Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.9142
Peer-review started: April 19, 2022
First decision: May 30, 2022
Revised: June 8, 2022
Accepted: July 22, 2022
Article in press: July 22, 2022
Published online: September 6, 2022
Processing time: 129 Days and 0.8 Hours
Tamsulosin, a selective α1-adrenergic receptor antagonist, is commonly used for treating neurogenic bladder in patients with spinal cord injury (SCI). No severe adverse events have been described with such tamsulosin use. To our knowledge, we report the first case of severe life-threatening hypotension as an adverse effect of tamsulosin in a person with SCI. Therefore, we report this case to inform that this severe adverse effect of tamsulosin can occur when treating patients with SCI.
A 59-year-old woman was diagnosed with cervical spinal cord myelopathy and was classified as American Spinal Injury Association Impairment Scale D, neurological level of injury C3. Because she suffered from voiding difficulty due to neurogenic bladder, we prescribed tamsulosin. Her vital signs remained stable, but occasional hypotensive symptoms followed defecation. We reduced the dose of tamsulosin, but after administering tamsulosin for 9 d, she experienced life-threatening hypotension with no evidence of hypovolemic shock, neurogenic shock, cardiogenic shock, or septic shock. A hypotensive condition induced by tamsulosin was the suspected cause, and her symptoms could be associated with adverse effects of tamsulosin. As symptoms resolved after stopping tamsulosin, and no other reason was found, we concluded that tamsulosin was the cause of her symptoms.
Caution for severe hypotension is needed when administering tamsulosin for neurogenic bladder in a patient with SCI.
Core Tip: Voiding difficulty is a common symptom of spinal cord injury (SCI) due to neurogenic bladder. Tamsulosin is commonly used for treating neurogenic bladder. However, this case demonstrated that tamsulosin can cause severe life-threatening hypotension. Thus, clinicians should be aware of this possible condition when treating neurogenic bladder in a patient with SCI.
- Citation: Lee JY, Lee HS, Park SB, Lee KH. Tamsulosin-induced life-threatening hypotension in a patient with spinal cord injury: A case report. World J Clin Cases 2022; 10(25): 9142-9147
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/9142.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.9142
Tamsulosin is a selective α1-adrenergic receptor antagonist that is widely used for benign prostatic hypertrophy (BPH). It also is effective against and commonly used for treating neurogenic bladder in patients with spinal cord injury (SCI). The usual dosage of tamsulosin is 0.2, 0.4, or 0.8 mg per day[1-3]. In Korea, tamsulosin is approved by insurance in both men and women who have neurogenic bladder as well as BPH. Furthermore, tamsulosin is an effective treatment for relief of lower urinary tract symptoms in women by improving the average flow rate and the residual volume after voiding[4,5].
Classically, α1-adrenergic receptor antagonists have been used for treating arterial hypertension. Among the three α1-adrenergic receptor subtypes (α1A, α1B, and α1D), α1B is thought to have an effect on vasoconstriction. The α1A-adrenergic receptor subtype is more specifically involved in the human prostate[1]. Tamsulosin is an α1-adrenergic receptor antagonist with high selectivity for α1A-adrenergic receptors and low selectivity for α1B-adrenergic receptors.
Well-known adverse effects of tamsulosin are dizziness, abnormal ejaculation, and asthenia[3,6]. Hypotension is another possible but rare adverse event[1-3,6]. Previous cases of severe hypotension induced by tamsulosin were people with BPH or undergoing a general anesthetic procedure[6,7]. To the best of our knowledge, there are no reported cases of tamsulosin-induced severe hypotension in people with SCI. Here, we present a unique case of a woman with SCI who suffered life-threatening hypo
A 59-year-old woman suffering from tetraparesis due to cervical spinal cord myelopathy presented with a sudden drop in blood pressure (BP) after tamsulosin use for 9 d.
A cervical spine magnetic resonance imaging (MRI) study was performed. The patient was diagnosed with cervical spinal cord myelopathy at the C3-C6 level and fractures at the C4-C6 level. She was classified as C3 AIS D (American Spinal Injury Association Impairment Scale). After surgery, she was transferred to the rehabilitation medicine department after Foley catheter insertion for urination.
Considering her completeness of injury (AIS D), we removed her Foley catheter to identify residual urine using clean intermittent catheterization (CIC). On the day of removal, she was able to self-void, but residual urine was in the range of 160-200 mL. On suspicion of voiding difficulty due to neurogenic bladder, a complication of her SCI, we scheduled a urodynamic study, but the patient refused due to discomfort. In the absence of a urodynamic study, we prescribed the recommended dose of 0.4 mg tamsulosin once daily[1,2]. For bladder management, we planned CIC at least 4 times/day, followed by self-voiding. Because she had been diagnosed with cervical spinal cord myelopathy, we encouraged a total fluid intake of approximately 2000 mL/day to prevent orthostatic hypotension[8].
After administering tamsulosin for 6 d, we reduced the dose to 0.2 mg once daily as her residual urine remained less than 200 mL. In addition to voiding difficulty, tamsulosin use produced other symptoms. Though her BP remained stable, she experienced occasional dizziness, likely due to orthostatic hypotension. She also experienced constipation and post-defecation symptoms such as nausea, lightheadedness, and sweating. We attributed these symptoms to orthostatic hypotension due to cervical myelopathy aggravated by tamsulosin[9]. We reduced the dose of tamsulosin to 0.2 mg and expect resolution of the symptoms.
After tamsulosin use for 9 d, her BP suddenly dropped to 70/40 mmHg. Before the event, she had difficulties defecating and experienced dizziness. Her heart rate was 50 beats/min (bpm), and body temperature (BT) was 36.3 ℃, although there was no alteration in consciousness. Despite treatment with normal saline hydration, supplemental O2, and norepinephrine, her BP decreased to 50/40 mmHg. We did not suspect hypovolemia as the underlying cause and administered atropine for possible cardiogenic shock. However, her electrocardiogram (EKG) showed sinus bradycardia and vasovagal syncope as potential causes of her symptoms. After several minutes, her BP increased to 120/80 mmHg, and all other vital signs returned to within normal range, including BT. No abnormal result was found in her laboratory tests, including urinalysis. We stopped tamsulosin immediately because of its possible hypotensive effect.
On the next day, her BP dropped to 60/40 mmHg after defecation. Once again, she remained conscious and alert. Atropine was administered but had no effect. Norepinephrine was administered, her BP increased to 100/60 mmHg, and heart rate ranged from 90 to 110 bpm.
After the second event, there was no additional event of hypotension or difficulty in defecation after stopping tamsulosin.
The patient had a medical history of diabetes and hypertension and use of associated medications for the previous 20 years. Her BP and blood glucose level had been well-controlled. She had not experienced previous orthostatic hypotension or hypoglycemia.
She had no family history of cervical cord disorder and other diseases.
Her motor strength was 3/5 grade in the upper extremities and 4/5 grade in the lower extremities. She suffered hypoesthesia below the C4 dermatome. Deep anal pressure and voluntary anal contraction were intact. According to physical examination, she was classified as C3 AIS D.
Her blood glucose level was 74, 84, 94, 74, and 75 mg/dL pre-meal and 122, 133, 102, 106, and 140 mg/dL on 2 h after a meal. We did not examine hemoglobin A1c because we thought that her blood glucose level was well-controlled.
At the first hypotensive event, the laboratory test results showed white blood counts (WBC) of 8500/mm3, C-reactive protein (CRP) of 0.77 mg/dL, and procalcitonin of 0.04 ng/mL; troponin-I, lactate dehydrogenase, and creatinine levels were normal.
