Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8718
Peer-review started: March 14, 2022
First decision: May 11, 2022
Revised: May 24, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 26, 2022
Processing time: 155 Days and 0.2 Hours
Myasthenia gravis (MG) is an autoimmune disorder caused by neuromuscular junction failure characterized by muscle weakness and fatigability. We herein report a case of MG that received intravascular laser irradiation of blood (ILIB) interventions and regained muscle power and better quality of life. To our knowledge, no previous study has investigated the benefits of ILIB treatment on patients with MG. We also evaluated the changes in brain perfusion scan and the MG activities of daily living (MG-ADL) and quantitative MG (QMG) scales.
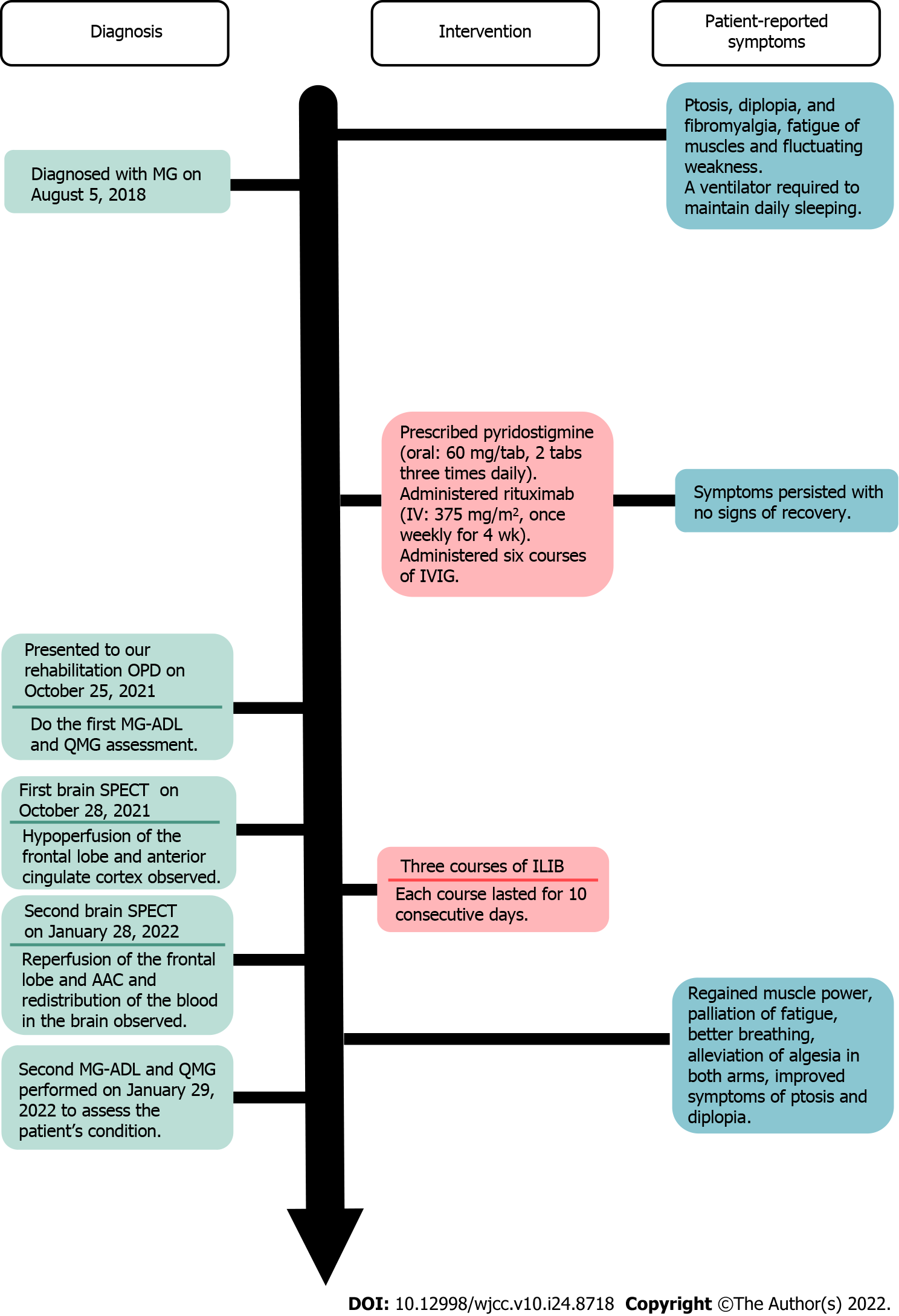
A 59-year-old man presented to our outpatient hospital experiencing ptosis, diplopia, fibromyalgia, muscle fatigue, and fluctuating weakness in his limbs for 1 year. Based on his history, physical examination, and laboratory investigations, the final diagnosis was a flare-up of MG with poor endurance and muscle fatigue. The patient agreed to receive ILIB. Brain single-photon emission computed tomography (SPECT) was performed both before and after ILIB therapy. After receiving three courses of ILIB, the brain SPECT images showed greatly increased perfusion of the frontal lobe and anterior cingulate gyri. The patient’s MG-ADL scale score decreased markedly from 17/24 to 3/24. The QMG scale score also decreased remarkably from 32/39 to 9/39. The symptoms of MG became barely detectable and the patient was able to perform his activities of daily living and regain muscle power.
