Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8547
Peer-review started: April 19, 2022
First decision: May 11, 2022
Revised: May 23, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: August 26, 2022
Processing time: 118 Days and 14.7 Hours
Most patients with primary hepatocellular carcinoma (HCC) have a history of chronic hepatitis B and usually present with varying degrees of cirrhosis. Owing to the special nature of liver anatomy, the blood vessel wall in the liver parenchyma is thin and prone to bleeding. Heavy bleeding and blood transfusion during hepatectomy are independent risk factors for liver cancer recurrence and death. Various clinical methods have been used to reduce intraoperative bleeding, and the Pringle method is most widely used to prevent blood flow to the liver.
To investigate the effect of half-hepatic blood flow occlusion after patients with HCC and cirrhosis undergo hepatectomy.
This retrospective study included 88 patients with HCC and liver cirrhosis who underwent hepatectomy in our hospital from January 2017 to September 2020. Patients were divided into two groups based on the following treatment methods: the research group (n = 44), treated with half-hepatic blood flow occlusion technology and the control group (n = 44), treated with total hepatic occlusion. Differences in operation procedure, blood transfusion, liver function, tumor markers, serum inflammatory response, and incidence of surgical complications were compared between the groups.
The operation lasted longer in the research group than in the control group (273.0 ± 24.8 min vs 256.3 ± 28.5 min, P < 0.05), and the postoperative anal exhaust time was shorter in the research group than in the control group (50.0 ± 9.7 min vs 55.1 ± 10.4 min, P < 0.05). There was no statistically significant difference in incision length, surgical bleeding, portal block time, drainage tube indwelling time, and hospital stay between the research and control groups (P > 0.05). Before surgery, there were no significant differences in serum alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin, and prealbumin levels between the research and control groups (P > 0.05). Conversely, 24 and 72 h after the operation the respective serum ALT (378.61 ± 77.49 U/L and 246.13 ± 54.06 U/L) and AST (355.30 ± 69.50 U/L and 223.47 ± 48.64 U/L) levels in the research group were significantly lower (P < 0.05) than those in the control group (ALT, 430.58 ± 83.67 U/L and 281.35 ± 59.61 U/L; AST, 416.49 ± 73.03 U/L and 248.62 ± 50.10 U/L). The operation complication rate did not significantly differ between the research group (15.91%) and the control group (22.73%; P > 0.05).
Half-hepatic blood flow occlusion technology is more beneficial than total hepatic occlusion in reducing liver function injury in hepatectomy for patients with HCC and cirrhosis.
Core Tip: There are differences in the selection of different blood-flow blocking techniques during hepatocellular carcinoma (HCC) surgery. We explore surgical effect of half hepatic blood flow occlusion and liver function recovery of patients with hepatocirrhosis HCC in hepatectomy.
- Citation: Liu D, Fang JM, Chen XQ. Clinical significance of half-hepatic blood flow occlusion technology in patients with hepatocellular carcinoma with cirrhosis. World J Clin Cases 2022; 10(24): 8547-8555
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8547.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8547
Hepatocellular carcinoma (HCC) accounts for 90% of all primary liver tumors[1]. Most patients with HCC often also have cirrhosis, and the recurrence of microvascular tumor thrombi has been increasing. Currently, radical surgery is the preferred treatment for HCC; however, massive intraoperative bleeding and blood transfusion may increase the incidence of postoperative complications, mortality, and the possibility of recurrence and metastasis of HCC[2,3]. The vascular distribution of the liver is more complex, and the blood supply is rich. Therefore, effective control of bleeding during hepatectomy has been the focus of liver surgery research[4]. In 1988, researchers proposed a method of vascular occlusion during hepatectomy that could be used to control intraoperative blood loss. The classical Pringle total hepatic occlusion and selective semi-hepatic occlusion techniques are mainly used in clinical practice[5]. However, there are differences in the selection of different blood-flow blocking techniques during tumor surgery. This study selected patients with HCC and liver cirrhosis scheduled to undergo hepatectomy in our hospital to explore the postoperative surgical effect of half hepatic blood flow occlusion and liver function recovery in this patient population.
This retrospective single-blinded study included 88 patients with HCC and liver cirrhosis who underwent hepatectomy from January 2017 to September 2020 in our hospital and were divided into an observation group and a control group (44 patients in each group). The inclusion criteria were based on the diagnostic criteria for patients with HCC in the code for the diagnosis and treatment of primary liver cancer (2011 edition)[6] as follows: confirmed preoperative computed tomography and magnetic resonance imaging examinations or confirmed liver biopsy, age of ≤ 75 years, mild cirrhosis, preoperative Child–Pugh liver function grade A or B[7], and tumor diameter of 2.0–6.0 cm. This study met the relevant requirements of the Medical Ethics Committee, and written informed consent was obtained from the patients. The exclusion criteria were as follows: patients with metastasis to other abdominal organs, with other malignant tumors, history of cerebrovascular or myocardial infarction within the last 6 mo, with history of parasitic diseases such as liver echinococcosis, and with anemia or malnutrition.
The research group included patients aged 50–75 years, with an average age of 60.6 ± 5.0 years (26 men and 18 women). Forty patients had preoperative Child-Pugh grade A and four patients had grade B. The mean lesion diameter was 5.18 ± 1.00 cm. History of hepatitis B virus infection was noted in 32 cases. The mean serum alpha-fetoprotein (AFP) value was 240.8 ± 75.6 ng/L. Regarding the surgical resection scope, there were ≥3 Liver segments in 18 cases and < 3 Liver segments in 26 cases. The control group included patients aged 48–75 years, with an average age of 61.3 ± 5.5 years (22 men and 22 women). Thirty-seven patients had preoperative Child-Pugh grade A and seven patients had grade B. The mean lesion diameter was 5.08 ± 1.30 cm. History of hepatitis B virus infection was noted in 27 cases, and the mean serum AFP value was 228.6 ± 66.3 ng/L. Regarding the surgical resection scope, there were ≥ 3 Liver segments in 14 cases and < 3 liver segments in 30 cases. There was no statistically significant difference in the baseline data between the groups (P > 0.05), as shown in Table 1.
| Normal information | Research group (n = 44) | Control group (n = 44) | t/χ2 value | P value |
| Age (yr) | 60.6 ± 5.0 | 61.3 ± 5.5 | -0.625 | 0.534 |
| Lesion diameter (cm) | 5.18 ± 1.00 | 5.08 ± 1.30 | 0.404 | 0.687 |
| Serum AFP (ng/L) | 240.8 ± 75.6 | 228.6 ± 66.3 | 0.805 | 0.423 |
| Sex | 0.733 | 0.392 | ||
| Male | 26 (59.09) | 22 (50.00) | ||
| Female | 18 (40.91) | 22 (50.00) | ||
| Child-Pugh stage | 1.252 | 0.263 | ||
| A stage | 40 (100) | 37 (84.09) | ||
| B stage | 4 (9.09) | 7 (15.91) | ||
| Hepatitis B virus infection | 1.286 | 0.257 | ||
| Yes | 32 (72.73) | 27 (61.36) | ||
| No | 12 (27.27) | 17 (38.64) | ||
| Surgical resection range | 0.786 | 0.375 | ||
| ≥ 3 liver segments | 18 (40.91) | 14 (31.82) | ||
| < 3 liver segments | 26 (59.09) | 30 (68.18) |
All patients underwent a right upper abdominal incision under general anesthesia. The site of the lesion and scope of resection were determined after entering the abdomen. All liver tissues were removed using forceps, and the duct with a larger wound surface was sutured and tied.
The control group was treated with the Pringle total hepatic blood flow occlusion technique, in which partial hepatectomy was performed after the hepatoduodenal ligament and entire hepatic blood flow were blocked with a normal drainage tube through the Venturi hole tightly the hepatoduodenal ligament to completely block the hepatic artery and portal vein. Every operation was controlled for 15 min, depending on the ease of operation and adjustment of the block number of operating time. If the liver tumor could not be removed within the period of closure, the operation had to be repeated for 5–10 min intermittently until the liver tumor was removed and blocked at most twice.
Half-hepatic blood flow occlusion technology was used in the research group. The first hepatic portal was dissected, and the left and right hepatic veins, left and right portal veins, and left and right hepatic ducts were bluntly separated to block the branches of the hepatic artery and portal vein on the affected side to form a local ischemic area. Hepatectomy was performed along the edge of this ischemic area. Vascular forceps were used to externally separate the hilum at the upper margin of the lateral sulcus of the affected side against the Glisson sheath. The fingers of the left hand were guided behind the hepatic portal, and the vascular forceps were threaded out from behind the Glisson sheath and blocked the affected side of the liver into the hepatic blood flow with the blocking band, for < 15 min each time, and blocked again 5 min after opening. After liver resection, the wound was treated, the blood flow pathway was opened, and an abdominal drainage tube was placed.
Operation time, incision length, surgical bleeding, portal block time, postoperative anal exhaust time, drainage tube indwelling time, and hospital stay were compared between the groups. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), prealbumin (PA), AFP, carcinoembryonic antigen (CEA), α-L fasosylase (AFU), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) levels before and after surgery, C-reactive protein (CRP) levels, and postoperative complications were also compared between the groups.
After surgery, 5 mL fasting venous blood was collected from the patient and centrifuged at 3000 rpm for 5 min; the supernatant was collected to detect serum ALT, AST, TBIL, and PA levels. TBIL levels were determined using the vanadate method. The kit was provided by Beijing Jiuqiang Biotechnology Co., Ltd. The normal reference range was 3.0–20.0 μmol/L. AST and ALT were continuously monitored by Ningbo Purui Biotechnology Co., LTD., with the normal reference range being 0–40 U/L. The PA immunotransmission turbidimetry kit was provided by Shanghai Shenfeng Biological Reagent Co., Ltd., with a normal reference range of 200–400 mg/L. The levels of AFP, AFU, and CEA in the serum were detected using a C6000 automatic immunochemiluminescence analyzer provided by Roche. All operations were carried out in strict accordance with the requirements of the kit manufactured by Wuhan Youersheng Bioengineering Co., LTD. The normal reference values of the tumor markers were: AFP < 15 ng/mL, AFU < 40 U/L, CEA < 5.0 ng/mL, and CA19-9 < 27 U/L.
SPSS 21.0 was used for data analysis. ALT, AST, TBIL, and other measurement data of the two groups are expressed as mean ± SD, and the t-test was used for inter-group comparisons. For enumeration data, the χ2 test was used for inter-group comparisons. Statistical significance was set at P < 0.05.
The operation group in the research group was longer than that of the control group (P < 0.05). Postoperative anal exhaust time in the research group was shorter than that in the control group (P < 0.05), with no statistically significant difference in incision length, surgical bleeding, portal block time, drainage tube indwelling time, and hospital stay between the operation group and the control group (P > 0.05, Table 2).
| Index | Research group (n = 44) | Control group (n = 44) | t value | P value |
| Operation time (min) | 273.0 ± 24.8 | 256.3 ± 28.5 | 2.932 | 0.004 |
| Incision length (cm) | 25.98 ± 1.55 | 26.14 ± 1.64 | -0.470 | 0.639 |
| Surgical bleeding (mL) | 626.9 ± 105.1 | 598.4 ± 97.0 | 1.322 | 0.190 |
| Portal block time (min) | 25.3 ± 4.1 | 23.5 ± 4.5 | 1.961 | 0.053 |
| Postoperative anal exhaust time (h) | 50.0 ± 9.7 | 55.1 ± 10.4 | -2.379 | 0.020 |
| Drainage tube indwelling time (d) | 3.84 ± 0.66 | 3.62 ± 0.71 | 1.505 | 0.136 |
| Hospital stay (d) | 13.6 ± 1.8 | 14.0 ± 2.2 | -0.933 | 0.353 |
Before surgery, there were no significant differences in serum ALT, AST, TBIL, and PA levels between the research and control groups (P > 0.05). Meanwhile, 24 h and 72 h after operation, the serum ALT and AST values in the research group were lower than those in the control group (P < 0.05, Table 3).
| Index | Research group (n = 44) | Control group (n = 44) | t value | P value |
| ALT (U/L) | ||||
| Preoperative | 33.04 ± 8.56 | 34.72 ± 8.11 | -0.945 | 0.347 |
| 24 h after operation | 378.61 ± 77.49a | 430.58 ± 83.67a | -3.023 | 0.003 |
| 72 h after operation | 246.13 ± 54.06a | 281.35 ± 59.61a | -2.903 | 0.005 |
| AST (U/L) | ||||
| Preoperative | 29.61 ± 7.21 | 27.30 ± 7.85 | 1.438 | 0.154 |
| 24 h after operation | 355.30 ± 69.50a | 416.49 ± 73.03a | -4.026 | 0.000 |
| 72 h after operation | 223.47 ± 48.64a | 248.62 ± 50.10a | -2.389 | 0.019 |
| TBIL (μmol/L) | ||||
| Preoperative | 15.92 ± 4.40 | 15.28 ± 4.71 | 0.659 | 0.512 |
| 24 h after operation | 32.85 ± 7.01a | 34.06 ± 8.43a | -0.732 | 0.466 |
| 72 h after operation | 20.46 ± 5.83a | 22.90 ± 6.15a | -1.910 | 0.059 |
| PA (mg/dL) | ||||
| Preoperative | 313.86 ± 46.91 | 320.74 ± 51.67 | -0.654 | 0.515 |
| 24 h after operation | 194.82 ± 32.65a | 188.57 ± 29.48a | 0.942 | 0.349 |
| 72 h after operation | 275.12 ± 41.81a | 269.84 ± 46.10a | 0.563 | 0.575 |
Before surgery, there were no significant differences in serum AFP, CEA, and AFU levels between the research group and the control group (P > 0.05). One month after surgery, the serum AFP, CEA, and AFU levels in the two groups were lower than those before surgery (P < 0.05), and there was no statistically significant difference between the two groups (P > 0.05, Table 4).
| Index | Research group (n = 44) | Control group (n = 44) | t value | P value |
| AFU (U/L) | ||||
| Preoperative | 66.41 ± 9.51 | 68.18 ± 10.84 | -0.814 | 0.418 |
| 1 mo after operation | 27.04 ± 6.44a | 29.95 ± 8.27a | -1.842 | 0.069 |
| AFP (ng/L) | ||||
| Preoperative | 240.8 ± 75.6 | 228.6 ± 66.3 | 0.805 | 0.423 |
| 1 mo after operation | 78.55 ± 18.04a | 82.01 ± 20.63a | -0.837 | 0.405 |
| CEA (μg/L) | ||||
| Preoperative | 18.58 ± 4.20 | 20.03 ± 4.81 | -1.506 | 0.136 |
| 1 mo after operation | 3.77 ± 0.89a | 4.01 ± 0.81a | -1.323 | 0.189 |
Before surgery, there were no significant differences in serum TNF-α, IL-6, and CRP levels between the research and control groups (P > 0.05). The levels of TNF-α and IL-6 in the research group were lower than those in the control group (P < 0.05, Table 5).
| Index | Research group (n = 44) | Control group (n = 44) | t value | P value |
| IL-6 (pg/mL) | ||||
| Preoperative | 54.23 ± 9.50 | 56.39 ± 9.11 | -1.089 | 0.279 |
| 24 h after operation | 97.41 ± 17.59 | 108.26 ± 18.25 | -2.839 | 0.006 |
| 72 h after operation | 70.55 ± 13.02 | 74.18 ± 14.40 | -1.240 | 0.218 |
| TNF-α (pg/mL) | ||||
| Preoperative | 68.33 ± 13.20 | 70.53 ± 12.65 | -0.798 | 0.427 |
| 24 h after operation | 148.12 ± 21.04 | 167.00 ± 24.28 | -3.898 | 0.000 |
| 72 h after operation | 98.40 ± 13.27 | 102.73 ± 15.19 | -1.424 | 0.158 |
| CRP (mg/L) | ||||
| Preoperative | 4.91 ± 1.53 | 5.34 ± 1.58 | -1.297 | 0.198 |
| 24 h after operation | 18.48 ± 3.75 | 20.14 ± 4.43 | -1.897 | 0.061 |
| 72 h after operation | 14.20 ± 3.36 | 15.38 ± 4.28 | -1.438 | 0.154 |
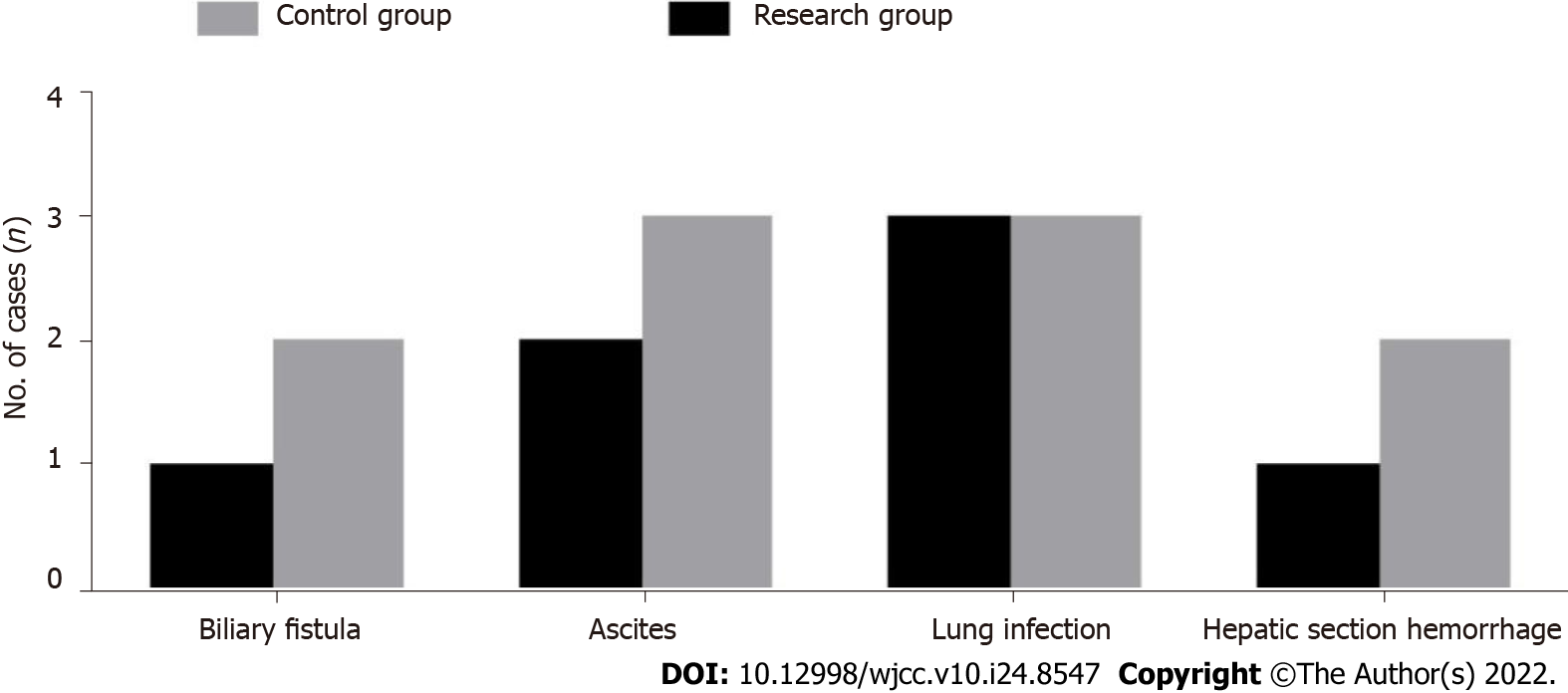
The operation complication rate of the research group was 15.91%, whereas that of the control group was 22.73%, and the difference was not statistically significant (P > 0.05, Figure 1).
Total hepatic blood flow occlusion during surgical resection can completely block blood return to the portal vein system, resulting in gastrointestinal tract hyperemia, impaired mucosal barrier function, and high susceptibility to bacterial and toxin infections. Long-term blocking of portal blood flow can lead to portal vein and superior mesenteric vein thromboses[8-10]. More importantly, the blood entering the liver causes ischemic reperfusion injury of the liver parenchyma and distal organs after the blood flow is restored, and the liver function is seriously impaired. Theoretically, local hemo-occlusion in the affected segment of the patient's liver is better, with minimal damage to liver function and in line with the concepts of anatomic hepatectomy and precise hepatectomy[11-13]. However, the operation process is complicated and requires mastery of color ultrasound-guided puncture technology, which has not been popularized. Although half-hepatic blood flow occlusion technology cannot directly block local liver blood flow from entering the liver, it retains all blood supply to the healthy side of the liver, and intraoperatively greatly reduces healthy-side liver parenchyma ischemia–reperfusion injury, causing minor damage to the liver; after surgery, liver function can be quickly restored, which is obviously advantageous in operations for hepatitis and hepatocirrhosis[14-17].
Our study showed that the operation lasted longer in the research group than in the control group. There was no statistically significant difference in incision length, surgical bleeding, portal block time, drainage tube indwelling time, and hospital stay between the operation and control groups, indicating that control of half-hepatic blood flow during the operation was similar to that during use of the complete hepatic occlusion technique, which could effectively reduce bleeding. The half-hepatic blood flow occlusion technique requires detailed intrathecal dissection of the first hilum of the liver, thus increasing the difficulty of the operation and the operative time. The technique of hepatic blood flow occlusion for hepatectomy can easily lead to liver ischemia and hypoxia, which can cause liver tissue damage and liver function impairment[18]. Meanwhile, 24 and 72 h after the operation, the serum ALT and AST levels in the research group were significantly lower than those in the control group, indicating that the half-hepatic blood flow occlusion technique is superior for postoperative liver function recovery. The reason is that half-hepatic occlusion does not markedly influence the hemodynamics, and the mesenteric blood still flows back to the systemic circulation, avoiding gastrointestinal hyperemia, intestinal bacterium and endotoxin translocation, intestinal ventricular membrane injury, and liver regeneration. After operation, healthy hepatic arteries and portal veins remain open, not affecting the blood supply, thus avoiding ischemia-reperfusion injury and having less impact on liver function, especially in patients with hepatocirrhosis and other liver-related diseases. The rate of surgical complications in the research group was lower than that in the control group. This may be because the single block time of the half-hepatic blood flow occlusion technique is long, and the portal vein and hepatic artery branches at the lesion site are directly ligated or even separated; therefore, there is sufficient time for liver parenchyma dissection, hemostasis of the liver section, and bile leakage of the section to reduce the occurrence of surgical complications. In the past, the clinical diagnosis of patients with liver cancer was mainly based on AFP levels. Although the operation was simple, the detection sensitivity was not high, and it was easy to miss the diagnosis. In this study, 1 mo after surgery, the levels of serum AFP, CEA, and AFU in both groups were lower than those before surgery (P < 0.05), and there was no statistically significant difference between the groups. The reason for the analysis was that the patients were relieved of tumor cell growth and other factors after surgery, and the expression levels of AFP, AFU, and CEA decreased significantly.
Hepatectomy has always been the primary choice for patients with HCC and liver cirrhosis. Intraoperative blood flow occlusion with a half approach to the liver and a complete approach to the liver are both safe and effective, and the choice between the two methods is controversial[19,20]. Therefore, the two methods were compared in this study. Changes in the operation process indicators were compared after the patients with hepatocirrhosis received different treatments. Postoperative liver function recovery and the occurrence of adverse reactions had certain reference values. Although half-hepatic blood flow occlusion is complicated and can prolong the operation time, it causes limited damage to liver function during the operation and is beneficial for the recovery of liver function after surgery, rendering it worthy of widespread clinical application. However, the sample size of this study was relatively small, and it is necessary to increase the sample size and detection indicators in future studies to verify the reliability of the results of this study.
In conclusion, half-hepatic blood flow occlusion technology is more beneficial than total hepatic occlusion in reducing liver function injury in patients with HCC and cirrhosis undergoing hepatectomy.
Hepatocellular carcinoma (HCC) accounts for 90% of all primary liver tumors.
Currently, radical surgery is the preferred treatment for HCC.
This study aimed to investigate the effect of half-hepatic blood flow occlusion after patients with HCC and cirrhosis undergo hepatectomy.
This retrospective single-blinded study included 88 patients with HCC and liver cirrhosis who underwent hepatectomy from January 2017 to September 2020 in our hospital and were divided into an observation group and a control group.
About 24 h and 72 h after the operation the respective serum alanine transaminase and aspartate aminotransferase levels in the research group were significantly lower than those in the control group.
Half-hepatic blood flow occlusion technology is more beneficial than total hepatic occlusion in reducing liver function injury in patients with HCC and cirrhosis undergoing hepatectomy.
However, the sample size of this study was relatively small, and it is necessary to increase the sample size and detection indicators in future studies.
| 1. | Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, van den Berg LH. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3:17071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 1013] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 2. | Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengeløv L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR; CA184-043 Investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 94] [Reference Citation Analysis (0)] |
| 3. | Huang X, Zeng Y, Xing X, Zeng J, Gao Y, Cai Z, Xu B, Liu X, Huang A, Liu J. Quantitative proteomics analysis of early recurrence/metastasis of huge hepatocellular carcinoma following radical resection. Proteome Sci. 2014;12:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Jin S, Dai CL. Hepatic blood inflow occlusion without hemihepatic artery control in treatment of hepatocellular carcinoma. World J Gastroenterol. 2010;16:5895-5900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Feng L, Wang L, Rong W, Wu F, Yu W, An S, Liu F, Tian F, Wu J. [Initial comparison of regional ischemic preconditioning and hemi-hepatic vascular inflow occlusion in resection of hepatocellular carcinoma]. Zhonghua Zhong Liu Za Zhi. 2015;37:186-189. [PubMed] |
| 6. | Marrero JA. Hepatocellular carcinoma. Curr Opin Gastroenterol. 2005;21:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Qin W, Wang L, Hu B, Leng S, Tian H, Luo H, Yao J, Chen X, Wu C, Chen G, Yang Y. A Novel Score Predicts HBV-Related Hepatocellular Carcinoma Recurrence After Hepatectomy: a Retrospective Multicenter Study. J Gastrointest Surg. 2019;23:922-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Tokumitsu Y, Sakamoto K, Tokuhisa Y, Matsui H, Matsukuma S, Maeda Y, Sakata K, Wada H, Eguchi H, Ogihara H, Fujita Y, Hamamoto Y, Iizuka N, Ueno T, Nagano H. A new prognostic model for hepatocellular carcinoma recurrence after curative hepatectomy. Oncol Lett. 2018;15:4411-4422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Carr BI. Introduction: hepatocellular carcinoma. Semin Oncol. 2012;39:367-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Barreiro-Vázquez JD, Miranda M, Barreiro-Vilanova MI, Diéguez FJ, Barreiro-Lois A. Characterization of the Normal Portal and Hepatic Blood Flow of Adult Holstein-Friesian Cows. Animals (Basel). 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Chau GY, Lui WY, King KL, Wu CW. Evaluation of effect of hemihepatic vascular occlusion and the Pringle maneuver during hepatic resection for patients with hepatocellular carcinoma and impaired liver function. World J Surg. 2005;29:1374-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Han H, Ji Z, Ding H, Zhang W, Zhang R, Wang W. Assessment of blood flow in the hepatic tumors using non-contrast micro flow imaging: Initial experience. Clin Hemorheol Microcirc. 2019;73:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Balci D, Ozcelik M, Kirimker EO, Cetinkaya A, Ustuner E, Cakici M, Inan B, Alanoglu Z, Bilgic S, Akar AR. Extended left hepatectomy for intrahepatic cholangiocarcinoma: hepatic vein reconstruction with in-situ hypothermic perfusion and extracorporeal membrane oxygenation. BMC Surg. 2018;18:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Jo HS, Kim DS, Jung SW, Yu YD, Choi SB, Kim WB, Han HJ, Song TJ. Clinical significance of post-hepatectomy hepatic failure in patients with liver metastases from colorectal cancer. Ann Hepatobiliary Pancreat Surg. 2018;22:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706-1718.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 894] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 16. | Fukuda A, Sakamoto S, Sasaki K, Narumoto S, Kitajima T, Hirata Y, Hishiki T, Kasahara M. Modified triangular hepatic vein reconstruction for preventing hepatic venous outflow obstruction in pediatric living donor liver transplantation using left lateral segment grafts. Pediatr Transplant. 2018;22:e13167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Xu HW, Liu F, Li HY, Wei YG, Li B. Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score-matched analysis. Surg Endosc. 2018;32:712-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (61)] |
| 18. | Hallager S, Ladelund S, Kjaer M, Madsen LG, Belard E, Laursen AL, Gerstoft J, Røge BT, Grønbaek KE, Krarup HB, Christensen PB, Weis N. Hepatocellular carcinoma in patients with chronic hepatitis C and cirrhosis in Denmark: A nationwide cohort study. J Viral Hepat. 2018;25:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | D'Ambrosio R, Aghemo A, Rumi MG, Degasperi E, Sangiovanni A, Maggioni M, Fraquelli M, Perbellini R, Rosenberg W, Bedossa P, Colombo M, Lampertico P. Persistence of hepatocellular carcinoma risk in hepatitis C patients with a response to IFN and cirrhosis regression. Liver Int. 2018;38:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 481] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Honda M, Japan; Mahmud N, United States S-Editor: Wang JL L-Editor: A P-Editor: Wang JL