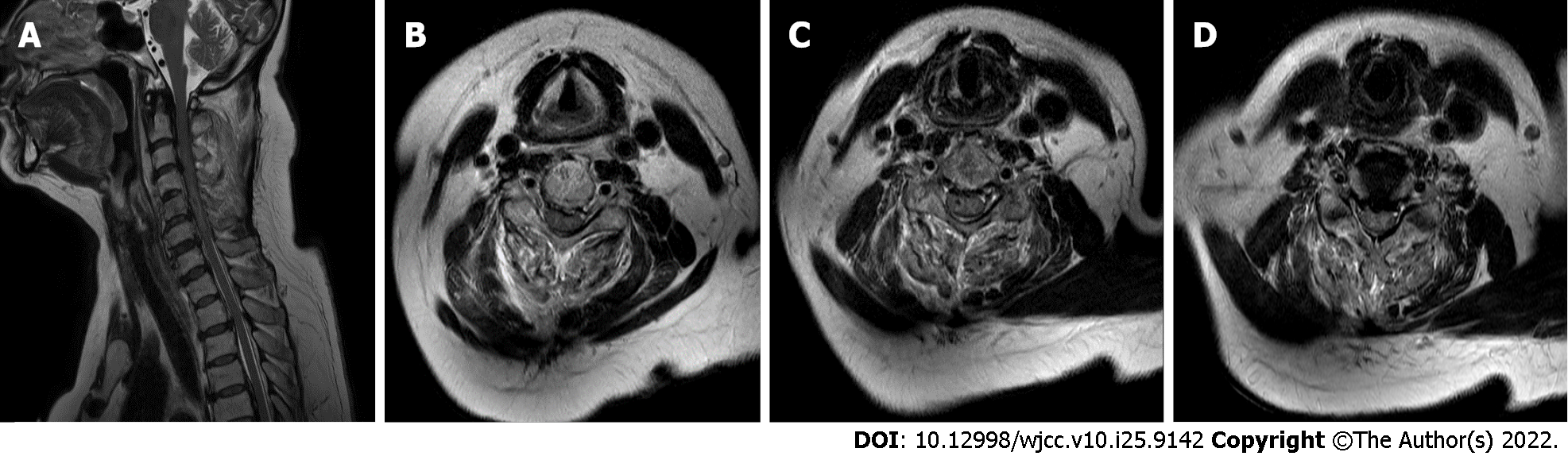
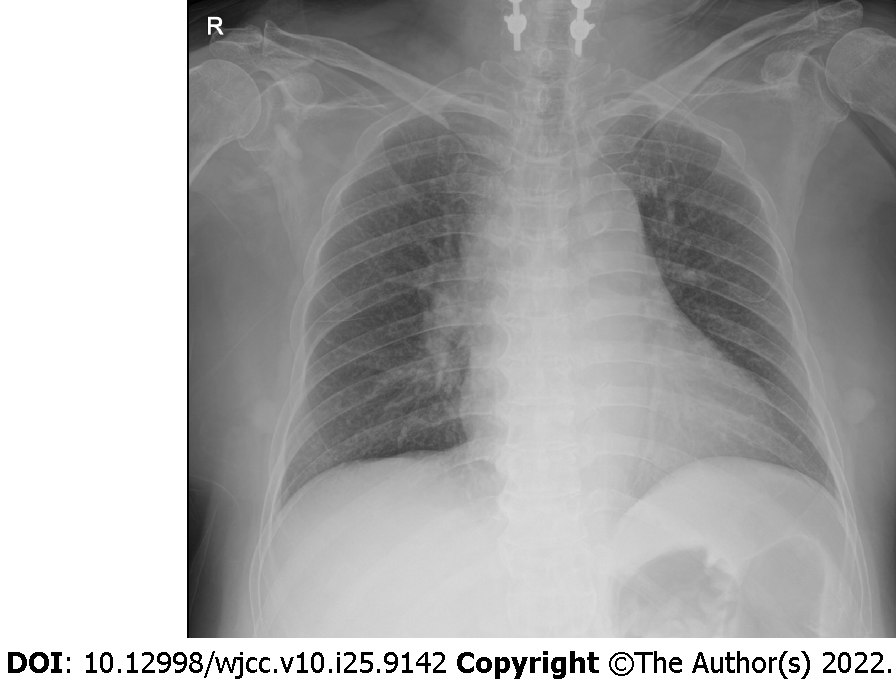
Cervical spine MRI revealed cord signal change at the C4-C6 levels in the emergency room (Figure 1). Chest and abdomen x-ray revealed no specific finding at the first hypotensive event (Figure 2).
As all symptoms resolved after stopping tamsulosin, we posited tamsulosin as the possible cause of her symptoms.
We stopped tamsulosin immediately after her severe event. To treat possible sepsis, we administered piperacillin-tazobactam after laboratory tests including blood, urine, and stool cultures including that for Clostridium difficile. After 2 d, no bacterial growth was observed, and piperacillin-tazobactam was stopped. Norepinephrine was administered and tapered for 2 d.
At 4 d from the event, laboratory test results showed WBC of 5600/mm3, CRP of 5.0 mg/dL, and procalcitonin of 1.80 ng/mL. Her kidney function was normal, with a creatinine level of 0.60 mg/dL. There was no persistent difficulty in defecation.
We prescribed bethanechol chloride for voiding difficulty, and the patient was discharged 10 days after the first event[10,11].
At present, tamsulosin is widely used for voiding difficulty in patients with SCI. Although tamsulosin can affect BP, associated life-threatening hypotensive events are not common. One case report discusses tamsulosin-induced severe hypotension during general anesthesia[7]. Another documented increased risk of severe hypotension when administering tamsulosin to people with BPH[6]. However, there are no reported cases of tamsulosin-induced severe side effects in people with SCI.
In this case, the patient had two specific issues that commonly accompany cervical cord myelopathy: Orthostatic hypotension and difficulty in defecation. Her other medications included antidiabetics and an angiotensin receptor blocker for underlying hypertension. These medications have no reported significant effect when co-administered with tamsulosin[2]. As mentioned earlier, she had no evidence of hypovolemic, neurogenic, cardiogenic, or septic shock. Treatment with only normal saline hydration showed no benefit to her symptoms, and no evidence of dehydration was found. Cardiogenic shock was a possible cause since her BP increased after administering atropine at the first event. However, her EKG showed sinus bradycardia and no evidence of cardiogenic shock. Her response to atropine might have been due to vasovagal syncope. No microorganisms were found in culture before administration of antibiotics, and no evidence of infection was found. Although elevated CRP and procalcitonin (PCT) levels are common in septic conditions, those circumstances can be seen in other conditions, such as in anaphylactic shock[12]. Also, CRP and PCT levels can be increased by various etiologies other than infection[13]. Although autonomic dysreflexia is seen in SCI patients, she had experienced no prior acute hypertensive emergency and reported no noxious stimulus. Rather than autonomic dysreflexia, loss of sympathetic tone might have contributed to bradycardia or hypothermia. Therefore, a non-infectious and normal pathologic response was the suspected reason for her shock[14,15].
Tamsulosin and malignant vasovagal syncope remained possible causes of her life-threatening hypotension. However, vasovagal syncope is a common disorder and does not usually cause life-threatening situations. The term “malignant vasovagal syncope” is used to explain a rare unexplained syncope that is recurrent and can cause serious disability[16,17]. Because the patient recovered soon after stopping tamsulosin and had no further severe events, malignant vasovagal syncope was excluded as the cause of her severe hypotension. Excluding all other possible causes and combined with the absence of an additional severe event, we concluded that tamsulosin was the reason for her symptoms.
Furthermore, considering her history of diabetes mellitus (DM), her shock might have been due to autonomic neuropathy. Diabetes-associated cardiovascular autonomic neuropathy affects the autonomic nerve that innervates the heart and blood vessels and can cause multi-organ failure[18]. However, autonomic neuropathy due to DM was less likely because her blood glucose level had been well-controlled.
Though no other such cases have been reported, use of tamsulosin could cause severe side effects in people with SCI. We focused on orthostatic hypotension and vasovagal symptoms as risk factors of severe adverse effects of tamsulosin in people with SCI. Cardiovascular complications, including orthostatic hypotension, are frequently seen in people with SCI and are often attributed to sympathetic nervous system disturbances, loss of supraspinal control, and peripheral α-adrenoreceptor hyperresponsiveness[19,20]. With disturbances in cardiovascular systems in people with SCI, adverse effects of tamsulosin are possible. In people with SCI suffering orthostatic hypotension and vasovagal symptoms, tamsulosin could affect BP based on underlying pathophysiology of orthostatic hypotension seen in SCI. Additionally, the duration of use of tamsulosin should be analyzed. Bird et al[6] showed that the incidence of severe hypotension increased during the first 8 wk after administering tamsulosin to people with BPH[6]. The patient in the presented case suffered severe hypotension after 9 days of treatment with tamsulosin. The phrase “first-dose phenomenon” could be applied with use of tamsulosin in patients with SCI as well as in patients with BPH[6].
There were some limitations in this case report. First, we tended to manage urinary retention too strictly when using tamsulosin. The patient was able to self-void, and residual urine was in the range of 160-200 mL. Though we could have managed her with self-voiding and timed CIC, considering her cervical myelopathy, we thought that strict management of urinary retention with tamsulosin was needed to prevent urinary tract infection and autonomic dysreflexia. Second, we could not perform urodynamic study because the patient refused. In general, urodynamic study should be performed and used as the basis for appropriate treatments to manage voiding difficulty of SCI patients. We should have persuaded the patient to proceed with the urodynamic study. Third, we did not examine hemoglobin A1c level of the patient. However, in DM patients, hemoglobin A1c level should be measured because it reflects the mean blood sugar over the previous weeks to months[21].
This is the first reported case of severe life-threatening hypotension induced by tamsulosin in an SCI patient. Hence, we report this case to increase awareness when administering tamsulosin for neurogenic bladder to patients with SCI. Additional studies should be performed in patients with SCI treated with tamsulosin to reveal possible severe side effects.
| 1. | Chapple C, Andersson KE. Tamsulosin: an overview. World J Urol. 2002;19:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Michel MC, Bressel HU, Goepel M, Rübben H. A 6-month large-scale study into the safety of tamsulosin. Br J Clin Pharmacol. 2001;51:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Abrams P, Amarenco G, Bakke A, Buczyński A, Castro-Diaz D, Harrison S, Kramer G, Marsik R, Prajsner A, Stöhrer M, Van Kerrebroeck P, Wyndaele JJ; European Tamsulosin Neurogenic Lower Urinary Tract Dysfunction Study Group. Tamsulosin: efficacy and safety in patients with neurogenic lower urinary tract dysfunction due to suprasacral spinal cord injury. J Urol. 2003;170:1242-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Meyer LE, Brown JN. Tamsulosin for voiding dysfunction in women. Int Urol Nephrol. 2012;44:1649-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Zhang HL, Huang ZG, Qiu Y, Cheng X, Zou XQ, Liu TT. Tamsulosin for treatment of lower urinary tract symptoms in women: a systematic review and meta-analysis. Int J Impot Res. 2017;29:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Bird ST, Delaney JA, Brophy JM, Etminan M, Skeldon SC, Hartzema AG. Tamsulosin treatment for benign prostatic hyperplasia and risk of severe hypotension in men aged 40-85 years in the United States: risk window analyses using between and within patient methodology. BMJ. 2013;347:f6320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Kumar D, Khan FA. Tamsulosin-induced severe hypotension during general anesthesia: a case report. J Med Case Rep. 2010;4:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Snapper H, Cheshire WP. Oral and intravenous hydration in the treatment of orthostatic hypotension and postural tachycardia syndrome. Auton Neurosci. 2022;238:102951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Bushkov FA. [Orthostatic hypotension in patients with posttraumatic cervical myelopathy]. Zh Nevrol Psikhiatr Im S S Korsakova. 2019;119:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Gaitonde S, Malik RD, Christie AL, Zimmern PE. Bethanechol: Is it still being prescribed for bladder dysfunction in women? Int J Clin Pract. 2019;73:e13248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Diokno AC, Koppenhoefer R. Bethanechol chloride in neurogenic bladder dysfunction. Urology. 1976;8:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kim YJ, Kang SW, Lee JH, Cho JH. Marked elevation of procalcitonin level can lead to a misdiagnosis of anaphylactic shock as septic shock. Int J Infect Dis. 2015;37:93-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005;351:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Balk RA. Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence. 2014;5:20-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 15. | Bonanno FG. Clinical pathology of the shock syndromes. J Emerg Trauma Shock. 2011;4:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Biffi M, Boriani G, Sabbatani P, Bronzetti G, Frabetti L, Zannoli R, Branzi A, Magnani B. Malignant vasovagal syncope: a randomised trial of metoprolol and clonidine. Heart. 1997;77:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Maloney JD, Jaeger FJ, Fouad-Tarazi FM, Morris HH. Malignant vasovagal syncope: prolonged asystole provoked by head-up tilt. Case report and review of diagnosis, pathophysiology, and therapy. Cleve Clin J Med. 1988;55:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Agashe S, Petak S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc J. 2018;14:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Claydon VE, Steeves JD, Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord. 2006;44:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 202] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 417] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Saudek CD, Brick JC. The clinical use of hemoglobin A1c. J Diabetes Sci Technol. 2009;3:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dai DL, China; Ichihara K, Japan; Wang G, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR