Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9390
Peer-review started: April 8, 2022
First decision: May 31, 2022
Revised: June 3, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: September 16, 2022
Processing time: 146 Days and 23.7 Hours
Congenital adrenal hyperplasia (CAH), which is caused by a mutation of the steroidogenic acute regulatory (StAR) gene. Affected patients are usually characterized by adrenal insufficiency in the first year of life, salt loss, glucocorticoid and mineralocorticoid deficiency, and female external genitalia, regardless of chromosomal karyotype. Patients with non-classical lipoid CAH usually develop glucocorticoid deficiency and mild mineralocorticoid deficiency at 2-4 years of age.
Herein, We report the case of a woman with non-classic lipoid CAH combined with Graves' disease. Her chromosome karyotype was 46, XX, and high-throughput sequencing revealed two missense variants in the StAR gene: c.229C > T (p.Q77X) and c.814C > T (p.R272C), which were inherited from both parents (non-close relatives). The patient was treated for Graves' disease in a timely manner and the dosage of glucocorticoid was adjusted during the treatment of Graves' disease.
This is the first case of non-classic lipoid CAH combined with Graves' disease reported in the Chinese population. In addition to conventional glucocorticoid replacement therapy, timely adjustments were made to the dosages of thyroid hormone and glucocorticoid to avoid adrenal crisis as a consequence of the increased demand and accelerated metabolism of glucocorticoids when the patient was diagnosed with Graves' disease.
Core Tip: Congenital lipoid adrenal hyperplasia (CAH) is the most severe form of CAH, which is caused by a mutation of the steroidogenic acute regulatory gene. This report describes the case of a 24-year-old woman with non-classic lipoid CAH combined with Graves' disease, which is the first of its kind in China. Additionally, the symptoms, presentation, and treatment of the comorbid condition have also been described. We believe that our study makes a significant contribution to the literature because it is an extremely rare case and, in this report, the clinical characteristics are comprehensively reported. Additionally, we provide guidance on how such a case could be effectively treated.
- Citation: Wang YJ, Liu C, Xing C, Zhang L, Xu WF, Wang HY, Wang FT. Congenital lipoid adrenal hyperplasia with Graves' disease: A case report. World J Clin Cases 2022; 10(26): 9390-9397
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9390.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9390
The steroidogenic acute regulatory protein (StAR) is a cytoplasmic enzyme containing 285 amino acids. It promotes the transfer of cholesterol from the outer mitochondrial membrane (OMM) to the inner mitochondrial membrane (IMM) and promotes the production of pregnenolone from cholesterol via the cholesterol side chain lyase[1]. The StAR accounts for about 86% of cholesterol input; only about 14% of cholesterol input occurs in ways unrelated to the StAR[2]. The human StAR gene is located on chromosome 8p11.2 and consists of seven exons. The loss-of-function mutation of the biallelic StAR gene can lead to congenital lipoid adrenal hyperplasia (CAH), which is an autosomal recessive genetic disease, and one of the lethal types of CAH, leading to a serious steroid production disorder and impaired gonad maturation and adrenal steroid hormone synthesis. Lipoid CAH was first described by Prader and Gurtner[3] in 1955. Like other forms of CAH, lipoid CAH can be subdivided into classic and non-classic forms. Because of the complete loss of StAR activity in classic lipoid CAH patients, symptoms of life-threatening Addison’s crisis usually occur shortly after birth, including salt loss, deficiency in serum steroids (glucocorticoids, mineralocorticoids, and sex steroids), increase in adrenocorticoid hormone (ACTH), increase in plasma renin activity, and an increase in the size of adrenal glands as a result of accumulation of cholesterol and cholesteryl esters. In contrast, patients with non-classic lipoid CAH with non-harmful genetic variants retain 10%-25% of StAR activity; therefore, when compared to those with classic lipoid CAH, they have a milder phenotype with a relatively late onset, wherein symptoms of glucocorticoid deficiency and mild deficiency of mineral glucocorticoids usually appear after 2-4 years of age or even into adulthood[4]. Here, we report a case of a woman with non-classic lipoid CAH combined with Graves' disease, who presented in the department of endocrinology, Shengjing Hospital of China Medical University. Through this case, we can understand the necessary dosage adjustment for glucocorticoid therapy in patients with low adrenal function in the case of abnormal thyroid function.
