is often referred to as corneal fibroma, myofibroma, and hypertrophic scars, and is easily misdiagnosed as corneal dermatoid tumor, ocular surface squa
Herein, we report the clinical features, histopathology, and anterior segment optical coherence tomography (AS-OCT) features in a case of giant corneal keloid presenting approximately 26 years after corneal trauma.
A 36-year-old man was referred to our hospital in December 2019 to address a corneal swelling found in his left eye 4 years prior. Over the past 2 years, the mass had rapidly increased until it protruded from the surface of the eye, preventing the eyelid from closing properly.
The patient reported having suffered an injury to the left eye at the age of 6-years-old; as the injury had not caused a corneal perforation, treatment was foregone. Since then, however, he had suffered from recurrent redness and a foreign body sensation, which led him to rub his eyes often.
The patient’s medical history was unremarkable.
Not available at this time.
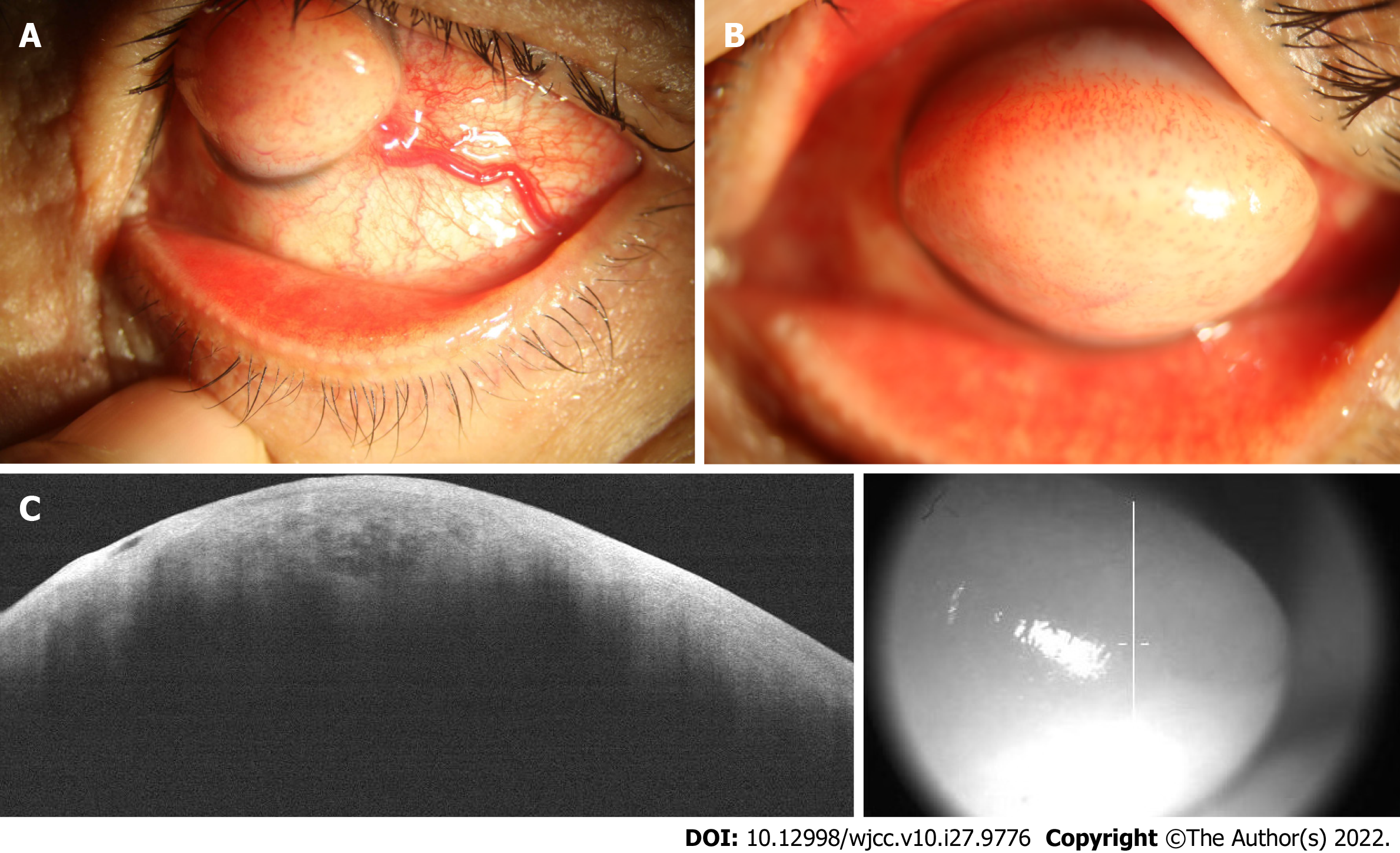
On initial examination, the best-corrected visual acuity (BCVA) was light perception/30 cm for the left eye and 20/20 for the right eye. Slit lamp showed conjunctival congestion with a thick nutrient vessel in the inferior temporal quadrant extending to the corneal limbal epithelium (Figure 1A). A white, tough, round, raised mass, 7 mm high and 9 mm in diameter, with numerous tiny vessels on the surface, localized in the center of the cornea, covered almost the entire cornea surface, preventing the eyelid from closing properly (Figure 1B). Anterior chamber details could not be visualized.
Not available at this time.
Ultrasonographic evaluation of the posterior segment yielded normal findings. AS-OCT showed irregular epithelial tissue and dense subepithelial tissue with a low density of blood vessel invasion, similar to a honeycomb-like structure (Figure 1C).
Detailed history-taking of the patient’s birth revealed that his mother had a spontaneous full-term delivery and no history of medications taken during pregnancy. No swelling was observed in any parts of the patient’s body other than the cornea. The results of routine blood, urine, and immunological examinations were negative.
Secondary corneal fibroproliferative mass.
Corneal keloid due to pathological examination.
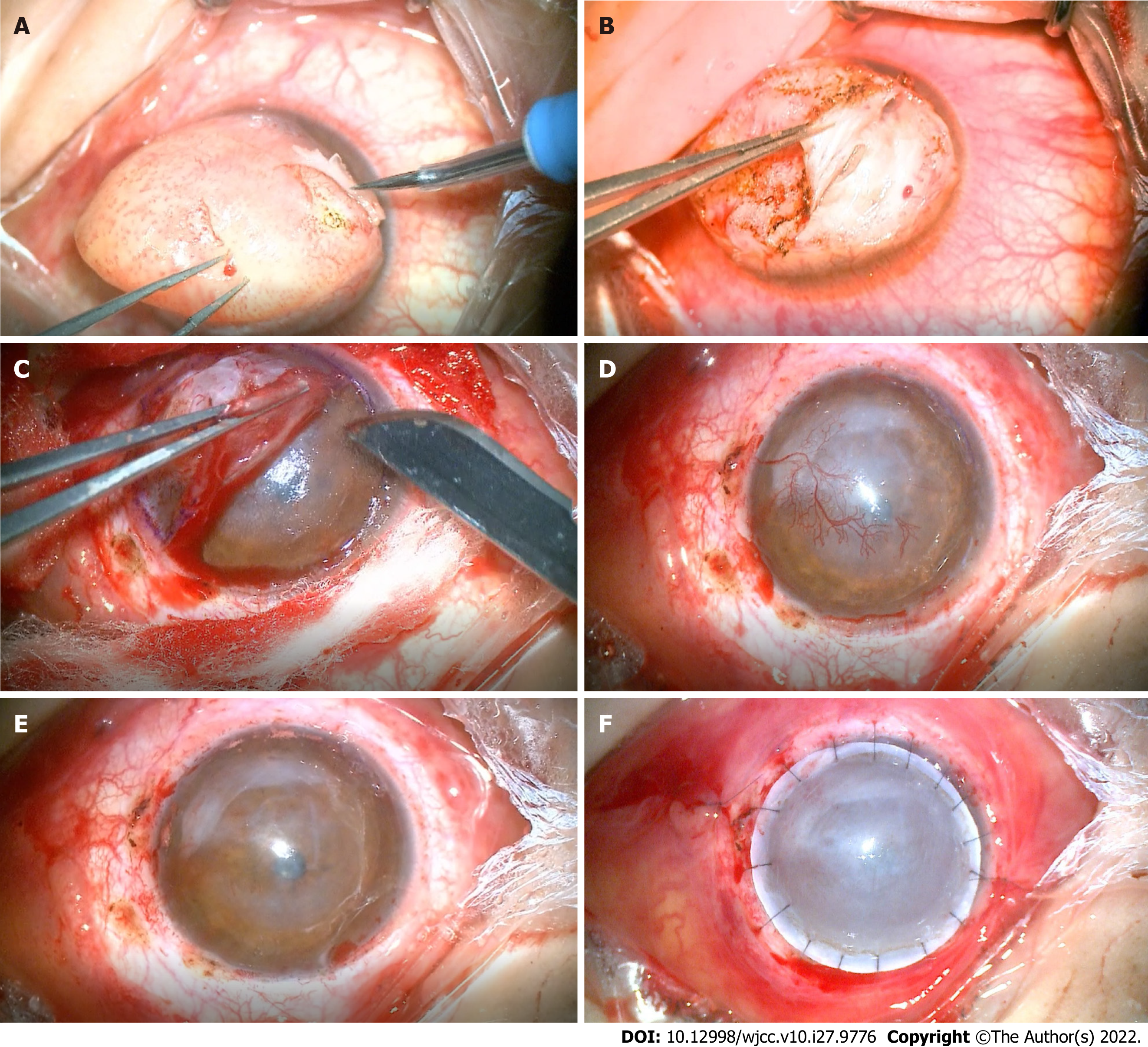
The lesion was removed via superficial keratectomy. Based on the depth of infiltration of the nodule, a deep anterior lamellar keratoplasty was performed (Figure 2). The excised mass was dissected, soaked in formalin, and sent for pathological examination.
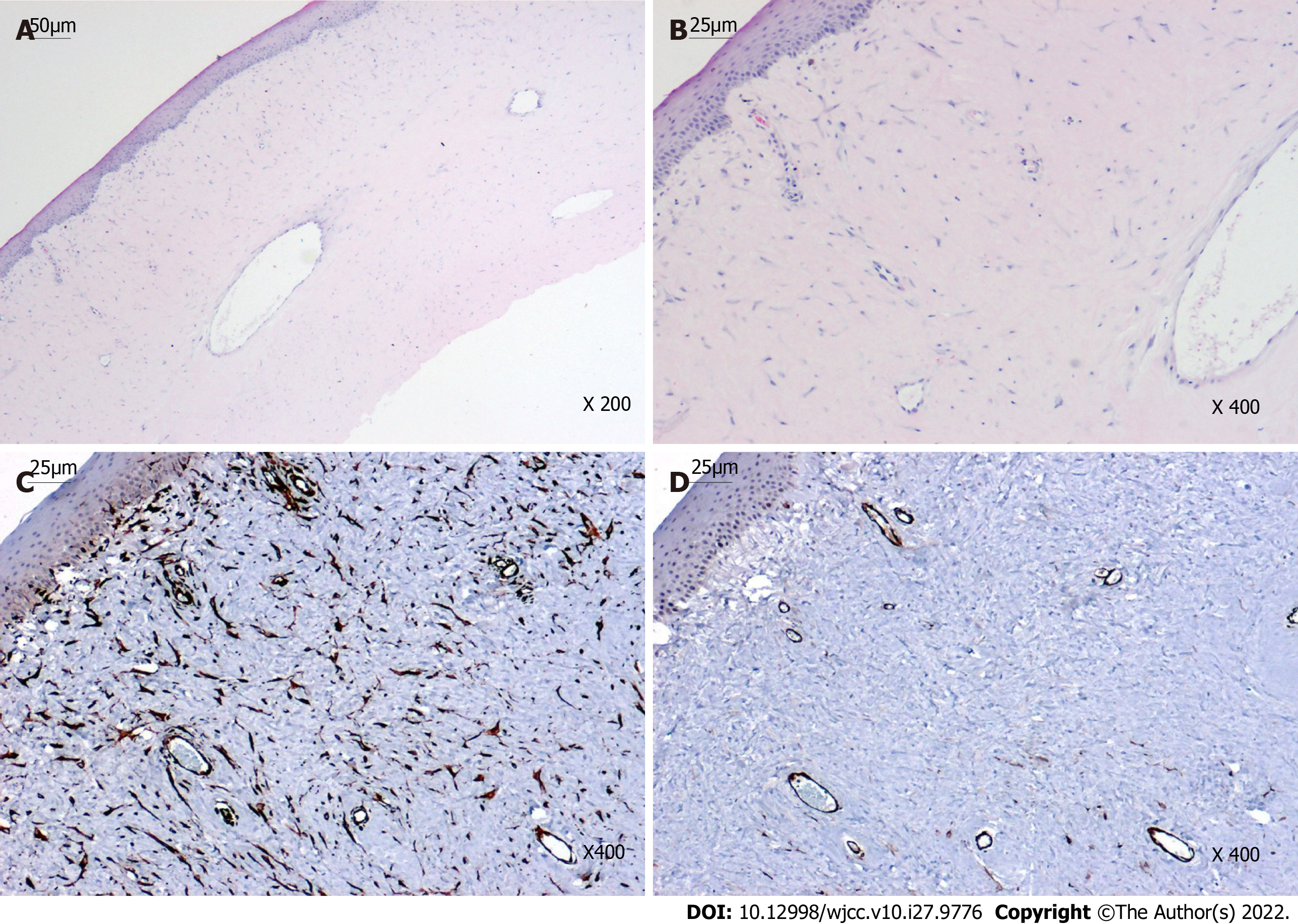
Histopathological examination revealed irregular epithelial hyperplasia without Bowman's layer and abundant subepithelial collagen fibers with irregular proliferation and degenerative changes, which were lined with fibroblasts and vascular cavities (Figures 3A and B). Immunohistochemical staining of the keloid showed diffuse positivity for vimentin and smooth muscle actin (SMA), highlighting the smooth muscle wall of the vasculature and myofibroblasts (Figures 3C and D).
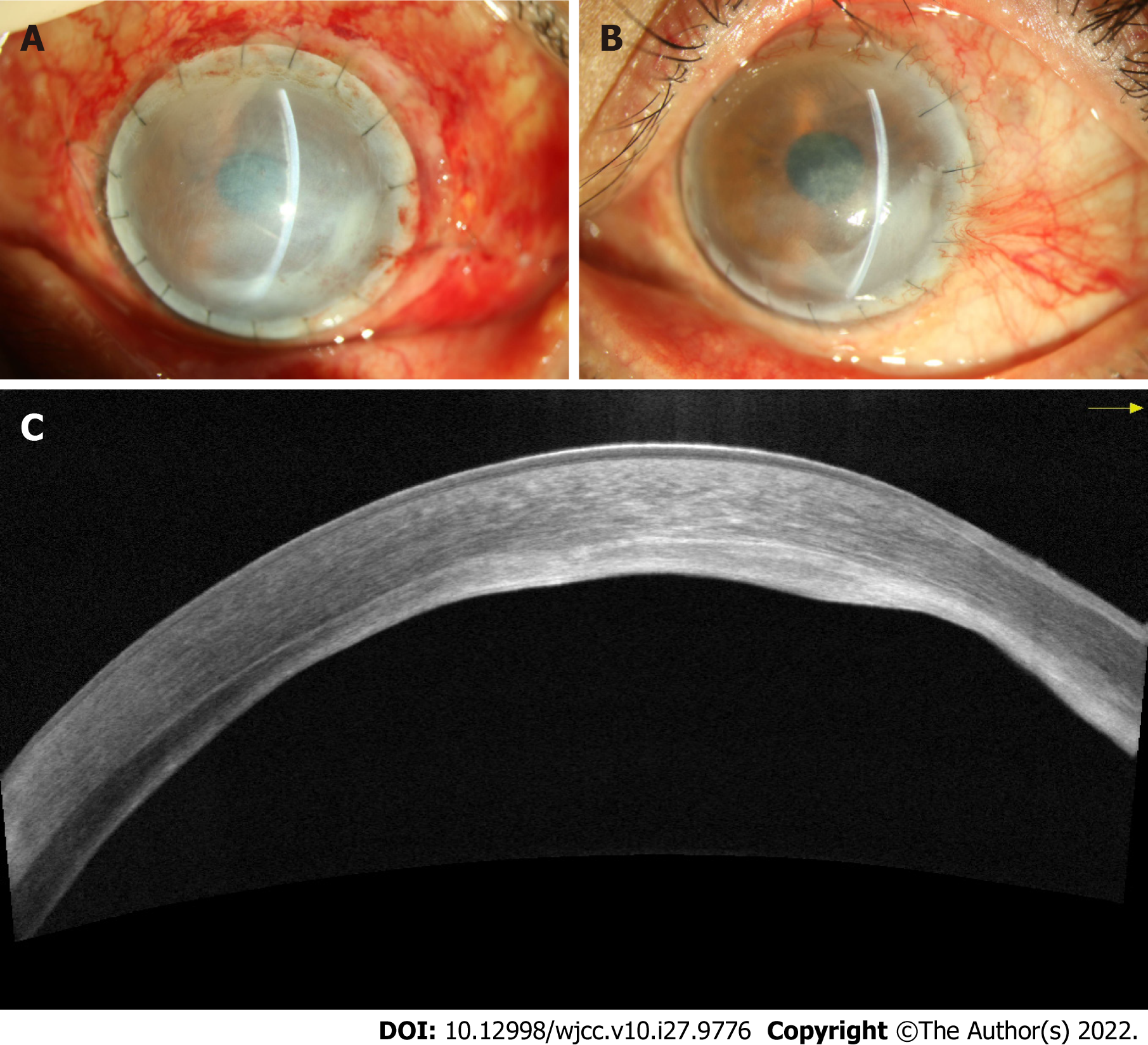
Postoperatively, the patient was prescribed topical steroids, antibiotics, and lubricants. On the first day after surgery, the BCVA was 20/100, and epithelialization of the ocular surface was completed 1 wk later (Figure 4A). There was no recurrence of swelling or post-operative complications during the 4 mo follow-up, and the patient reported satisfaction with the postoperative visual acuity and appearance (Figure 4B). AS-OCT showed that the corneal graft was closely attached to the bed, and the residual stroma thinness was about 100 μm (Figure 4C).
