Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.9004
Peer-review started: March 14, 2022
First decision: April 8, 2022
Revised: April 24, 2022
Accepted: July 29, 2022
Article in press: July 29, 2022
Published online: September 6, 2022
Processing time: 165 Days and 5.1 Hours
Kaposi sarcoma and post-transplant lymphoproliferative disorder have been occasionally reported in post-liver transplant patients. However, the simultaneous occurrence of these two diseases in the same lymph nodes is very rare.
We report the case of a 19-mo-old boy, who presented with intermittent fever and enlarged cervical lymph nodes after liver transplantation. Six cervical lymph nodes were biopsied, and the histopathological examinations revealed multifocal hyperplasia of spindle cells around small blood vessels, extravasated erythrocytes, and heavy infiltration of plasma cells in the cortex and medulla of the lymph nodes. The immunohistochemical analyses of spindle cells revealed positive expression of CD34, CD31, erythroblast transformation-specific-related gene, friend leukemia integration 1, and human herpesvirus-8. The lymphoproliferative lesions expressed CD38, CD138, and multiple myeloma 1. Epstein-Barr encoded RNA in situ hybridization demonstrated Epstein-Barr virus-positive lymphoid cells. Finally, we diagnosed the coexistence of Kaposi sarcoma and post-transplant lymphoproliferative disorder (plasmacytic hyperplasia) in the same lymph nodes. Treatment strategy included anti-CD20 monoclonal antibody (rituximab) and discontinuation of the immunosuppressant therapies. Lymph node biopsies during follow-up examinations revealed lymphoid hyperplasia.
The rare coexistence of Kaposi sarcoma and post-transplant lymphoproliferative disorder in the same lymph nodes post-liver transplantation possibly associates with immunodeficiency and Epstein-Barr virus and human herpesvirus-8 coinfection.
Core Tip: We report a rare case of coexistent Kaposi sarcoma and post-transplant lymphoproliferative disorder in the same lymph nodes after a pediatric liver transplant. The definitive diagnosis was based on histopathological examination of the lymph nodes. The patient recovered after discontinuation of the immunosuppressants and application of anti-CD20 monoclonal antibody (rituximab) therapy. In conclusion, the concurrent occurrence of these two disorders may be associated with immunodeficiency as well as Epstein-Barr virus and human herpesvirus-8 coinfection.
- Citation: Zhang SH, Chen GY, Zhu ZJ, Wei L, Liu Y, Liu JY. Coexistent Kaposi sarcoma and post-transplant lymphoproliferative disorder in the same lymph nodes after pediatric liver transplantation: A case report. World J Clin Cases 2022; 10(25): 9004-9011
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/9004.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.9004
Post-liver transplantation patients suffer from an increased risk of developing various lesions, including Kaposi sarcoma (KS)[1,2] and post-transplant lymphoproliferative disorder (PTLD)[3-6]. KS mainly involves the skin and occasionally the lymph nodes and stomach[1,2], while PTLD can occur in multiple organs, including the lymph nodes and the gastrointestinal tract[5] after liver transplantation. However, these two different disorders rarely coexist in the same lymph node. In fact, to date, there have been only 7 case reports[7-12], particularly of acquired immunodeficiency syndrome (AIDS), non-AIDS, and kidney transplantation patients, describing the co-occurrence of KS and lymphoid tissue lesions in the same lymph node. These cases are summarized in Table 1. To the best of our knowledge, there is no case report regarding the concurrent occurrence of KS and PTLD in the same lymph nodes of a pediatric liver transplant patient.
| Case | Ref. | Age (yr) | Sex | Past illness | Type of lymphoid tissue lesion | Treatment | Outcome |
| 1 | Licci et al[7] | 59 | Male | AIDS | DLBCL | ND | ND |
| 2 | Licci et al[7] | 58 | Male | None | TCRLBCL | ND | ND |
| 3 | Fernandes et al[8] | 18 | Female | None | TCRLBCL | ND | ND |
| 4 | Paksoy[9] | 30 | Male | AIDS | DLBCL | ND | ND |
| 5 | Kankaya et al[10] | 57 | Male | None | NLPHL | Without any therapy | Alive (8 yr) |
| 6 | Ngan and Kuo[11] | 61 | Female | None | CHL | Chemotherapy | Alive (13 mo) |
| 7 | Sabeel et al[12] | 30 | Female | ESRD PTLD | B-NHL Subtype: ND | Discontinued cyclosporine; intravenous acyclovir | Deceased (5 wk) |
In our hospital, the overall incidence of PTLD and KS in post-pediatric liver transplant recipients was 5.4% (43/789) and 0.13% (1/789), respectively, from 2013 to 2021. Only 1 of these cases had KS complicated with PTLD in the same lymph nodes. Herein, we report this unusual case of KS and PTLD coexistence in the same lymph nodes of a 19-mo-old boy after liver transplantation.
