Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8375
Peer-review started: March 30, 2022
First decision: June 16, 2022
Revised: June 25, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: August 16, 2022
Processing time: 123 Days and 18.1 Hours
A biliary inflammatory myofibroblastic tumor (IMT) is a rare type of mesen
Here, we retrospectively describe a 10-month-old infant who was admitted to our hospital due to stubborn jaundice. The patient responded poorly to routine medical treatment and his clinical manifestations and laboratory tests lacked specificity, so we turned to repeated ultrasound scans and other imaging examinations. As both hepatosplenic ultrasonography and diffusion-weighted magnetic resonance imaging demonstrated a space-occupying lesion, an exploratory laparotomy was performed. The final diagnosis made over two mo after the disease onset was infant biliary cirrhosis caused by a biliary IMT, which partially infiltrated into the liver. This infant is the youngest case of biliary IMTs that has been reported till now. The patient underwent an incomplete resection of the mass and Kasai Portoenterostomy. However, because of cirrhosis, he also received a paternal liver transplant. Since some IMTs show malignant properties, we proceeded with a three-year of follow-up; however, no recurrence or metastasis has been noted.
Neoplastic disease such as IMTs should be considered when routine medical treatment of obstructive jaundice is not successful. Observation of dynamic imaging changes is helpful for diagnosis. Periodic follow-up is necessary for IMTs.
Core Tip: Biliary inflammatory myofibroblastic tumor (IMT) is a rare type of mesenchymoma. Diagnosis is difficult because IMTs often exhibit nonspecific clinical symptoms. We describe a biliary IMT in a 10-month-old male patient who manifested as stubborn obstructive jaundice. This is the youngest case of biliary IMTs that have been reported till now. This case highlights that neoplastic disease should be considered when routine medical treatment of obstructive jaundice is not successful. Observation of dynamic imaging changes is helpful to find out occupying lesions. Timely diagnosis and treatment are crucial and periodic follow-up is necessary due to the malignant properties of IMTs.
- Citation: Huang Y, Shu SN, Zhou H, Liu LL, Fang F. Infant biliary cirrhosis secondary to a biliary inflammatory myofibroblastic tumor: A case report and review of literature. World J Clin Cases 2022; 10(23): 8375-8383
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8375.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8375
An inflammatory myofibroblastic tumor (IMT), which was mistaken for a non-neoplastic process upon its discovery, is now recognized as a neoplastic disease. Now, an emerging consensus is that IMT is a rare borderline mesenchymal neoplasm, which has tendencies towards recurrence and local infiltration, as well as metastasis[1-4]. IMT was characterized using histopathology by the proliferation of myofibroblastic and fibroblastic spindle cells accompanied by an inflammatory infiltrate of plasma cells, lymphocytes, and/or eosinophils[5]. The exact etiology and pathogenesis of IMT remain unclear. Scientists believe that cytogenetic abnormalities play an important role. Apart from its biological characteristics, the discovery that IMT may harbor chromosomal rearrangements at the 2p23 Locus, where the gene site for anaplastic lymphoma kinase (ALK) is, also firmed the borderline neoplasm property of IMT[6-8]. According to several case reports, approximately 30 kinds of genetic rearrangement have been found in IMT, most of which are ALK fusion genes[9-12]. ALK can be detected in about 50% of IMTs[13]. A study revealed that several ALK-negative IMTs harbor the receptor tyrosine kinase encoded by ROS-1 or platelet-derived growth factor receptor-β kinase fusions by using next generation sequencing[14]. Infection etiology has also been proposed due to the systemic symptoms in part of patients. A study using by in situ hybridization showed that Epstein-Barr virus (EBV) RNAs were detected in spindled and round cells of extranodal (splenic and hepatic) and nodal inflammatory pseudotumor (IPT) respectively[15]. A research study from Spain found human herpesvirus 8 (HHV-8) DNA segments in five lung IMTs, a limb IMT, and a retroperitoneal lymph node IMT. It also subsequently detected HHV-8 mRNAs of several open reading frames encoded in latent stage of viral replicative cycle in these lung IMTs, suggesting that HHV-8 may play an important role in the pathogenesis of IMT[16,17].
Original description of this lesion was IPT, which was detected in 1939 as a primary lung tumor. After that, diverse extrapulmonary locations were successively reported. The most frequently reported anatomic sites are the lung, abdominopelvic region, and retroperitoneum[1]. Biliary IMT is rarely reported. IMT has a predilection for children and adolescents, while biliary IMT usually occur in adults. IMTs usually appear as circumscribed solitary or multinodular masses[18]. The clinical presentation varies markedly depending on the site at which the tumors originate. Fifteen to thirty percent of patients have systemic manifestations including fever, weight loss and general malaise, which may be caused by the tumor-mediated release of Interleukin 6[13,16,17,19,20]. Most biliary IMTs begin with obstructive jaundice.