Corneal keloid is a rare clinical disease, classified as congenital, primary, or secondary depending on the etiology[1]. The earliest case of corneal keloid was reported by Szokalski in 1865[9], and since then approximately 80 cases of corneal keloid have been reported in the literature[2]. Most of the reported cases have occurred secondary to trauma, inflammation, or surgery, and the duration of onset has varied from a few months to several years. Herein, we reported a case of giant corneal keloid presenting approximately 26 years after corneal trauma.
There is no uniformity in the pathogenesis of corneal keloid, according to the collective cases reported. Some scholars believe that keloids result from the replacement of embedded iris and inflammatory exudate by fibrovascular tissue[9]. However, in our case, the patient had no history of penetrating corneal injury, so we believe that the keloid originated from the injury and persistent eye rubbing, which led to excessive corneal repair.
Under normal conditions, an intact corneal epithelial basement membrane (EBM) protects the corneal stroma from the presence of myofibroblasts. Once the EBM is damaged, cytokines such as transforming growth factor beta-1, TGFβ2, and platelet-derived growth factor of corneal epithelial origin enter the stroma layer, contributing to the proliferation and differentiation of myofibroblast precursors[10]. If the EBM is repaired within 1-3 wk, the fibroblast precursors do not mature[11]. We assumed that the EBM could not repair the damage in this patient due to frequent eye rubbing and cytokines that were continuously secreted to stimulate myofibroblast activation. As this persisted for years, secretion of large amounts of extracellular matrix, in turn, led to the formation of a giant corneal keloid. Thus, both vimentin and SMA staining within the keloid were positive.
The enlargement of the swelling also depends on the blood vessel supply. The growth of neovascularization into the cornea is a complex process, in which multiple mediators such as inflammatory regulators, cytokines, growth factors, and matrix metalloproteinases interact. The neovascularization itself can assist dendritic cell (DC) migration into the central area[12]. DCs promote keloid proliferation by secreting a series of cytokines that activate inflammatory pathways in the cornea[13].
After being diagnosed with corneal keloids, 82.6% of patients underwent surgery, these include superficial keratectomy (SK), lamellar excision/keratoplasty, penetrating keratoplasty (PK), sclerokeratoplasty, and enucleation[6, 14-16]. SK remains the preferred choice of most physicians, with 70% of patients undergoing the procedure receiving SK. However, according to the literature, a non-dissected mass leads to recurrence in 40% of patients[3]. Bakhtiari performed SK in 1 patient, after which the corneal opacification recurred, so he had to undergo 2 SK procedures combining PK and mitomycin C, followed by a femtosecond laser–assisted deep anterior lamellar keratoplasty[15]. Therefore, we recommend choosing surgery based on the depth of the infiltrated mass, with the aim of complete removal of the mass from the corneal stroma. For lesions located in the corneal stroma that do not breach the Descemet's membrane, SK combined with amniotic membrane graft or deep lamellar corneal graft may be an option. For lesions that infiltrate into the endothelium or do not reach the endothelium but cause corneal endothelial edema, PK surgery is an option. In our case, the corneal keloid and neovascularization infiltrated the deep stromal layer of the cornea without breaking through the Descemet’s membrane; thus, the deep anterior lamellar keratoplasty procedure was selected.
Corneal keloids can occur up to 26 years secondary to non-penetrating corneal trauma. The genesis of such keloids may be related to EBM injury with cytokine clustering. AS-OCT plays an important role in early diagnosis of the disease, but confirmation of the diagnosis still relies on histopathological examination. Surgery is the treatment modality of choice for corneal keloid, and the operator should choose the procedure based on depth of infiltration.
| 1. | Vanathi M, Panda A, Kai S, Sen S. Corneal keloid. Ocul Surf. 2008;6:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Gupta J, Gantyala SP, Kashyap S, Tandon R. Diagnosis, Management, and Histopathological Characteristics of Corneal Keloid: A Case Series and Literature Review. Asia Pac J Ophthalmol (Phila). 2016;5:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Palko JR, Arfeen S, Farooq AV, Reppa C, Harocopos GJ. Corneal keloid presenting forty years after penetrating injury: Case report and literature review. Surv Ophthalmol. 2019;64:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Nair AG, Kenia H, Gopinathan I, Mehta SV, Mehta VC. Corneal fibroma: An uncommon stromal tumor. Indian J Ophthalmol. 2018;66:699-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Zimmermann L, Reinhard T, Lange C, Heegaard S, Auw-Haedrich C. Corneal Myofibroma (Keloid) in a Young Patient with Neurofibromatosis Type 2. Ocul Oncol Pathol. 2017;3:247-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Lee HK, Choi HJ, Kim MK, Wee WR, Oh JY. Corneal keloid: four case reports of clinicopathological features and surgical outcome. BMC Ophthalmol. 2016;16:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Alkatan HM, Al-Arfaj KM, Hantera M, Al-Kharashi S. Healed corneal ulcer with keloid formation. Saudi J Ophthalmol. 2012;26:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 8. | Miyamoto R, Sakimoto T, Homma T, Tanaka Y, Sugiura T, Yamagami S. A rare case of corneal keloid occurred 30 years after pterygium surgery and 3 years after cataract surgery. Am J Ophthalmol Case Rep. 2020;20:100901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Smith HC. Keloid of the Cornea. Trans Am Ophthalmol Soc. 1940;38:519-538. [PubMed] |
| 10. | Medeiros CS, Marino GK, Santhiago MR, Wilson SE. The Corneal Basement Membranes and Stromal Fibrosis. Invest Ophthalmol Vis Sci. 2018;59:4044-4053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Lassance L, Marino GK, Medeiros CS, Thangavadivel S, Wilson SE. Fibrocyte migration, differentiation and apoptosis during the corneal wound healing response to injury. Exp Eye Res. 2018;170:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Narumi M, Kashiwagi Y, Namba H, Ohe R, Yamakawa M, Yamashita H. Contribution of corneal neovascularization to dendritic cell migration into the central area during human corneal infection. PLoS One. 2014;9:e109859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Forrester JV, Xu H, Kuffová L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol Rev. 2010;234:282-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Mejía LF, Acosta C, Santamaría JP. Clinical, surgical, and histopathologic characteristics of corneal keloid. Cornea. 2001;20:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Bakhtiari P, Agarwal DR, Fernandez AA, Milman T, Glasgow B, Starr CE, Aldave AJ. Corneal keloid: report of natural history and outcome of surgical management in two cases. Cornea. 2013;32:1621-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Jung JJ, Wojno TH, Grossniklaus HE. Giant corneal keloid: case report and review of the literature. Cornea. 2010;29:1455-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hegazy AA, Egypt; Shekouhi R, Iran S-Editor: Wang LL L-Editor: A P-Editor: Wang LL