A 19-mo-old Asian boy with intermittent fever for 2 mo was admitted to the hospital.
The patient presented with facial edema and diarrhea after 6 mo of undergoing liver transplant surgery.
The patient had a history of past illness, including congenital biliary atresia, cholestatic cirrhosis, and hepatic encephalopathy. He was diagnosed with congenital biliary atresia at the age of 1.5 mo. A Kasai procedure had been performed on the patient at the age of 2 mo to treat his congenital biliary atresia. However, the procedure failed to reach the desired outcome, and he ultimately received a living donor liver transplant at the age of 13 mo. After liver transplantation, the young boy was treated with intravenous methylprednisolone as induction therapy, followed by an immunosuppressive regimen of tacrolimus (FK506) + methylprednisolone. The methylprednisolone dose was gradually reduced and ultimately discontinued 3 mo after the liver transplantation.
His father had provided the donor liver. Additionally, while his mother had a history of virus infection (hepatitis B), the virus was not transmitted to the child.
Physical examination upon admission showed several swollen lymph nodes that were palpable in both the anterior and posterior regions of the patient’s neck.
Laboratory investigations showed: hemoglobin, 64 g/L; white blood cells, 6.4 × 109; platelets, 6.4 × 109; alanine transaminase, 42 U/L; aspartate aminotransferase, 62 U/L; alkaline phosphatase, 314 U/L; and gamma-glutamyl transferase, 47 U/L. The patient was serum Epstein-Barr virus (EBV)-DNA positive, which was negative before liver transplantation.
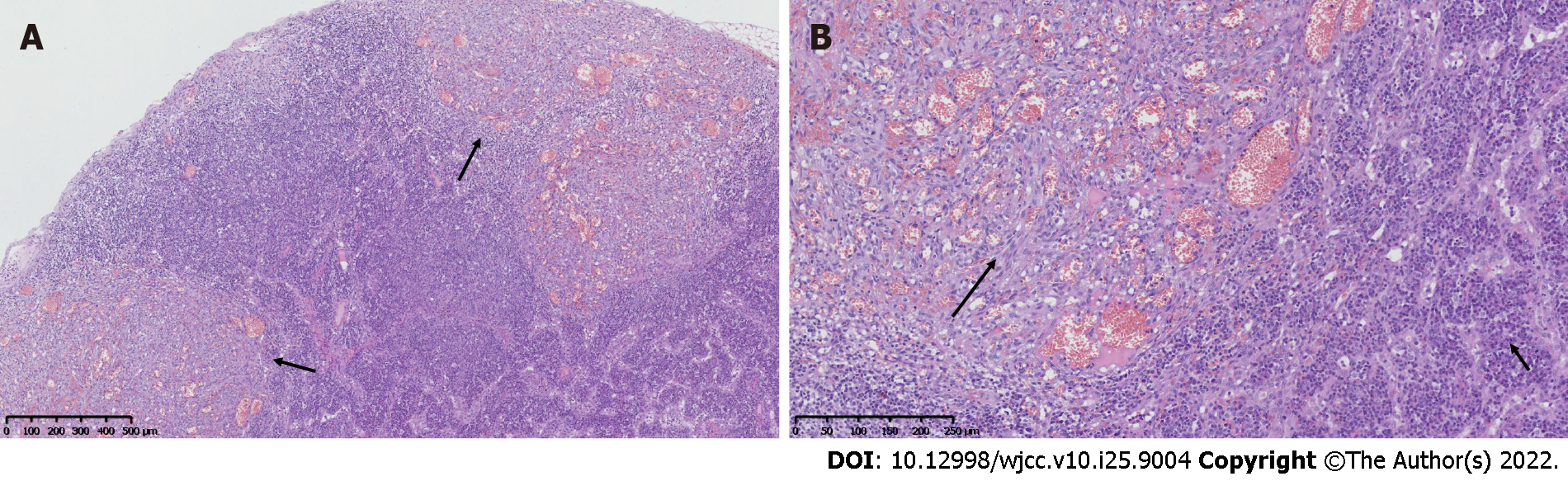
Clinicians suspected PTLD of lymph nodes. To decide the next therapeutic plan, six of the patient’s left cervical lymph nodes were excised. The histopathological examinations revealed multifocal hyperplasia of spindle cells around small blood vessels, particularly beneath the capsules of the lymph nodes (Figure 1A). Additionally, extravasated erythrocytes and a relatively scanty amount of inflammatory infiltrate were present. The spindle cells had minimal atypia (Figure 1B), and mitotic figures were absent. The cortical and medullary areas were infiltrated by numerous plasma cells along with small lymphocytes and eosinophils (Figure 1B).