A 24-year-old woman with both lipoid CAH and Graves’ disease presented to the department of endocrinology, Shengjing Hospital of China Medical University with a complaint of regular follow-up and hormone medication dosage adjustment.
The patient receives regular follow-ups in the outpatient division of the endocrinology department in our hospital, and the hormone dosage is adjusted according to the results of relevant biochemical examinations.
23 years ago, the skin of the patient gradually darkened, with obvious skin folds, palm lines, and gingival pigmentation. 21 years ago, due to her coarse voice, boyishness, preference for salt, love of drinking, poor appetite, and a higher rate of growth and development than her peers, she was brought to the pediatrics department of our hospital. Examinations revealed that serum cortisol (COR) levels were low and ACTH was high. The patient was subsequently diagnosed with “CAH.” After treatment with hydrocortisone, her skin pigmentation improved slightly. 9 years ago, the treatment was changed to dexamethasone according to her doctor's advice, and her skin pigmentation improved significantly. Furthermore, her growth rate was noted to be the same as that of her peers. 3 years ago, the patient went to an endocrine clinic due to palpitations and weight loss. There, she was diagnosed with Graves' disease. After treatment with methimazole, the patient developed a rash all over her body. Subsequently, she stopped methimazole and received 131 iodine radiation therapy.
The patient was born at full term without any birth injuries. Her parents were non-consanguineous and had no family history of hereditary diseases.
Physical examination showed blood pressure of 120/80 mmHg, heart rate of 70 beats/min, height of 1.71 m, weight of 70 kg, and a BMI of 23.9 kg/m2. Skin folds; palm lines; lip and gingival pigmentation; thyroid I swelling; no armpit hair; and Tanner scale: Breast development stage V, pubic hair stage V, and normal external genitalia were noted.
The patient's blood routine examination, liver and kidney function, serum ions, and blood glucose were all normal. The results of the relevant hormones of the patient are shown in Table 1. Except for slightly lower progesterone, other sex hormones were normal. The patient had low serum COR, low aldosterone (12.240 pg/mL; normal, 70-300 pg/mL), and high plasma renin (16.137 ng/mL; normal, 0.10-6.56 ng/mL). However, ACTH levels fluctuated as the exogenous glucocorticoids supplemented dosage changed.