ILIB might have beneficial effects on MG, and brain SPECT images provided direct evidence of a positive correlation between ILIB and clinical performance.
Core Tip: Myasthenia gravis (MG) is an autoimmune disease without effective treatments. We herein report a case of intractable MG administered a novel therapy, intravascular laser irradiation of blood interventions (ILIB), which regained muscle power and improved quality of life. To our knowledge, this is the first study investigating the benefits of ILIB treatment in patients with MG. We also evaluated the changes in brain perfusion scan and MG activities of daily living scale, and quantitative MG scale scores. We report this case to trigger further research and facilitate the recovery of patients with MG.
- Citation: Lan CH, Wu YC, Chiang CC, Chang ST. Effects of intravascular photobiomodulation on motor deficits and brain perfusion images in intractable myasthenia gravis: A case report. World J Clin Cases 2022; 10(24): 8718-8727
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8718.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8718
Myasthenia gravis (MG) is an autoimmune disease that occurs due to the failure of neuromuscular transmission resulting from antibodies against the acetylcholine receptor, muscle-specific kinase, lipoprotein-related protein 4, or agrin in the postsynaptic membrane at the neuromuscular junction[1-4]. During the initial lifetime course of MG, patients often present with ocular symptoms of ptosis and diplopia[5,6]. About half of these patients progress to generalized disease within 2 years[7]. The clinical manifestation of this disorder is fluctuation and variable weakness in the ocular, bulbar, limb, and respiratory muscles, which lead to major symptoms such as dysarthria, dysphagia, fatigable chewing, fatigable limb, or axial weakness. Skeletal muscle fatigue manifests as weakened muscle contractile force[8-10].
The conventional treatment of MG usually includes a combination of symptomatic therapy with acetylcholinesterase inhibitors (e.g., pyridostigmine), immunosuppressive drugs, and immunotherapy using either intravenous immunoglobulin (IVIG) or plasma exchange in selected patients thymectomy[10-14]. The common therapeutic goal of these treatments is to help patients return to normal function as soon as possible while minimizing the side effects. However, patients receiving these treatments often experience adverse side effects and have difficulties in recovering[15].
A novel alternative therapy is intravascular photobiomodulation, also known as intravascular laser irradiation of blood (ILIB). ILIB treatment utilizes a helium-neon laser with a wavelength of 632.8 nm (red light). An optic fiber is inserted into a superficial vein to deliver the laser light[16]. ILIB is considered an alternative treatment for diseases such as chronic spinal cord injury, cerebral stroke, traumatic brain injury, rheumatoid arthritis, chronic Sjögren’s syndrome, fibromyalgia, and chronic pain conditions, due to its effects in increasing microcirculation and improving oxygen supply[17-21]. Before this case, the usefulness of ILIB in patients with MG had not been reported. This study presents a case diagnosed with MG that was treated with ILIB therapy, which resulted in regained muscle power and improved quality of life.
A 59-year-old man experienced ptosis, diplopia, fibromyalgia, muscle fatigue, and fluctuating limb weakness for 1 year. Worsening weakness in bilateral lower limbs caused walking disability. The patient then came to our outpatient hospital in hopes of regaining muscle power and better walking ability.
The patient’s symptoms had started 1 year ago with recurrent episodes of weakness in four limbs. His eyelid dropped soon after he woke up and double vision appeared spontaneously. He also required a ventilator to maintain his daily sleeping.
The patient was initially diagnosed with MG on August 5, 2018, accompanied by severe ptosis, diplopia, and fibromyalgia. He visited a neurologist in a local medical center, who prescribed acetylcholinesterase inhibitor, pyridostigmine (oral: 60 mg/tab, 2 tabs three times daily), for 1 year. Later, the patient was treated with immunosuppressive drugs; namely, rituximab (intravenously: 375 mg/m2, once weekly for 4 wk), and received six courses of IVIG to improve his conditions. However, the severe ptosis, diplopia, and fibromyalgia persisted without any sign of recovery. The patient reported no improvements in the weaknesses of his bilateral lower limbs, which caused walking disability and muscle fatigue, which severely affected his daily life.
The patient had no significant personal or family history.
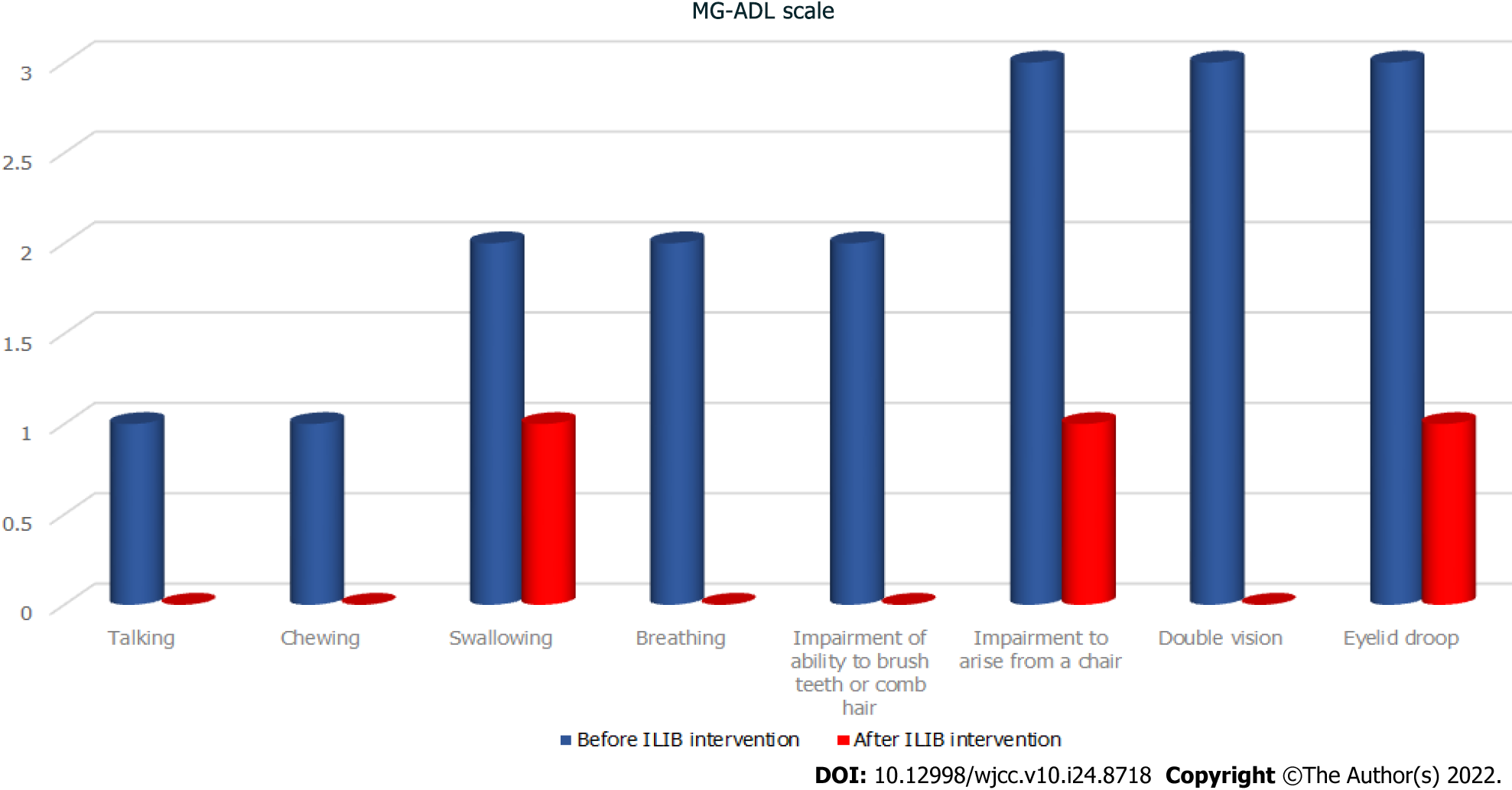
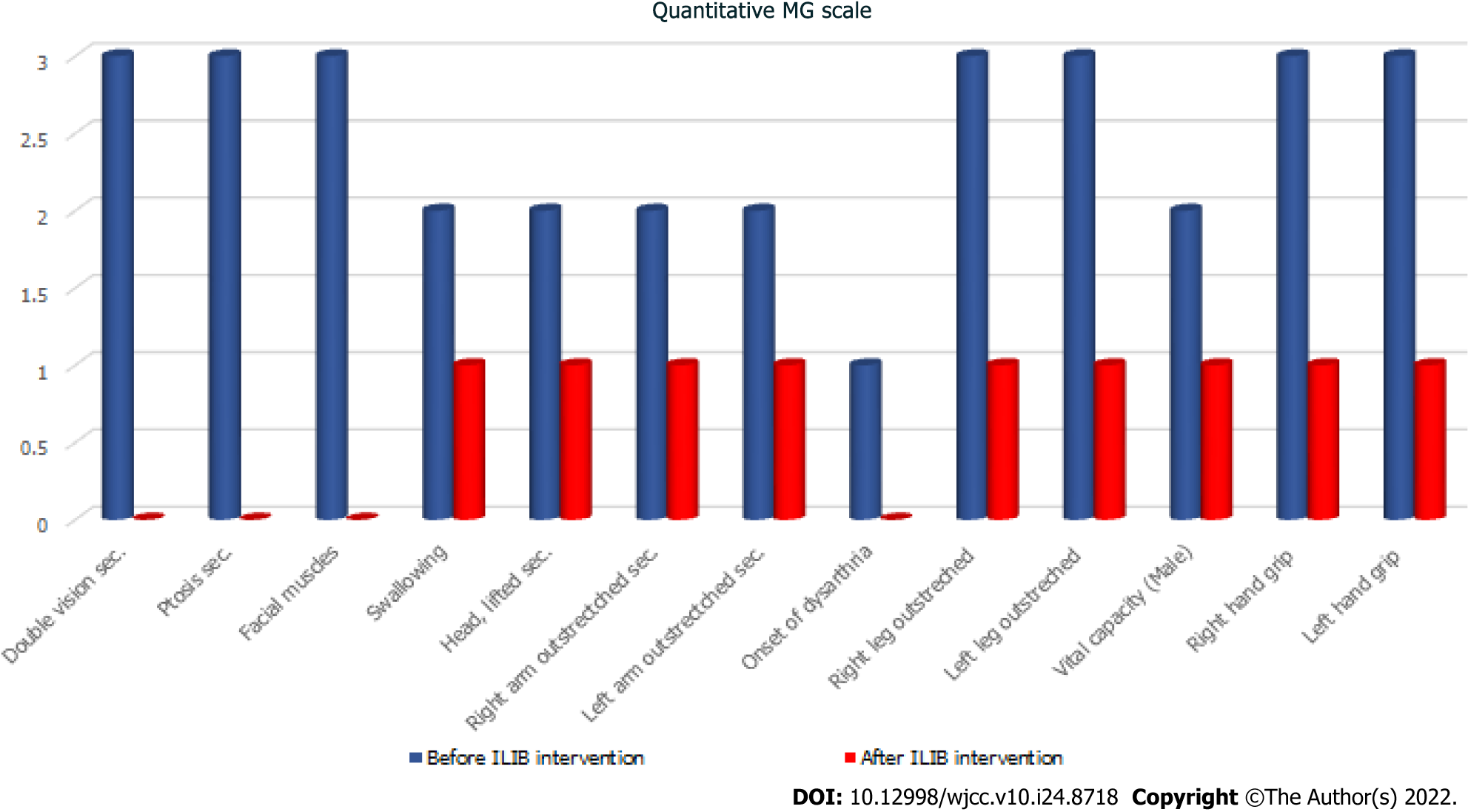
During his visit, the MG activities of daily living scale (MG-ADL scale)[22] total score was 20/24 (Figure 1, Table 1), while that for the quantitative MG scale (QMG scale)[23] was 32/39 (Figure 2, Table 2). The patient’s vital signs were within the normal ranges. Repetitive stimulation test at 3 Hz revealed decremental responses (11.8%) in both orbicularis oculi muscles but not in the right abductor pollicis brevis muscle, which suggested the need to consider post-synaptic neuromuscular junctional disorder.
| MG-ADL scale | Before ILIB intervention | After ILIB intervention |
| Talking | 1 | 0 |
| Chewing | 1 | 0 |
| Swallowing | 2 | 1 |
| Breathing | 2 | 0 |
| Impairment of ability to brush teeth or comb hair | 2 | 0 |
| Impairment to arise from a chair | 3 | 1 |
| Double vision | 3 | 0 |
| Eyelid droop | 3 | 1 |
| Total | 17 | 3 |
| Quantitative MG scale | Before ILIB intervention | After ILIB intervention |
| Double vision sec. | 3 | 0 |
| Ptosis sec. | 3 | 0 |
| Facial muscles | 3 | 0 |
| Swallowing | 2 | 1 |
| Head, lifted sec. | 2 | 1 |
| Right arm outstrectched sec. | 2 | 1 |
| Left arm outstrectched sec. | 2 | 1 |
| Onset of dysarthria | 1 | 0 |
| Right leg outstreched | 3 | 1 |
| Left leg outstreched | 3 | 1 |
| Vital capacity (male) | 2 | 1 |
| Right hand grip | 3 | 1 |
| Left hand grip | 3 | 1 |
| Total | 32 | 9 |
Laboratory evaluation revealed positivity for acetylcholine receptor antibody (5.97 nmol/L). The blood biochemistry examinations revealed mildly decreased complement C3 (74.8 mg/dL) levels compared to normal values (79–152 mg/dL). The urine analysis showed normal values.
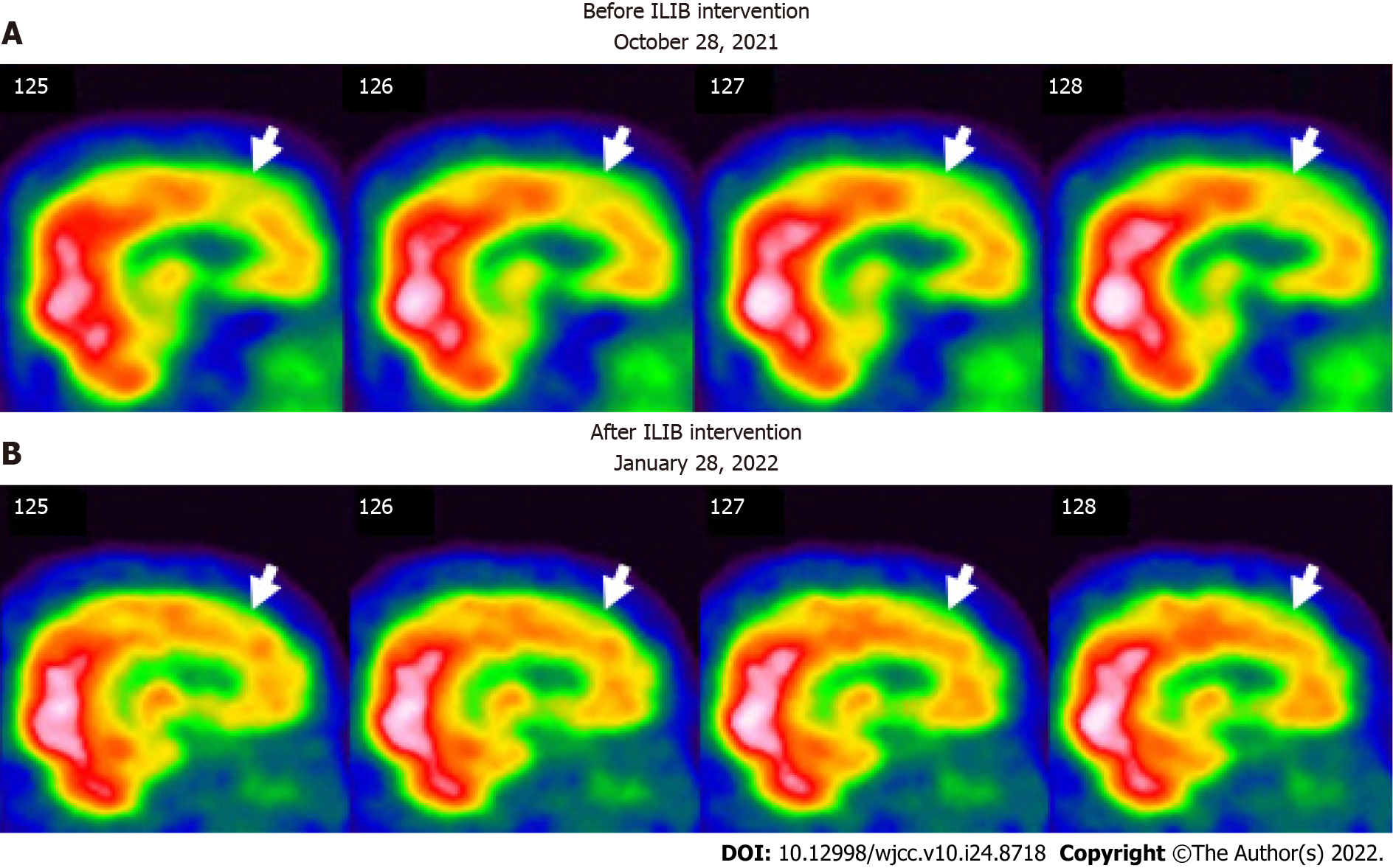
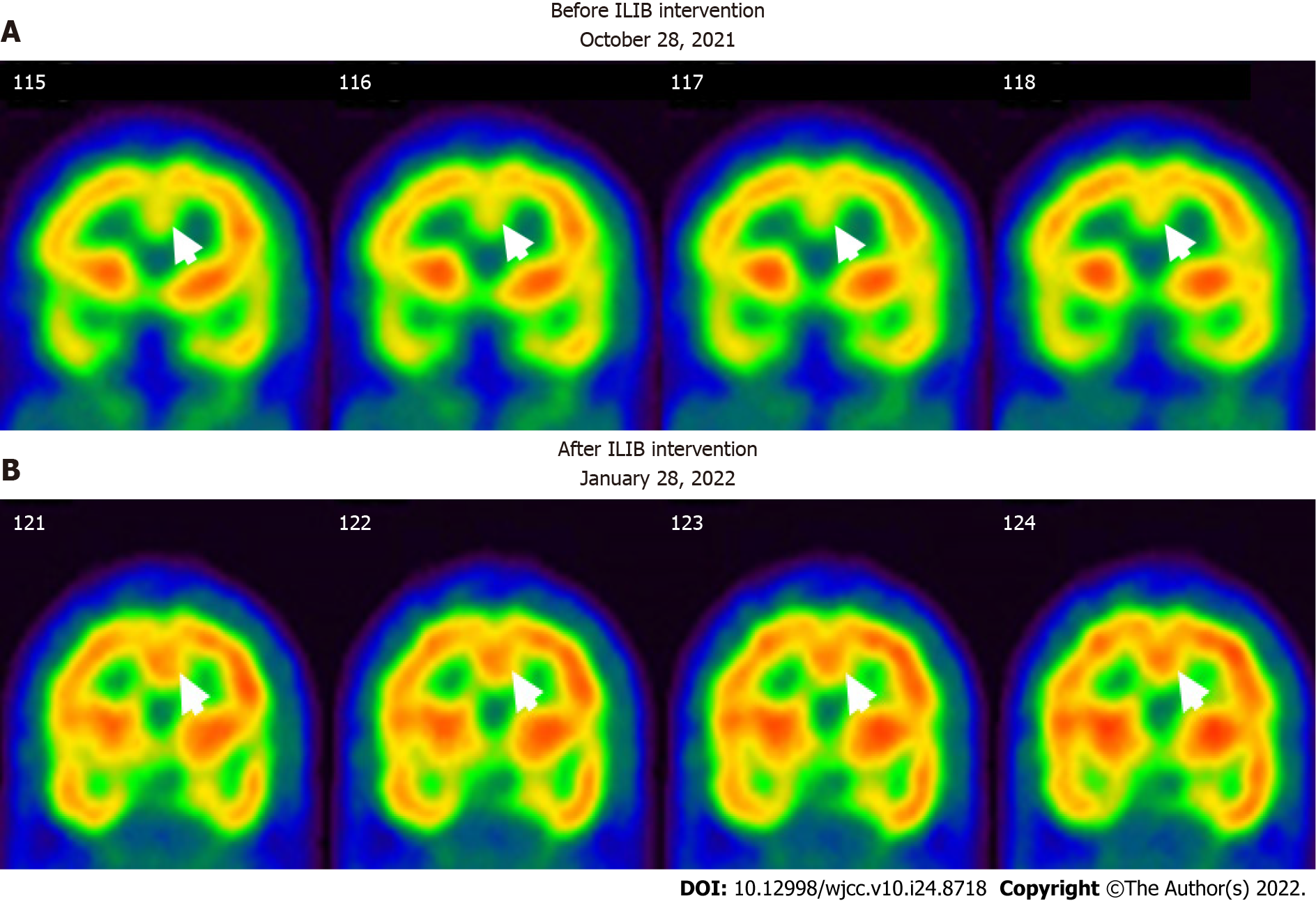
No abnormalities were noted on electrocardiograms and chest X-rays. Regional cerebral perfusion (rCBF) scans by brain single-photon emission computed tomography (SPECT) were performed 30 min after the intravenous injection of 30.8 mCi Tc-99m ECD. Easy Z-score Imaging System was used for statistical analysis. The first brain SPECT showed decreased rCBF in the frontal regions and anterior cingulate gyri (Figures 3 and 4). Magnetic resonance imaging of the chest showed no evidence of a thymic mass.
The results of the antinuclear autoantibodies (ANA) test were negative. The levels of RA factor (serum) were within normal limits (< 20 IU/mL). The levels in anti-cyclic citrullinated peptide (anti-CCP) screening were also normal (0.6 U/mL). The patient was negative for HLA-B27. Finally, the result of the myositis 16-specific Ag panel was unremarkable.
Based on the patient’s history, physical examinations, and laboratory investigations, the final diagnosis of the presented case was a flare-up of MG with poor endurance and muscle fatigue (Figure 5).
The patient was advised to undergo ILIB and agreed. He received three courses of ILIB (60 min each session for 10 consecutive days per course, with a rest interval of 1–2 wk). A helium-neon (He-Ne) laser illuminator (TAIEX He-Ne Laser, YJ-ILIB-5, Bio-ILIB Human Energy Corporation, Taiwan) was applied with a wavelength of 632.8 nm, energy of 12.6 to 14.4 J, energy density of 6428.57 J/cm2, power output of 2.5–3.5 w/cm2, power intensity of 1.79–2.04 w/cm2, and irradiation time of 3600 s/session[20]. The laser power was adjusted depending on the patient’s responses.
After completing three courses of ILIB, the patient’s double vision and eyelid-dropping were remarkably improved, with his MG-ADL scale total score decreasing from 17/24 to 3/24. The weaknesses in both upper and lower limbs changed as anticipated. In the QMG scale, the time for both arms outstretched (90° standing) increased from 0–10 s to 90–240 s, while that for both legs’ outstretched (45° supine) increased from 0–10 s to 90–240 s. The patient’s breathing also improved. The algesia of both arms caused by fibromyalgia was also alleviated. Notably, the second total score of the QMG Scale decreased from 32/39 to 9/39. The second brain SPECT showed increased activities in the frontal regions and anterior cingulate gyri (Figures 3 and 4). The patient is content with the efficacy of ILIB treatment.
After several years of conventional treatments, including acetylcholinesterase inhibitors and immunosuppressive drugs without subjective improvement, the relatively rapid improvement in motor and respiratory function during ILIB treatment in our case suggests that the patient benefited significantly from ILIB therapy. This patient also made great clinical progress, according to his MG ADL and QMG scale scores. This is the first case report on the novel treatment of MG with ILIB. This is also the first study to describe the benefit of ILIB as a treatment for impaired motor function and analgesia by SPECT imaging of a patient with MG.
We used regional perfusion brain SPECT images to assess brain function before and after the ILIB intervention. We observed two intriguing findings in this study of a patient with MG.
First, we observed remarkably increased perfusion in the motor areas of the frontal lobe (Figure 3), which indicated a strong relationship between motor function and frontal lobe activity. Recent studies showed that ILIB therapy improved regional cerebral blood flow and provided faster repair of the affected nervous system through increased ATP production[24,25]. The motor cortex comprises three different areas of the frontal lobe, immediately anterior to the central sulcus. These areas are the primary motor cortex (Brodmann’s area 4), the premotor cortex (PMC), and the supplementary motor area (SMA)[26]. Our SPECT images showed significantly increased perfusion in both the SMA and PMC after ILIB therapy, suggesting that blood flow might re-perfuse, contributing to the improvement in muscle weakness and fatigue.
Second, the SPECT images also showed higher activity in the anterior cingulate cortex (ACC) after ILIB therapy (Figure 4), which indicated a correlation between pain relief and the ACC. Davis et al[27] reported that the signal intensity changes within the ACC were correlated with pain intensity, sensory, cognitive processes, and motor function including voluntary movement[28]. Hence, higher activities in the ACC, considered a complicated integrative center, showed a re-distribution of blood flow in the brain, which resulted in pain relief in both arms and regained power in the skeletal muscles.
Furthermore, ILIB is considered a treatment that facilitates circulation in the frontal area of the cortex, especially in this case. ILIB may enhance muscular strength and relieve fluctuating and variable fatigue[29,30]. ILIB also plays a role as an immunomodulator through direct or indirect effects on the immune system[21], which was unbalanced in our case with MG. The post-treatment imaging study revealed that ILIB effectively facilitated circulation around the frontal area of the cortex, which improved the clinical symptoms of this patient with MG.
However, the role of the peripheral mechanism in contributing to the recovery of muscle power cannot be ignored. To sustain muscle contraction, ATP needs to be regenerated at a rate complementary to ATP demand. Three major ways are used to replenish ATP in muscle. These systems-phosphagen, glycolytic, and mitochondrial respiration-differ in the substrates used, products, maximal rate of ATP regeneration, capacity of ATP regeneration, and associated contributions to fatigue[31,32]. In addition, ILIB therapy has been reported to promote total cellular ATP synthesis, and antioxidant activity, which contributes to the alleviation of chronic conditions, such as chronic spinal cord injury and fibromyalgia[17,20]. Therefore, we believe that ILIB therapy might enhance muscle power by increasing ATP synthesis in the peripheral mechanism.
MG is an autoimmune disease that previously lacked effective treatments and detailed brain perfusion images. Our case showed the feasible management of this condition with ILIB treatment. The brain perfusion scan demonstrated increased activity in the prior deficit of the brain lesion. ILIB might have beneficial effects on MG and SPECT images could be a good monitor for any deficits in the brain.
| 1. | Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet. 2001;357:2122-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 349] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 2. | Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116:2843-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 456] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | Koneczny I, Herbst R. Myasthenia Gravis: Pathogenic Effects of Autoantibodies on Neuromuscular Architecture. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 4. | Zhang B, Tzartos JS, Belimezi M, Ragheb S, Bealmear B, Lewis RA, Xiong WC, Lisak RP, Tzartos SJ, Mei L. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol. 2012;69:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 5. | Dresser L, Wlodarski R, Rezania K, Soliven B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 6. | Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 490] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 7. | Kupersmith MJ, Latkany R, Homel P. Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Arch Neurol. 2003;60:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Hehir MK, Silvestri NJ. Generalized Myasthenia Gravis: Classification, Clinical Presentation, Natural History, and Epidemiology. Neurol Clin. 2018;36:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 9. | Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM. Myasthenia gravis. Nat Rev Dis Primers. 2019;5:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 608] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 10. | Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8:475-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 611] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 11. | Gold R, Schneider-Gold C. Current and future standards in treatment of myasthenia gravis. Neurotherapeutics. 2008;5:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Kumar V, Kaminski HJ. Treatment of myasthenia gravis. Curr Neurol Neurosci Rep. 2011;11:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14:1023-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 866] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 14. | Gajdos P, Chevret S, Toyka KV. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev. 2012;12:CD002277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Schneider-Gold C, Gilhus NE. Advances and challenges in the treatment of myasthenia gravis. Ther Adv Neurol Disord. 2021;14:17562864211065406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Moskvin SV. Low-Level Laser Therapy for Herpesvirus Infections: A Narrative Literature Review. J Lasers Med Sci. 2021;12:e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Huang SF, Tsai YA, Wu SB, Wei YH, Tsai PY, Chuang TY. Effects of intravascular laser irradiation of blood in mitochondria dysfunction and oxidative stress in adults with chronic spinal cord injury. Photomed Laser Surg. 2012;30:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Chiran DA, Weber M, Ailioaie LM, Moraru E, Ailioaie C, Litscher D, Litscher G. Intravenous laser blood irradiation and tocilizumab in a patient with juvenile arthritis. Case Rep Med. 2014;2014:923496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Yang WH, Lin SP, Chang ST. Case report: Rapid improvement of crossed cerebellar diaschisis after intravascular laser irradiation of blood in a case of stroke. Medicine (Baltimore). 2017;96:e5646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Momenzadeh S, Abbasi M, Ebadifar A, Aryani M, Bayrami J, Nematollahi F. The intravenous laser blood irradiation in chronic pain and fibromyalgia. J Lasers Med Sci. 2015;6:6-9. [PubMed] |
| 21. | Liu EY, Chang ST. Benefits of intravascular laser irradiation of blood on motor and sensory recovery viewing from brain function images: Portrait of a case with chronic Sjögren’s syndrome, transverse myelitis, and Guillain-Barré syndrome. Biomed J Sci Tech Res. 2019;14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Muppidi S. The myasthenia gravis--specific activities of daily living profile. Ann N Y Acad Sci. 2012;1274:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52:1487-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 408] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Dompe C, Moncrieff L, Matys J, Grzech-Leśniak K, Kocherova I, Bryja A, Bruska M, Dominiak M, Mozdziak P, Skiba THI, Shibli JA, Angelova Volponi A, Kempisty B, Dyszkiewicz-Konwińska M. Photobiomodulation-Underlying Mechanism and Clinical Applications. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 420] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 25. | Tafur J, Mills PJ. Low-intensity light therapy: exploring the role of redox mechanisms. Photomed Laser Surg. 2008;26:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Schlaug G, Renga V. Transcranial direct current stimulation: a noninvasive tool to facilitate stroke recovery. Expert Rev Med Devices. 2008;5:759-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol. 1997;77:3370-3380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 295] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Kwan CL, Crawley AP, Mikulis DJ, Davis KD. An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain. 2000;85:359-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Tomé RFF, Silva DFB, Dos Santos CAO, de Vasconcelos Neves G, Rolim AKA, de Castro Gomes DQ. ILIB (intravascular laser irradiation of blood) as an adjuvant therapy in the treatment of patients with chronic systemic diseases-an integrative literature review. Lasers Med Sci. 2020;35:1899-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Wang X, Tian F, Soni SS, Gonzalez-Lima F, Liu H. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep. 2016;6:30540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 31. | Baker JS, McCormick MC, Robergs RA. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J Nutr Metab. 2010;2010:905612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 32. | Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nat Metab. 2020;2:817-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 724] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Han J, China; Liu YC, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