In the present article, we describe a biliary IMT in a 10-month-old male patient who manifested as obstructive jaundice and fever. The final diagnosis was made more than two mo after disease onset, and the patient had progressed to cirrhosis. This is the youngest case of biliary IMT reported till now.
A 10 mo old male patient presented to our hospital with recurrent jaundice, accompanied by decreased appetite and dark urine.
The patient had presented with jaundice for one month. Associated symptoms included decreased appetite and dark urine, without fever and clay-colored stools. About two weeks previously, the patient was admitted to a local medical institution. Laboratory assessment showed liver dysfunction and conjugated hyperbilirubinemia. Ultrasonography revealed that the left liver lobe was enlarged, and that the right was shrunken. This was accompanied by Glisson’s system expansion and low-echo of the surrounding tissues, as well as by intrahepatic biliary dilatation. After more than ten days of ineffective treatment, including glutathione and ademetionine for jaundice, the infant became feverish and was therefore referred to our hospital.
The past medical history of the patient was unremarkable.
This patient was the product of a normal pregnancy and delivery, and had no history of neonatal pathologic jaundice. The family history was also unremarkable.
Physical examination showed mild jaundice of the skin and sclera. Palpation of the abdomen revealed a blunted liver edge about 2 cm below the costal margin and 5 cm below the xiphoid process with medium level texture. The spleen was not detected under the costal margin, and there was no sign of ascites.
A routine blood test revealed lymphocytosis and mild anemia, with hemoglobin levels of 102 g/L, and normal blood platelet levels (298 × 109/L). C-reactive protein and erythrocyte sedimentation rate levels remained within the normal range. Blood biochemistry testing showed high levels of aminotransferases, with alanine aminotransferase measured at 159 U/L and aspartate aminotransferase at 164 U/L. Also, the patient displayed conjugated hyperbilirubinemia, consisting of total bilirubin levels of 87.5 μmol/L and direct bilirubin levels of 75.5 μmol/L, as well as hyperlipemia with triglyceride levels of 4.34 mmol/L, and hyperammonemia with plasma ammonia concentration of 176 μmol/L. Other liver function indicators such as alkaline phosphatase (977 U/L), gamma-glutamyl transpeptidase (1960 U/L), serum albumin (36.1 g/L), globulin (30.8 g/L), and total bile acid (173.6 umol/L), were also been detected. Tumor markers such as alpha fetoprotein (6.47 ng/mL) and CA19-9 (8.27 U/mL) were negative. Blood levels of IgG, IgA, IgM, and complement C3 and C4, were normal. The blood levels of lactic acid and ammonia were higher than anticipated, but there was no evidence of metabolic acidosis. Moreover, urine organic acids assay and blood tandem mass spectrometry, which are used to diagnose metabolic diseases, showed normal results. Blood coagulation was normal. Hepatitis B surface antigen, e antigen, and antibodies to hepatitis B core antigen were all negative. Hepatitis C antibodies were also negative. EBV derived VCA-IgM and IgG were positive, and EA-IgG and NA-IgG were negative. Anti-human cytomegalovirus IgM and uric viral inclusion bodies were negative.
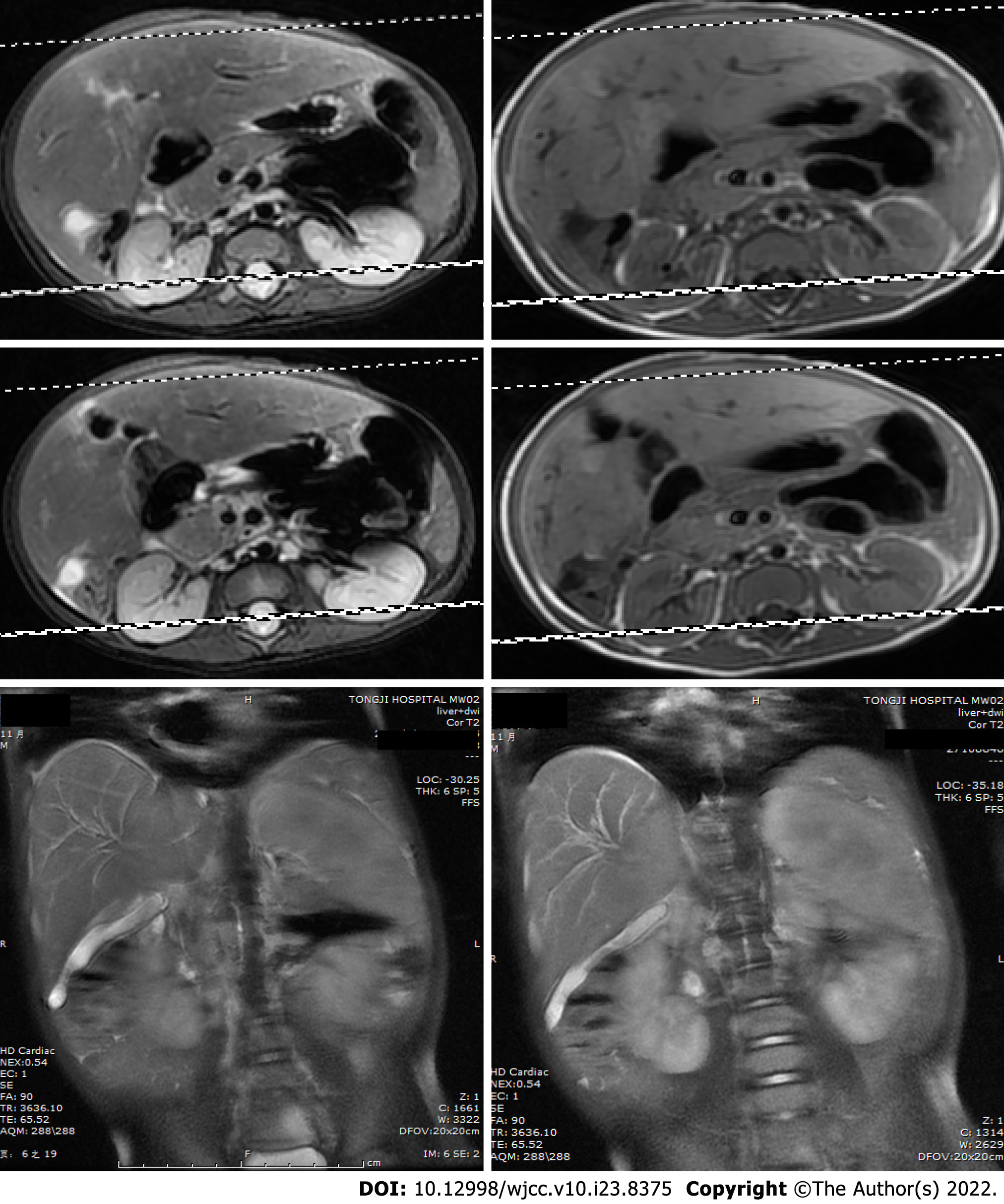
Hepatosplenic ultrasonic showed abnormal liver morphology with inhomogeneous parenchyma, and multiple irregular anechoic tubular structures at the porta hepatis and intra-hepatic portal veins. The inner diameters of gallbladder cross section were 4.9cm × 1.4cm. There was no sign of intrahepatic or extrahepatic bile ducts dilatation. The spleen was 3.4 cm thick. Overall, the hepatosplenic ultrasonic indicated cavernous transformation of the portal vein and splenomegaly. Routine medical treatment of cholestatic hepatitis including glutathione, diammonium glycyrrhizinate, and ursodesoxycholic acid had poor effect. Reevaluation of the patient’s condition using hepatosplenic ultrasonic examination revealed a new radiographic finding characterized by a cystic mass between the liver and kidney. Diffusion-weighted magnetic resonance imaging (DWI-MRI) of the abdomen showed that the patient’s intrahepatic bile ducts were dilated, and that the number of blood vessels of the porta hepatis were increased. The gallbladder could not be clearly seen. Short T1 and long T2 signals were found between the liver and the right kidney, which were displayed as hyperintense on the DWI (Figure 1).
A bone marrow biopsy was also performed to exclude niemann-pick disease and gaucher disease.
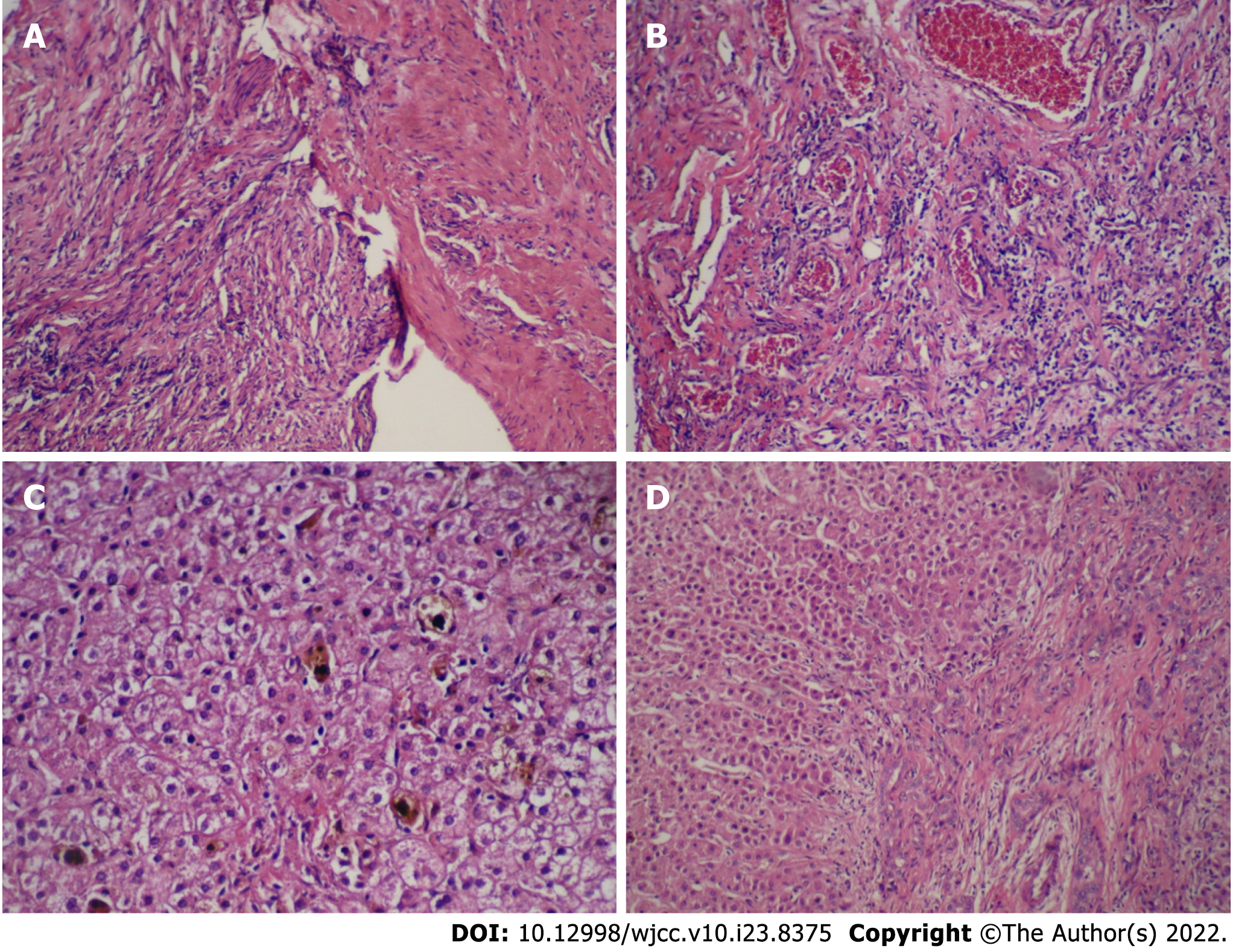
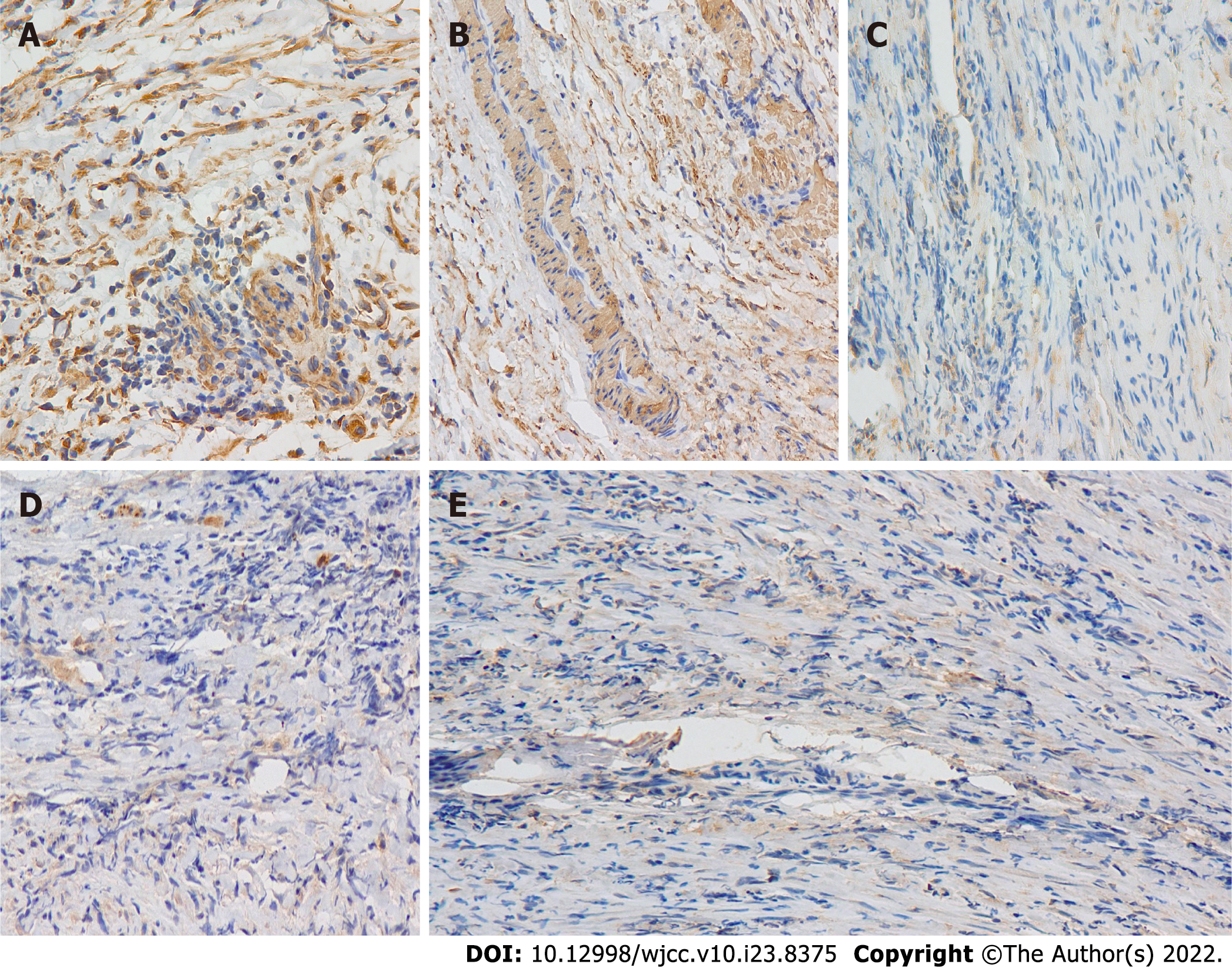
According to the imaging features, we speculated that the lesion was a tumor. Thus an exploratory laparotomy was performed. The liver was swollen and hard, and the gallbladder was enlarged (6 cm × 2 cm × 1.5 cm). A stiff mass measuring 2 cm × 2 cm × 2.5 cm located in the junction between the cystic gall duct and the common bile duct, infiltrating into the liver, was observed. Since there was no clear border between the tumor and the normal tissue of the liver, the patient received incomplete resection of the mass and cholecystectomy; Kasai Portoenterostomy was also performed to allow for bile drainage. Intraoperative histopathology of the mass showed fibrous tissue proliferation and a well-differentiated glandular epithelium. Postoperative pathology verified that the mass had undergone spindle cell and fibrous tissue proliferation, inflammatory cellular infiltration and small vessel congestion and expansion without cytologic atypia, coinciding with a diagnosis of an IMT (Figure 2A and B). Liver biopsy showed that in the portal area, bile canaliculus hyperplasia, hepatic fibrosis, and lymphocyte infiltration accompanied by hepatocyte degeneration and cholestasis could be observed (Figure 2C and D), indicating that the patient had progressed to liver cirrhosis. On Immunohistochemistry, the lesion was positive for smooth muscle actin and vimentin, and negative for desmin, S100, and ALK1 (Figure 3).
The final diagnosis of the presented case is infant biliary cirrhosis caused by a biliary IMT, which partially infiltrated into the liver.
After drugs such as glutathione, diammonium glycyrrhizinate, and ursodesoxycholic acid were administered to protect the liver and lower the levels of aminotransaminase and bilirubin, the symptoms were tentatively relieved. The patient also received antibiotics for a concurrent respiratory tract infection, presenting as a fever and a cough. However, the previous symptoms recurred soon after and gradually worsened, being accompanied with intermittent clay-colored stools. We reevaluated the condition by hepatosplenic ultrasonic examination and DWI-MRI of the abdomen and found out a cystic mass. Therefore, an exploratory laparotomy was performed about ten weeks after disease onset. The postoperative diagnosis was biliary IMT. Since there was no clear border between the tumor and the normal tissue of the liver, the patient received incomplete resection of the mass and Kasai Portoenterostomy.
Unfortunately, the obstructive jaundice progressed just one week after the operation. Two mo later, the patient received a paternal liver transplant because of the cirrhosis. After three years of follow up, no recurrence or metastasis has been noted.
Biliary IMT is rarely reported and usually occurs in adults. Heretofore, there are only 16 documented cases of biliary IMT, of which one is published in Russian (Table 1)[21-26]. Among these studies, the age of onset varies from 6 to 70 years, and 5 of the 16 cases were male. The ratio of children was 31.2%.
| Ref. | Age/Sex | Therapies | Infiltration | Follow up | Prognosis | Definition |
| Coffin et al[1], 1995 | 6/M | Pancreaticoduodenectomy and celecoxib | No | 5 mo | NR | IPT |
| Badea et al[2], 2015 | 13/F | Extra hepatic bile duct Excision | No | 21 mo | NR | IPT |
| Panagiotopoulos et al[4], 2015 | 43/M | Gallbladder and cystic duct excision | Yes | 11 mo | LM? | IPT |
| Griffin et al[6], 1999 | 58/F | Pancreatoduodencectomy | Yes | - | IPT | |
| Walsh et al[22], 1998 | 71/F | Oral 5-fluorouracil | Yes | 21 years | LR | IMT |
| Coffin et al[7], 2001 | 50/M | Common bile duct excision | Yes | 17 years | PM | IMT |
| Venkataraman et al[29], 2003 | 51/F | pancreaticoduodenectomy | No | 2 years | NR | IPT |
| Sekaran et al[23], 2006 | 17/F | Left hepatectomy | Yes | 6 weeks | NR | IMT |
| Honda et al[11], 2019 | 55/F | Kaush-Whipple resection | No | 4.5 years | LR | IMT |
| Vargas-Madueno et al[9], 2018 | 51/F | Pancreaticoduodenectomy | No | - | - | IMT |
| Cheek et al[10], 2020 | 55/M | Extra hepatic bile duct | No | 14 mo | NR | IPT |
| Subhash et al[24], 2012 | 21/F | Left liver and caudate lobe excision, extra hepatic biliary excision | No | - | - | IPT |
| Fletcher et al[5], 2013 | 70/F | Extra hepatic bile duct excision | Yes | 8 mo | NR | IPT |
| Pang et al[8], 2016 | 12/F | Debulking, corticosteroid, celecoxib | No | 9 mo | NR | IMT |
| Verma et al[21], 2018 | 24/F | Extra hepatic bile duct excision, Etoricoxib | No | 12 mo | NR | IMT |
| Karimi et al[26], 2018 | 12/F | Limited hepatic resection | No | - | - | IMT |
| Present case | 10-mo-old/M | Limited tumor excision and Kasai Portoenterostomy; Liver transplantation | Yes | 3 years | NR | IMT |
Here we report the youngest male patient of biliary IMT reported till now, aged 10 mo. Similar to other cases of biliary IMTs, this patient presented with obstructive jaundice because of compression of the common bile duct. Another prominent clinical manifestation was fever. However, both obstructive jaundice and fever are non-specific manifestations. Following this, the laboratory investigations also generated nonspecific results, which added to the difficulty of obtaining a timely diagnosis. Further investigations revealed positive EBV-VCA-IgM suggesting a recent infection with EBV. In infants, the common causes of obstructive jaundice are congenital biliary atresia, congenital metabolic disease, and viral infections. In our case, we excluded the first two differential diagnoses. However, we considered that refractory, recurrent obstructive jaundice could not solely be due to an EBV infection. Although hepatic or biliary neoplasms are infrequent in children, they should not be ignored. Imaging examination may help in finding harboring masses. Ultrasonography, including conventional ultrasound, contrast-enhanced ultrasound, and strain elastography, should be used within the primary detection of such masses, given their non-invasive nature[2]. This patient underwent three inconsecutive hepatosplenic ultrasonographic examinations, which all revealed different findings. This should serve to remind us of the importance of dynamic imaging for monitoring a patient. Further imaging examination such as computed tomography (CT) and MRI may also be required. Since intrahepatic IMT usually has similar features upon CT and MRI with cholangiocarcinoma, a differential diagnosis by image examination is unreliable[26-29]. Clinicians are trying more discerning methodologies. By comparing different imaging parameters, Chang et al[28] found that IMT often shows early target appearance on unenhanced T1-weighted imaging and early dynamic phases of gadoxetic acid-enhanced MRI. This differs from intrahepatic cholangiocarcinoma, which shows target appearance on the later phases and DWI[28]. The biliary IMT in this case was a circumscribed solitary mass with infiltration to liver tissue. Coffin and colleges have described three basic histological patterns of IMT: A myxoid/vascular pattern, a compact spindle cell pattern, and a hypocellular fibrous (fibromatosislike) pattern. This case coincided with the second one, which usually characterized by a cellular proliferation of spindle cells with a fascicular or storiform architecture in a collagenous stroma[1,13].
The recommended, definitive treatment for well-defined masses is surgical resection. Most IMTs have a favorable prognosis after complete excision. The usage of corticosteroids and molecular-targeted agents, such as the ALK inhibitor crizotinib and the ROS inhibitor ceritinib, are permitted[7,30,31]. There are no appropriate marks that are associated with and can predict prognosis, although some articles suggest that an ALK-positive tumor may be related to local recurrence[3,7]. For extrapulmonary IMT, the recurrence rate is 25%, and metastasis occurs in less than 5% of cases[13].
Although ALK staining was negative in our case, the resection operation was ineffective partially because of the tumor infiltration and irreversible cirrhosis. Thus the patient eventually developed cirrhosis shortly after, due to ineffective medical treatment. This can probably be attributed to the fact that the course of the disease was probably longer, but early symptoms such as mild jaundice were difficult to be noted by the parents. This case study highlights the importance of early identification and surgical treatment of neoplastic disease when routine medical treatment of obstructive jaundice is not effective.
Biliary IMT is rarely reported and usually occurs in adults, sometimes in children. We report the youngest male patient of biliary IMT reported till now. Making a definite diagnosis usually difficult due to non-specific manifestations. Monitoring by image examination may help in finding harboring masses. Timely diagnosis and early surgical resection is meaningful because some IMTs show malignant properties such as infiltration, recurrence, and metastasis.
The authors thank Department of pathology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for kindly presenting the ALK1 antibody.
| 1. | Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1046] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 2. | Badea R, Veres AA, Andreica V, Caraiani C, Al-Hajjar N, Sechel R, Chiorean L. Inflammatory myofibroblastic tumor of the gallbladder: imaging aspects. J Med Ultrason. 2015;42:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 642] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Panagiotopoulos N, Patrini D, Gvinianidze L, Woo WL, Borg E, Lawrence D. Inflammatory myofibroblastic tumour of the lung: a reactive lesion or a true neoplasm? J Thorac Dis. 2015;7:908-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (1)] |
| 5. | Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. WHO classification of tumours of soft tissue and bone. Pathology and Genetics of Tumours of Soft Tissue and Bone 4th edition. Lyon: IARC Press, 2013. |
| 6. | Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776-2780. [PubMed] |
| 7. | Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 410] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Pang R, Merritt NH, Shkrum MJ, Tijssen JA. Febrile Illness in an Infant With an Intracardiac Inflammatory Myofibroblastic Tumor. Pediatrics. 2016;137:e20143544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Vargas-Madueno F, Gould E, Valor R, Ngo N, Zhang L, Villalona-Calero MA. EML4-ALK Rearrangement and Its Therapeutic Implications in Inflammatory Myofibroblastic Tumors. Oncologist. 2018;23:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Cheek EH, Fadra N, Jackson RA, Davila JI, Sukov WR, Uckerman MT, Clayton A, Keeney GL, Halling KC, Torres-Mora J, Schoolmeester JK. Uterine inflammatory myofibroblastic tumors in pregnant women with and without involvement of the placenta: a study of 6 cases with identification of a novel TIMP3-RET fusion. Hum Pathol. 2020;97:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Honda K, Kadowaki S, Kato K, Hanai N, Hasegawa Y, Yatabe Y, Muro K. Durable response to the ALK inhibitor alectinib in inflammatory myofibroblastic tumor of the head and neck with a novel SQSTM1-ALK fusion: a case report. Invest New Drugs. 2019;37:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Zarei S, Abdul-Karim FW, Chase DM, Astbury C, Policarpio-Nicolas MLC. Uterine Inflammatory Myofibroblastic Tumor Showing an Atypical ALK Signal Pattern by FISH and DES-ALK Fusion by RNA Sequencing: A Case Report. Int J Gynecol Pathol. 2020;39:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 513] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 14. | Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, Borinstein SC, Ross JS, Stephens PJ, Miller VA, Coffin CM. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 338] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 15. | Arber DA, Kamel OW, van de Rijn M, Davis RE, Medeiros LJ, Jaffe ES, Weiss LM. Frequent presence of the Epstein-Barr virus in inflammatory pseudotumor. Hum Pathol. 1995;26:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 222] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Gómez-Román JJ, Ocejo-Vinyals G, Sánchez-Velasco P, Nieto EH, Leyva-Cobián F, Val-Bernal JF. Presence of human herpesvirus-8 DNA sequences and overexpression of human IL-6 and cyclin D1 in inflammatory myofibroblastic tumor (inflammatory pseudotumor). Lab Invest. 2000;80:1121-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 17. | Gómez-Román JJ, Sánchez-Velasco P, Ocejo-Vinyals G, Hernández-Nieto E, Leyva-Cobián F, Val-Bernal JF. Human herpesvirus-8 genes are expressed in pulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). Am J Surg Pathol. 2001;25:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Souid AK, Ziemba MC, Dubansky AS, Mazur M, Oliphant M, Thomas FD, Ratner M, Sadowitz PD. Inflammatory myofibroblastic tumor in children. Cancer. 1993;72:2042-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Rohrlich P, Peuchmaur M, Cocci SN, Gasselin ID, Garel C, Aigrain Y, Galanaud P, Vilmer E, Emilie D. Interleukin-6 and interleukin-1 beta production in a pediatric plasma cell granuloma of the lung. Am J Surg Pathol. 1995;19:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Azuno Y, Yaga K, Suehiro Y, Ariyama S, Oga A. Inflammatory myoblastic tumor of the uterus and interleukin-6. Am J Obstet Gynecol. 2003;189:890-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Verma R, Saha A, Saha K. Inflammatory Myofibroblastic Tumor of the Mid Common Bile Duct Masquerading as Cholangiocarcinoma. J Gastrointest Cancer. 2019;50:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Walsh SV, Evangelista F, Khettry U. Inflammatory myofibroblastic tumor of the pancreaticobiliary region: morphologic and immunocytochemical study of three cases. Am J Surg Pathol. 1998;22:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Sekaran A, Lakhtakia S, Pradeep R, Santosh D, Gupta R, Tandan M, Reddy DB, Rao GV, Reddy DN. Inflammatory myofibroblastic tumor of biliary tract presenting as recurrent GI bleed (with video). Gastrointest Endosc. 2006;63:1077-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Subhash R, Arunkumar ML, Natesh B, Raji L. Inflammatory pseudotumor of the biliary tract. Indian J Pathol Microbiol. 2012;55:413-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Shatveryan GA, Bagmet NN, Ratnikova NP, Chardarov NK, Hrustaleva MV, Dolzhansky OV, Hovrin VV, Galyan TN. Inflammatory myofibroblastic tumor of common bile duct. Khirurgiia (Mosk). 2018;7:51-54. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Karimi M, Tizmaghz A, Shabestanipour G. An interesting case of inflammatory myofibroblastic tumor presenting as cholangiocarcinoma. Int J Surg Case Rep. 2018;47:38-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Yoon KH, Ha HK, Lee JS, Suh JH, Kim MH, Kim PN, Lee MG, Yun KJ, Choi SC, Nah YH, Kim CG, Won JJ, Auh YH. Inflammatory pseudotumor of the liver in patients with recurrent pyogenic cholangitis: CT-histopathologic correlation. Radiology. 1999;211:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Chang AI, Kim YK, Min JH, Lee J, Kim H, Lee SJ. Differentiation between inflammatory myofibroblastic tumor and cholangiocarcinoma manifesting as target appearance on gadoxetic acid-enhanced MRI. Abdom Radiol (NY). 2019;44:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Venkataraman S, Semelka RC, Braga L, Danet IM, Woosley JT. Inflammatory myofibroblastic tumor of the hepatobiliary system: report of MR imaging appearance in four patients. Radiology. 2003;227:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Alan O, Kuzhan O, Koca S, Telli TA, Basoglu T, Ercelep O, Filinte D, Sengul Y, Arikan H, Kaya S, Babacan NA, Dane F, Yumuk PF. How long should we continue crizotinib in ALK translocation-positive inflammatory myofibroblastic tumors? J Oncol Pharm Pract. 2020;26:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Li Y, Chen X, Qu Y, Fan JM, Li Y, Peng H, Zheng Y, Zhang Y, Zhang HB. Partial Response to Ceritinib in a Patient With Abdominal Inflammatory Myofibroblastic Tumor Carrying a TFG-ROS1 Fusion. J Natl Compr Canc Netw. 2019;17:1459-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elsayed MO, United Kingdom; Gupta T, India; Yeoh SW, Australia S-Editor: Wang LL L-Editor: A P-Editor: Wang LL