| Date | COR (8:00) (µg/dL) | ACTH (8:00) (pg/mL) | Prg (ng/mL) | PRL (ng/mL) | SHB (nmol/L) | T (ng/mL) | LH (mIU/mL) | FSH (mIU/mL) | E2 (pg/mL) | FT3 (pmol/L) | FT4 (pmol/L) | TSH (µIU/mL) | TRAb (IU/L) |
| 6.2-19.4 | 7.2-63.3 | 0.31-1.52 | 3.34-26.72 | 18.2-135.5 | < 0.1-0.75 | 2.12-10.89 | 3.85-8.78 | 27-122 | 2.63-5.71 | 9.01-19.05 | 0.3-4.8 | 0-1.75 | |
| 2013/8/21 | < 0.4 | 35.39 | |||||||||||
| 2015/7/29 | < 0.1 | 26.93 | |||||||||||
| 2016/1/21 | 0.05 | 133.6 | 0.27 | 6.38 | < 0.1 | 6.28 | 6.21 | 42 | |||||
| 2016/5/4 | 0.01 | 8.94 | |||||||||||
| 2017/12/7 | 18.97 | 38.06 | 0.0012 | 39.84 | |||||||||
| 2017/12/26 | 12.58 | 29.71 | 0.0002 | ||||||||||
| 2018/2/4 | 9.45 | 20.53 | 0.0009 | ||||||||||
| 2018/2/26(131I treatment) | 0.213 | 131.1 | 7.31 | 16.03 | 0.0002 | ||||||||
| 2018/12/4 | < 0.018 | 156.8 | < 0.1 | 16.22 | 62.1 | < 0.1 | 4.92 | 6.02 | 34 | 3.65 | 11.15 | 39.7133 | 38.3 |
| 2019/1/21 | < 0.054 | 3.01 | 3.66 | 14.59 | 1.0949 | ||||||||
| 2019/4/8 | < 0.054 | 3.55 | 4.08 | 15.02 | 2.8629 | ||||||||
| 2019/5/5 | < 0.054 | 46.32 | |||||||||||
| 2019/7/29 | < 0.054 | 120.2 | < 0.1 | 9.62 | 92.1 | < 0.1 | 9.63 | 7.76 | < 20 | 4.39 | 13.31 | 5.4609 | |
| 2019/9/24 | < 0.054 | 61.24 | 4.95 | 14.72 | 1.2851 | ||||||||
| 2019/10/28 | < 0.054 | 35.4 | 0.12 | 9.54 | 70.1 | < 0.1 | 6.27 | 7.18 | 34 | 4.63 | 15.07 | 0.646 | |
| 2020/8/11 | 0.71 | 199.7 | 0.22 | 7.2 | 96.5 | < 0.1 | 61.15 | 10.27 | 146 | 4.52 | 14.81 | 1.0839 | |
| 2021/4/19 | 0.682 | 134.4 | 0.59 | 8.45 | 83.8 | < 0.1 | 9.26 | 7.91 | 58.16 | 3.56 | 13.80 | 4.9828 | 3.66 |
| 2021/11/29 | 1.11 | 699.6 | < 0.1 | 17.06 | 77.8 | < 0.1 | 13.9 | 11.67 | 72.33 | 3.13 | 13.58 | 16.7861 |
A uterine ultrasound revealed that the size of the uterus was 7.2 cm × 4.5 cm × 2.7 cm, the endometrial thickness was about 0.6 cm, the left ovary was 3.4 cm × 2 cm, and the right ovary was 3.6 cm × 2.3 cm. Enhanced magnetic resonance imaging of the pituitary gland showed that the volume of the pituitary gland was increased significantly. Enhanced computed tomography of the adrenal glands showed punctate calcification of the left adrenal gland.
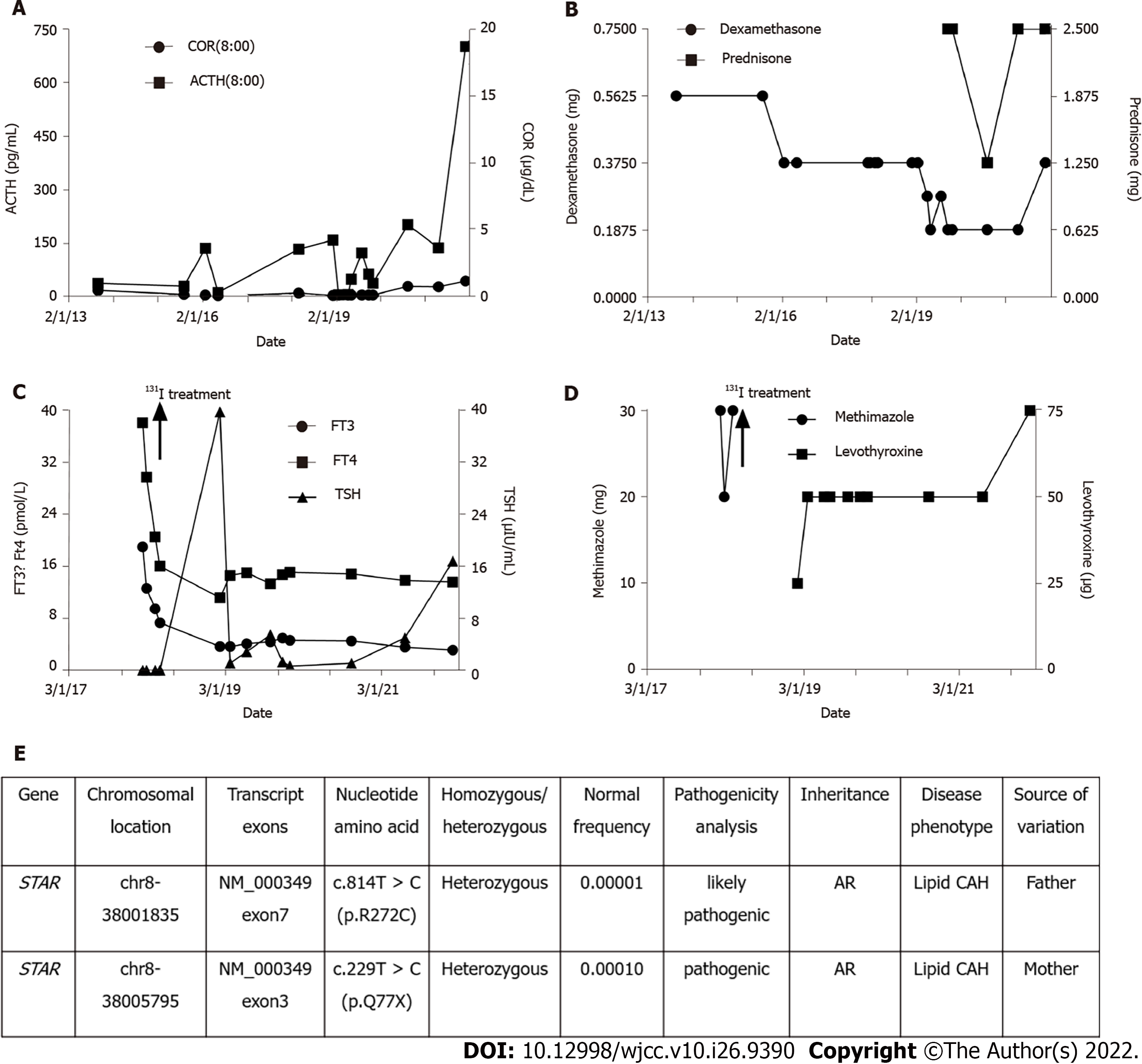
Her chromosome karyotype was determined as 46 XX, and high-throughput sequencing revealed two missense variants in the StAR gene: c.229C > T (p.Q77X) and c.814C > T (p.R272C) inherited from both parents (non-consanguineous) which was consistent with lipoid CAH. The levels of free triiodothyronine (FT) 3, FT4, and thyrotropin receptor antibody (TRAb) were significantly increased, while the level of thyroid stimulating hormone (TSH) was significantly reduced. Thyroid ultrasound showed that the thyroid gland was diffusely diseased and the blood supply of the thyroid was significantly increased. As per these findings, a diagnosis of Graves' disease was made in the patient.
Combined with the patient’s medical history, the final diagnosis was lipoid CAH and Graves’ disease.
The patient's thyroid function was altered with methimazole treatment and 131 iodine radiotherapy. When the patient developed subclinical hypothyroidism after 131 iodine radiotherapy, we promptly adopted levothyroxine replacement therapy and adjusted the dosage of glucocorticoids. The specific drug dosage is shown in Figure 1.
The patient had a follow up at the endocrinology clinic of our hospital on November 29, 2021. Since ACTH and TSH were significantly increased, we adjusted her medication regimen to dexamethasone 0.375 mg orally before bed, prednisone 1.25 mg orally in the morning and 1.25 mg orally in the evening to supplement glucocorticoids, and levothyroxine 75 μg orally every morning to supplement thyroid hormones.
In this case report, we described the complete medical history, examination, and treatment of a 46, XX female patient diagnosed with lipoid CAH. The genetic analysis of high-throughput sequencing revealed the variation of the StAR gene inherited from both parents. The patient gradually developed atypical clinical features of adrenal insufficiency (AI) during childhood, such as mild hyponatremia and hyperpigmentation, which improved after treatment with glucocorticoids. The patient developed Graves' disease in adulthood and was treated with antithyroid hormone drugs. After 131 iodine radiotherapy and adjustment of glucocorticoid dosage, her condition gradually stabilized.
CAH is a group of autosomal recessive genetic diseases caused by impaired adrenal steroid production due to pathogenic variants in genes encoding steroid-producing enzymes or cofactors. Adrenal insufficiency occurs with or without hyperandrogenism[5]. According to reports, the global incidence of CAH is 1/14000-1/18000[5], and the incidence in China is 1/20815–1/25757[6]. According to the particular defective enzyme’s genes[7], CAH can be divided into seven types, of which, 21-hydroxylase deficiency is the most common type and accounts for 90%-95% of the cases, 11β-hydroxylase deficiency accounts for about 5%-8% of the cases, while 17-hydroxylase deficiency and 3β-hydroxysteroid dehydrogenase deficiency accounts for less than 1% of the cases. Other types of CAH are relatively rare in the world population (lipoid CAH: About 200 cases, P450 oxidoreductase deficiency: About 130 cases, cholesterol side chain lyase deficiency: Only about 30 cases). Lipoid CAH caused by mutations in the StAR gene is the most serious form of CAH and is rare. However, so far, about 85 pathogenic variants in the StAR gene have been reported. These mutations usually cluster in exons 5-7[2]. Lipoid CAH is more common in Japanese and Korean populations where the heterozygous carrier rate is about one in 300, and p.Q258X is the most common mutant allele[8]. Other mutant genes include p.R182L, p.R182I, p.R182H, p.L260P, p.R188C, p.N148K, p.A218V, p.E169K, p.L275P, c.201_202delCT, c.772C > T, c.556A > G, c.179-15G > T, c.695delG, and c.306 + 3_c.306 + 6delAAGT[4,9], among which mutations related to non-classic lipoid CAH retain about 20%-30% of normal StAR activity[2].We reported a case of a patient whose high-throughput sequencing revealed two missense variants in the StAR gene: c.229C > T (p.Q77X) and c.814C >T (p.R272C), which were inherited from both parents. The c.229C > T (p.Q77X) mutation has been reported in previous lipoid CAH cases, whereas the c.814C > T (p.R272C) mutation has not been previously reported in lipoid CAH cases. It is, therefore, a possible pathogenic mutation site[4].
STAR is a basic participant in steroid production. It transfers cholesterol from the OMM to the IMM, where CYP11A1 can convert cholesterol to pregnenolone[10]. The complex pathophysiology of StAR deficiency can be explained by the “two-hit disease model” proposed by Miller, where the absence of StAR is the first blow, leading to the loss of most (but not all) steroid production. The reduction of adrenal and testicular steroid production leads to a compensatory increase in ACTH and luteinizing hormone, which increases the biosynthesis of low-density lipoprotein receptors, the uptake of low-density lipoprotein cholesterol, and the de novo synthesis of cholesterol. Over time, this type of intracellular cholesterol will accumulate, causing damage to mitochondria and cells due to overloading of accumulated cholesterol, cholesterol esters, and their auto-oxidation products. Insufficient steroid production is the second blow and will eventually destroy the residual StAR-independent steroid production in the steroid-producing cells[11]. Lipoid CAH is the most serious disorder of steroid production. Affected newborns cannot produce large amounts of any steroid, which is manifested by high ACTH levels, increased plasma renin activity, and hyperemic adrenal glands, which contain excessive cholesterol and its derivatives[12]. Regardless of the allosome present, all patients with classic lipoid CAH have female external genitalia, and severe adrenal AI, salt wasting crisis, and hyperpigmentation are present within 1 mo after birth[2]. Lack of aldosterone will increase renal salt uptake and plasma levels of renin and lack of COR will cause excessive secretion of ACTH through negative feedback from the hypothalamus and pituitary gland. Since ACTH is a growth factor of the adrenal cortex, this will lead to mediation through its melanocortin 2 receptor and will cause adrenal hyperplasia. In addition, excessive ACTH can stimulate the melanocortin 1 receptor and cause hyperpigmentation of the skin[13]. The Leydig cells in the fetal testes of the classic lipoid CAH 46, XY karyotype are destroyed during early pregnancy, eliminating the biosynthesis of testosterone, thereby leading to sexual development disorders and the development of female external genitalia. Sertoli cells continue to produce Mullerian duct inhibitory hormones appropriately; therefore, fetuses with a 46, XY phenotype lack the cervix, uterus, and fallopian tubes[14]. A fetus with classic lipoid CAH 46, XX karyotype has normal genitalia at birth, and fetal ovaries produce very little steroids. Therefore, the genitalia is not affected until puberty is stimulated by gonadotropins. At this time, steroids not related to StAR are produced. This results in enough estrogen to bring about appropriate characteristics of puberty in women with lipoid CAH. Breast development and anovulatory menstrual cycles occur in early puberty. Continuous gonadotropin stimulation can lead to cholesterol accumulation and cell damage. Although estrogen is produced by StAR-independent steroid production at the beginning of the menstrual cycle, progesterone synthesis is impaired in the latter part of the menstrual cycle, but low estrogen levels may be not enough to promote ovulation and/or embryo implantation[15]. Patients with non-classic lipoid CAH have mild hypoadrenal function, which may appear at any time from childhood to adulthood; hence, some of them are mistakenly diagnosed with familial glucocorticoid deficiency[1]. Unlike classic lipoid CAH, non-classic 46, XY males usually have normal-appearing male external genitalia. However, patients' mineralocorticoid secretion may be slightly impaired, serum electrolytes are normal, plasma renin is elevated, and some patients may have mild hypogonadotropic hypogonadism[14]. Our case is of a 24-year-old woman with non-classic lipoid CAH who received hydrocortisone and dexamethasone treatment. Her growth rate was noted to be the same as that of her peers. She had normal external genitalia, a normal uterine ultrasound pattern, and a normal menstrual cycle. Since the patient has not been pregnant, we could not assess her fertility.
As we know, the thyroid hormone can accelerate the metabolism of COR. In individuals with adequate adrenal function, the thyrotoxic state will not only increase the production of COR, but also shorten the half-life of COR due to the increase in turnover rate, which has a net effect on normal circulating COR levels. Due to the increased stress and metabolic demands of thyrotoxicosis, the demand for COR increases. Patients with AI cannot produce an adrenal response by increasing COR levels when thyroid hormones are too high, which puts them at risk of adrenal crisis. According to reports, individuals with autoimmune primary AI will develop recurrent adrenal crisis after developing autoimmune thyrotoxicosis[16]. Similarly, there is sufficient evidence that undiagnosed primary or secondary AI in hypothyroid patients who have started to supplement with thyroid hormone may have an adrenal crisis[17]. Kim et al[18] reported a case of a 23-year-old man with Graves’ thyrotoxicosis. He was diagnosed with atypical 11β-hydroxylase deficiency after hypokalemia and hypertension, and imaging revealed adrenal hyperplasia. Fredette et al[19] described a case of a pediatric patient with CAH who experienced recurrent adrenal crisis and hyperandrogenism after the onset of Graves’ thyrotoxicosis, proving the effect of excess thyroid hormone on glucocorticoid metabolism in the body. Due to the timely treatment of Graves' disease, the patient in our report did not induce an adrenal crisis as a consequence of increased demand and accelerated metabolism of glucocorticoids during Graves' disease, which may lead to a relative lack of glucocorticoids in the body. The patient was switched to 131 iodine radiotherapy for Graves' disease due to the development of a skin rash when taking methimazole. After the change in treatment, the rash gradually subsided, but the patient developed subclinical hypothyroidism. During the follow-up period, we adjusted the supplementary dose of thyroid hormone and glucocorticoid in time to ensure that the condition of the patient was stable.
Treatment of lipoid CAH includes physiological replacement of glucocorticoids and mineralocorticoids and salt supplementation in the neonatal period. The dose of glucocorticoid is same as the dose used in primary adrenal insufficiency, which is lower than the dose for patients with 21-hydroxylase deficiency because it is not necessary to inhibit excessive adrenal androgen production[14]. Salt supplements are usually stopped after the patient reaches 1 year of age, and mineralocorticoids are gradually reduced because the density of mineralocorticoid receptors in the renal epithelium increases with age and the diets of older children and adults tend to contain more sodium. The dose of mineralocorticoid should be adjusted according to blood pressure and plasma renin[14]. Lipoid CAH patients with 46, XY karyotype have female external genitalia and usually undergo orchiectomy and are raised as women. On reaching puberty, sex hormone replacement therapy is needed. Lipoid CAH patients with 46, XX karyotype undergo puberty and develop regular menstrual cycles, but they usually develop ovarian cysts and infertility. We recommend using current fertility techniques as early as possible, such as egg freezing, to provide patients with the opportunity to conceive[20].
In conclusion, our study summarized the clinical characteristics of a case of non-classic lipoid CAH with Graves' disease, including clinical manifestations, auxiliary examinations, and diagnosis. High-throughput sequencing detected 2 rare mutation sites: c.229C > T (p.Q77X) and c.814C > T (p.R272C), which enriched the mutation spectrum of the lipoid CAH gene. Due to the early diagnosis and timely treatment with glucocorticoid supplementation, the patient's development was normal. During the regular outpatient follow-up, the patient was diagnosed with Graves' disease and timely adjustments were made to the dosages of thyroid hormone and glucocorticoid to avoid adrenal crisis as a consequence of the increased demand and accelerated metabolism of glucocorticoids during Graves' disease.
| 1. | Flück CE. MECHANISMS IN ENDOCRINOLOGY: Update on pathogenesis of primary adrenal insufficiency: beyond steroid enzyme deficiency and autoimmune adrenal destruction. Eur J Endocrinol. 2017;177:R99-R111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Miller WL. Disorders in the initial steps of steroid hormone synthesis. J Steroid Biochem Mol Biol. 2017;165:18-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 3. | PRADER A, GURTNER HP. The syndrome of male pseudohermaphrodism in congenital adrenocortical hyperplasia without overproduction of androgens (adrenal male pseudohermaphrodism). Helv Paediatr Acta. 1955;10:397-412. [PubMed] |
| 4. | Zhang T, Ma X, Wang J, Jia C, Wang W, Dong Z, Ye L, Sun S, Hu R, Ning G, Li C, Lu W. Clinical and molecular characterization of thirty Chinese patients with congenital lipoid adrenal hyperplasia. J Steroid Biochem Mol Biol. 2021;206:105788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SE, White PC. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103:4043-4088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 729] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 6. | Li Z, Huang L, Du C, Zhang C, Zhang M, Liang Y, Luo X. Analysis of the Screening Results for Congenital Adrenal Hyperplasia Involving 7.85 Million Newborns in China: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2021;12:624507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390:2194-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 8. | Kang E, Kim YM, Kim GH, Lee BH, Yoo HW, Choi JH. Mutation Spectrum of STAR and a Founder Effect of the p.Q258* in Korean Patients with Congenital Lipoid Adrenal Hyperplasia. Mol Med. 2017;23:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Li Z, Liang Y, Du C, Yu X, Hou L, Wu W, Ying Y, Luo X. Clinical applications of genetic analysis and liquid chromatography tandem-mass spectrometry in rare types of congenital adrenal hyperplasia. BMC Endocr Disord. 2021;21:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Miller WL. Steroidogenesis: Unanswered Questions. Trends Endocrinol Metab. 2017;28:771-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Miller WL. Minireview: regulation of steroidogenesis by electron transfer. Endocrinology. 2005;146:2544-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Katharopoulos E, Di Iorgi N, Fernandez-Alvarez P, Pandey AV, Groessl M, Dubey S, Camats N, Napoli F, Patti G, Lezzi M, Maghnie M, Flück CE. Characterization of Two Novel Variants of the Steroidogenic Acute Regulatory Protein Identified in a Girl with Classic Lipoid Congenital Adrenal Hyperplasia. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kallali W, Gray E, Mehdi MZ, Lindsay R, Metherell LA, Buonocore F, Suntharalingham JP, Achermann JC, Donaldson M. Long-term outcome of partial P450 side-chain cleavage enzyme deficiency in three brothers: the importance of early diagnosis. Eur J Endocrinol. 2020;182:K15-K24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Miller WL. MECHANISMS IN ENDOCRINOLOGY: Rare defects in adrenal steroidogenesis. Eur J Endocrinol. 2018;179:R125-R141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Gomes LG, Bachega TASS, Mendonca BB. Classic congenital adrenal hyperplasia and its impact on reproduction. Fertil Steril. 2019;111:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Naik D, Jebasingh KF, Thomas N. Delayed Diagnosis of Graves' Thyrotoxicoisis Presenting as Recurrent Adrenal Crisis in Primary Adrenal Insufficiency. J Clin Diagn Res. 2016;10:OD20-OD22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Higham CE, Johannsson G, Shalet SM. Hypopituitarism. Lancet. 2016;388:2403-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 18. | Kim JH, Park G, Kim SY, Bae HY. Thyrotoxic periodic paralysis with Graves' disease leading to the discovery of a hidden nonclassic 11β hydroxylase deficiency. Intern Med. 2013;52:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Fredette ME, Topor LS. Graves' Thyrotoxicosis Leading to Adrenal Decompensation and Hyperandrogenemia in a Pediatric Patient with Salt-Wasting Congenital Adrenal Hyperplasia. Case Rep Endocrinol. 2018;2018:2359205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Chatziaggelou A, Sakkas EG, Votino R, Papagianni M, Mastorakos G. Assisted Reproduction in Congenital Adrenal Hyperplasia. Front Endocrinol (Lausanne). 2019;10:723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casella C, Italy; Covantsev S, Russia; Zavaleta MJC, Peru S-Editor: Wang DM L-Editor: A P-Editor: Wang DM