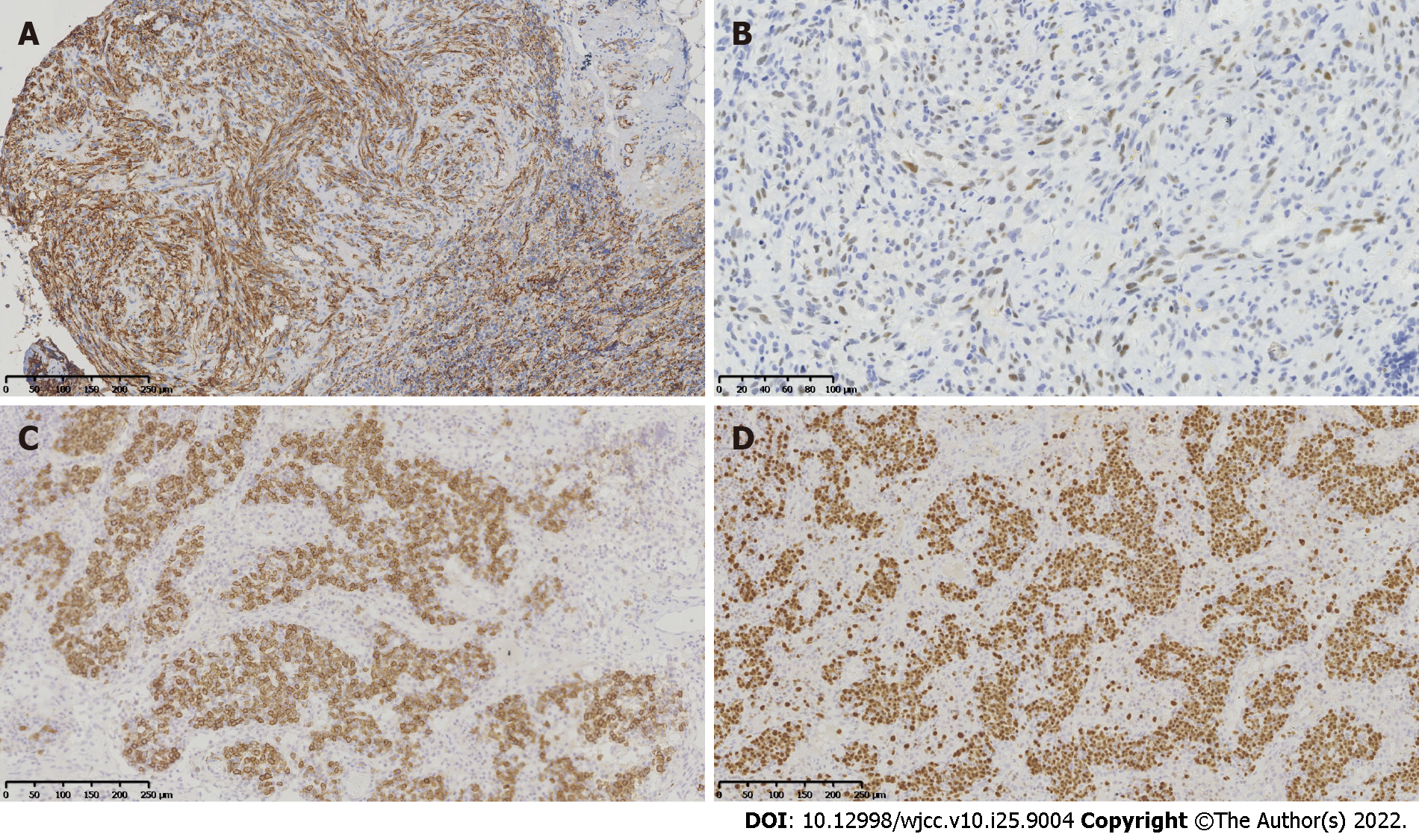
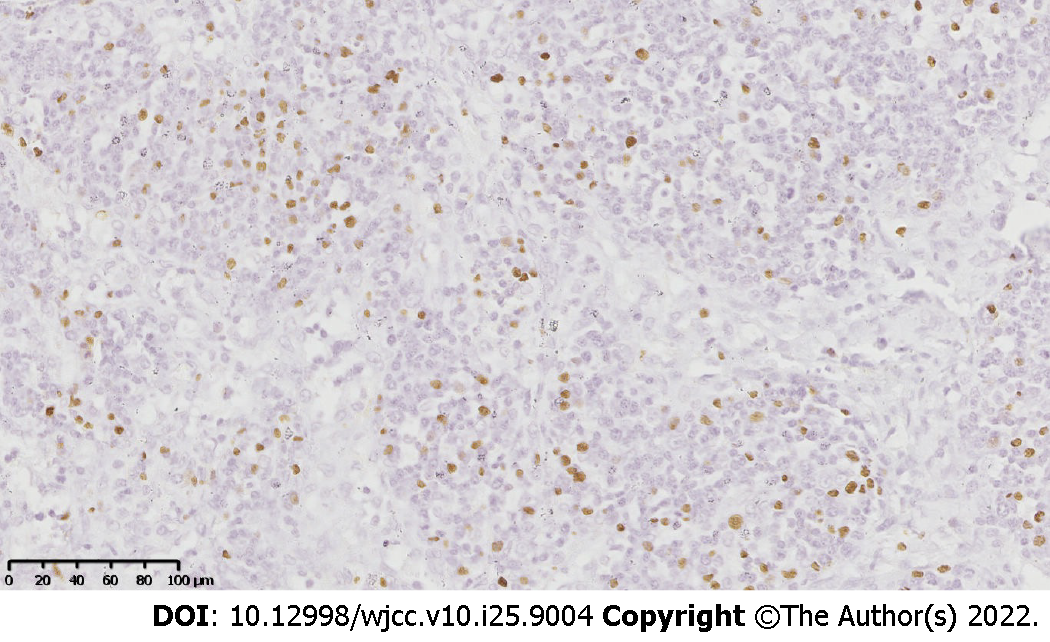
Immunohistochemical staining of the lymph node tissues revealed notable phenotypic features of the spindle cells, such as a diffuse expression of CD34 (Figure 2A), CD31, erythroblast transformation-specific-related gene, and friend leukemia integration 1 transcription factor, as well as a partially-positive nuclear staining for human herpesvirus-8 (HHV8; Figure 2B). In the lymphoproliferative lesions, CD38, CD138 (Figure 2C), and multiple myeloma 1 (Figure 2D) were highly expressed, while CD20 was partially expressed. Moreover, the kappa/lambda ratio was approximately 1:1. Incidentally, the spindle cells and the lymphoproliferative lesions exhibited proliferation rates of approximately 25% and 10%, respectively. The Epstein-Barr encoded RNA in situ hybridization revealed EBV-positive lymphoid cells (Figure 3). The B-cell clonality was evaluated using PCR amplification, and the results revealed IgH, IgK, and IgL polyclonal gene rearrangements.
Ultrasonography and computed tomography scanning revealed that multiple cervical lymph nodes were enlarged on both sides.
The results confirmed concurrent KS and non-destructive PTLD, particularly plasmacytic hyperplasia, within the same lymph nodes of the patient.
The patient received anti-CD20 monoclonal antibody (rituximab) therapy (1 cycle), and his immunosuppression therapy was discontinued. After 1 cycle of rituximab treatment, the patient’s EBV-DNA replication load reduced, but transaminase levels increased. Meanwhile, a liver needle biopsy indicated drug-induced liver injury; therefore, rituximab was not continued. Tacrolimus (FK506) was initiated again 14 d after its discontinuation. The patient’s condition improved gradually.
The patient was discharged in a stable condition 33 d after admission. However, at the ages of 60 and 71 mo, fever as well as lymph node enlargement were detected as a result of a telephone follow-up call and subsequent hospitalization. Lymph node biopsies were repeated on both occasions to exclude the relapse of PTLD and/or KS. However, all pathological results indicated lymphoid hyperplasia, and the recurrence of KS and PTLD was discarded as a possibility. The patient (109-mo-old) has been followed up for 8 years; he is in good health and attends school normally. The timeline of the patient diagnosis, treatment, and follow-up are summarized in Table 2.
| Age (mo) | Patient condition |
| 1.5 | Diagnosis: Congenital biliary atresia |
| 2 | Treatment: Kasai operation |
| 13 | Diagnosis: Cholestatic cirrhosis and hepatic encephalopathy. Treatment: Living donor liver transplantation |
| 19 | Diagnosis: Concurrent KS and non-destructive PTLD within the same lymph nodes. Treatment: Anti-CD20 monoclonal antibody (rituximab) therapy (1 cycle), discontinuation of immunosuppression |
| 60 | Diagnosis: Lymphoid hyperplasia. Treatment: Lymph node excised |
| 71 | Diagnosis: Lymphoid hyperplasia. Treatment: Lymph node excised |
| 109 | Follow-up: Alive |
Long-term use of immunosuppressive agents increases the risk of different diseases, including KS[1,2] and PTLD[3-6], in post-liver transplantation patients. However, to date, there are only 7 case reports[7-12], describing the co-occurrence of KS and lymphoid tissue lesions in the same lymph node (Table 1). The ages of these patients ranged from 18-years-old to 61-years-old, including 4 cases in non-AIDS patients, 2 cases in AIDS patients, and 1 case after kidney transplantation. There were 5 cases of coexistent with non-Hodgkin’s lymphoma and 2 cases coexistent with Hodgkin’s lymphoma. Our case is different from reported cases mentioned above. Our case describes a liver transplant patient, occurring in a child, with a type of lymphoid tissue lesion associated with PTLD, plasmacytic hy
In 1872, Moritz Kaposi[13] first described KS as a type of localized and invasive endothelial cell tumor. Based on clinical and epidemiological characteristics, KS can be divided into four types[14], namely classic, endemic, iatrogenic, and AIDS-related KS. Among them, iatrogenic KS is mainly observed in patients undergoing immunosuppressive therapy after a solid organ transplantation as well as in patients treated with immunosuppressants, notably corticosteroids, for various diseases. In fact, the incidence of iatrogenic KS is 500 times greater among organ transplant recipients as compared to that in the general population[15]. Specifically, KS has been reported in 2.00%-2.16% of adult liver transplant recipients[1,2,16], whereas its occurrence in pediatric liver transplant recipients is rare, with only a few case reports of individual recipients[17-19]. The incidence of KS in our series of liver graft recipients was 0.13% (1/789) from 2013 to 2021 in our department.
Typically, KS lesions occur on the skin, but they can also appear in internal organs and lymph nodes. However, the sole occurrence of KS in the lymph nodes is relatively rare. The KS distribution in lymph nodes can be unifocal or multifocal with a pathomorphology similar to that of KS in the skin, which includes the presence of red blood cells within slit-like spaces formed by spindle cells. The immunophenotype of KS is characterized by the positive expression of vascular endothelial cell markers, including CD31, CD34, erythroblast transformation-specific-related gene, and other relevant antigens.
Incidentally, HHV8 has an etiological role in KS, and all KS cases show almost invariable nuclear expression of HHV8, regardless of their epidemiological subtypes[14]. Hence, HHV8-positivity helps to confirm the diagnosis of KS as well as differentiate it from other vascular lesions. In our case, the immunohistochemical analyses of the spindle cells demonstrated the positive expression of CD34, CD31, erythroblast transformation-specific-related gene, and HHV8, and combined with the pathomorphological features, the diagnosis of KS was confirmed.
Incidentally, recipients of solid organ transplant or stem cell allograft can develop PTLD, which includes lymphoid or plasmacytic proliferations, as a consequence of immunosuppression[20]. Approximately 2.3%-4.3% of adult liver transplant recipients develop PTLD[3-5], whereas its incidence is as high as 9.7% in pediatric liver transplant recipients[5]. In our hospital, the overall incidence of PTLD in post-pediatric liver transplant recipients was 5.4% (43/789) from 2013 to 2021. It was slightly higher than that reported in adult liver transplantation patients. The majority of PTLD cases are “early onset PTLD,” i.e., occurring within 12 mo of liver transplantation[21]. On the contrary, “late PTLD” includes those cases that occur > 12 mo after liver transplantation. In our patient, PTLD occurred 6 mo after the liver transplant surgery, thereby making it a case of “early onset PTLD.”
PTLD is most commonly associated with EBV infection since it plays an important etiological role in PTLD. Previous studies have reported that close monitoring of the viral load of EBV in liver transplant patients is helpful to assess the risk of developing PTLD[6]. According to the “World Health Organization Classification of Tumours of Haematopietic and Lymphoid Tissues” in 2017[20], PTLD can be divided into non-destructive, polymorphic, monomorphic, and classic Hodgkin’s lymphoma-like PTLD. Our case was diagnosed as the non-destructive childhood PTLD, particularly plasmacytic hyperplasia, which tends to occur more in young individuals than the other types of PTLD.
Currently, there is no effective antiviral drug or standard treatment protocol for KS, and its treatment strategy depends on the disease staging, form of disease progression, distribution, clinical type, and patient’s immune status[19]. In cases of post-transplantation KS, if the lesion is confined to one site, then the most appropriate treatment plan is to reduce or discontinue the use of immunosuppressants, followed by a “wait and watch” period. Non-destructive PTLD often regresses spontaneously with discontinuation or reduction of immunosuppression; otherwise, it can be successfully treated by surgical excision. Our patient was followed up for the treatment of KS and was using anti-CD20 monoclonal antibody (rituximab) therapy and discontinuation of immunosuppression for the treatment of PTLD.
The association between two diseases in the same lymph nodes remains undetermined. Whether the two lesions within the same lymph nodes have a common pathogenic mechanism or if they emerged coincidently is still unclear. We believe that the patient’s immunosuppressed status after liver transplantation and the coincident EBV and HHV8 infections were the causes of the simultaneous occurrence of PTLD and KS in the same lymph nodes.
This case portrays a rare coexistence of KS and PTLD in the same lymph nodes of a pediatric post-liver transplant patient. The definitive diagnosis required histopathological analyses. In conclusion, the patient’s immunodeficient status combined with EBV and HHV8 coinfection may be associated with the concurrent occurrence of these two diseases in the same lymph nodes.
We would like to thank Teng XJ and Lan S of our department for performing the in situ hybridization as well as PCR analyses and providing us with the results.
| 1. | Di Benedetto F, Di Sandro S, De Ruvo N, Berretta M, Masetti M, Montalti R, Ballarin R, Cocchi S, Potenza L, Luppi M, Gerunda GE. Kaposi’s sarcoma after liver transplantation. J Cancer Res Clin Oncol. 2008;134:653-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Besnard V, Euvrard S, Kanitakis J, Mion F, Boillot O, Françès C, Faure M, Claudy A. Kaposi’s sarcoma after liver transplantation. Dermatology. 1996;193:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Tajima T, Hata K, Haga H, Nishikori M, Umeda K, Kusakabe J, Miyauchi H, Okamoto T, Ogawa E, Sonoda M, Hiramatsu H, Fujimoto M, Okajima H, Takita J, Takaori-Kondo A, Uemoto S. Post-transplant Lymphoproliferative Disorders After Liver Transplantation: A Retrospective Cohort Study Including 1954 Transplants. Liver Transpl. 2021;27:1165-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Mumtaz K, Faisal N, Marquez M, Healey A, Lilly LB, Renner EL. Post-transplant lymphoproliferative disorder in liver recipients: Characteristics, management, and outcome from a single-centre experience with > 1000 liver transplantations. Can J Gastroenterol Hepatol. 2015;29:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Jain A, Nalesnik M, Reyes J, Pokharna R, Mazariegos G, Green M, Eghtesad B, Marsh W, Cacciarelli T, Fontes P, Abu-Elmagd K, Sindhi R, Demetris J, Fung J. Posttransplant lymphoproliferative disorders in liver transplantation: a 20-year experience. Ann Surg. 2002;236:429-36; discussion 436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Liu Y, Sun LY, Zhu ZJ, Wei L, Qu W, Wang L, Yuan LL, Zeng ZG. Post-transplant lymphoproliferative disorder after paediatric liver transplantation. Int J Clin Pract. 2021;75:e13843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Licci S, D’Antonio A, Boscaino A, Morelli L, Piscioli F, Abbate I, Donnorso RP, Del Nonno F. Non-Hodgkin lymphomas concurrent with HHV8-associated Kaposi’s sarcoma in the same lymph node in AIDS and non-AIDS patients. Acta Haematol. 2007;118:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Fernandes F, Eloy C, Carimo A, Pinto P, Graves S, Simões J, Carrilho C, Lopes JM. Simultaneous presentation of Kaposi sarcoma and HHV8-associated large B-cell lymphoma in the same lymph node: A rare diagnosis in an HIV-negative patient. Am J Case Rep. 2013;14:263-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Paksoy N. Simultaneous occurrence of Kaposi sarcoma and tuberculosis; Kaposi sarcoma and lymphoma in the same lymph node: a report on two HIV-positive patients from Zimbabwe. Rev Soc Bras Med Trop. 2019;52:e20180188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Kankaya D, Kaygusuz G, Kuzu I, Savaş B, Bakanay ŞM, Özcan M. Simultaneous occurrence of Kaposi’s sarcoma and nodular lymphocyte predominant subtype of Hodgkin’s lymphoma in the same lymph node. Turk J Haematol. 2009;26:201-203. [PubMed] |
| 11. | Ngan KW, Kuo TT. Simultaneous occurrence of Hodgkin’s lymphoma and Kaposi’s sarcoma within the same lymph nodes of a non-AIDS patient. Int J Surg Pathol. 2006;14:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Sabeel A, Qunibi W, Alfurayh O, al-Meshari K. Simultaneous development of Kaposi’s sarcoma and lymphoma in a renal transplant recipient. Am J Kidney Dis. 1998;31:706-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kaposi M. Idiopatisches multiples pigmentsarkom der haut. Arch Dermatol Syph. 1872;3:265-273. [RCA] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 530] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Fletcher CD, Hogendoorn PC, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon: IARC, 2013: 151-154. |
| 16. | García-Astudillo LA, Leyva-Cobián F. Human herpesvirus-8 infection and Kaposi’s sarcoma after liver and kidney transplantation in different geographical areas of Spain. Transpl Immunol. 2006;17:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Yuksekkaya HA, Arikan C, Yazici A, Baran M, Aydogdu S, Kilic M. Successful treatment of a child having generalized Kaposi’s sarcoma after living donor liver transplantation with conversion to sirolimus. Pediatr Transplant. 2009;13:375-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Celtik C, Unüvar A, Aydoğan A, Gökçe S, Oztürk G, Güllüoğlu M, Yılmaz G, Türkoğlu S, Anak S, Sökücü S, Durmaz O. Human herpes virus type 8-associated Kaposi sarcoma in a pediatric liver transplant recipient. Pediatr Transplant. 2011;15:E100-E104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Şen HS, Ateş BT, Yılmazbaş P, Ocak S, Kırımlıoğlu H, Gökçe S, Acarlı K. Successful treatment of pediatric post-liver transplant Kaposi`s sarcoma with paclitaxel. Turk J Pediatr. 2020;62:858-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC, 2017: 586. |
| 21. | Khedmat H, Taheri S. Early onset post transplantation lymphoproliferative disorders: analysis of international data from 5 studies. Ann Transplant. 2009;14:74-77. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Khosravi M, Iran; Ni X, China; Rezus E, Romania; Wang JY, Taiwan S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP