Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8352
Peer-review started: March 13, 2022
First decision: June 16, 2022
Revised: June 25, 2022
Accepted: July 5, 2022
Article in press: July 5, 2022
Published online: August 16, 2022
Processing time: 140 Days and 23.6 Hours
Incontinentia pigmenti (IP) is a rare X-linked dominant genetic disorder that can be fatal in male infants. It is a disease that affects many systems of the human body. In addition to characteristic skin changes, patients may also have patho
An 11-year-old female patient suffered intermittent limb convulsions for five months and was sent to hospital. In the initial stage, the patient was considered to have primary epilepsy. Further investigation of the patient's medical history, physical examination and imaging examination led to the diagnosis of IP combined with intracranial space-occupying lesions, and secondary epilepsy. The patient was treated with craniotomy, and postoperative pathology revealed an IAC. The patient recovered well after craniotomy and had no obvious surgery-related complications. During the follow-up period, the patient did not have recurrent epilepsy symptoms.
IP is a multi-system disease that presents with typical skin lesions at birth, but the long-term prognosis of this disease depends on the involvement of systems other than the skin, especially nervous system and ocular lesions.
Core Tip: We report a rare case of incontinentia pigmenti (IP) with an intracranial arachnoid cyst (IAC). The initial diagnosis in this patient was considered to be primary epilepsy. Following admission, her medical history, physical examination and imaging examination led to the diagnosis of IP complicated by intracranial space-occupying lesions and was treated by craniotomy. Postoperative pathology revealed an IAC. During follow-up, the patient recovered well and did not experience recurrence of epilepsy. By recording the diagnosis and treatment history of this patient in detail, and reviewing relevant literature, discussing the characteristics of patients with IP and IAC will help to improve diagnosis and treatment, and improve the overall quality of life of such patients.
- Citation: Li WC, Li ML, Ding JW, Wang L, Wang SR, Wang YY, Xiao LF, Sun T. Incontinentia pigmenti with intracranial arachnoid cyst: A case report. World J Clin Cases 2022; 10(23): 8352-8359
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8352.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8352
Incontinentia pigmenti (IP) or Bloch-Sulzberger syndrome, is a rare X-linked dominant inherited systemic disease affecting primarily the skin but also other neuroectodermal tissues such as teeth, hair, eyes, and the central nervous system. IP is the consequence of a mutation in the IKBKG gene (formerly known as NEMO or nuclear factor kappa essential modulator). Because it is an X-linked dominant disorder, it is usually fatal in males and is therefore only seen in female infants. In addition reports suggest that the incidence of IP is 0.7 per 100000 individuals[1,2].
IP with intracranial space-occupying lesions is extremely rare. To date, no cases of IP with an intracranial arachnoid cyst (IAC) have been reported. We here report the first case of IP with an IAC in order to share our experience in managing patients with IP with IAC and help improve diagnosis and treatment and improve the overall quality of life of such patients.
An 11-year-old female patient was sent to the emergency department due to intermittent limb convulsions for 5 mo and loss of consciousness with limb twitching for 1 h.
The patient was sent to the emergency department of the First Affiliated Hospital of Xinxiang Medical University for treatment due to a sudden loss of consciousness with limb twitching for 1 h. The patient presented with sudden epilepsy symptoms without obvious inducement 5 mo ago, and the specific manifestations included turning up of the eyes, twitching of the limbs, no foaming at the mouth, and no incontinence. The above symptoms were relieved without treatment after approximately 30 s. Limb movement was subsequently normal, and there were no residual symptoms such as headache and dizziness. Over the past 5 mo, there were 4 intermittent attacks. The patient was hospitalized for treatment.
Approximately 10 d after birth, she developed a rash on her body, and with advance of age, her skin color gradually deepened. At present, the child’s psychomotor development showed no obvious abnormalities compared with normal children of the same age.
The father was healthy, and the mother also had similar skin lesions in childhood, which almost completely disappeared around the age of 8 years. At present, only a small area of pigmentation remains on the inner side of her left forearm. The mother denied a history of exposure to radioactive substances during pregnancy and a history of taking medication. However, she had a previous miscarriage (details unknown).
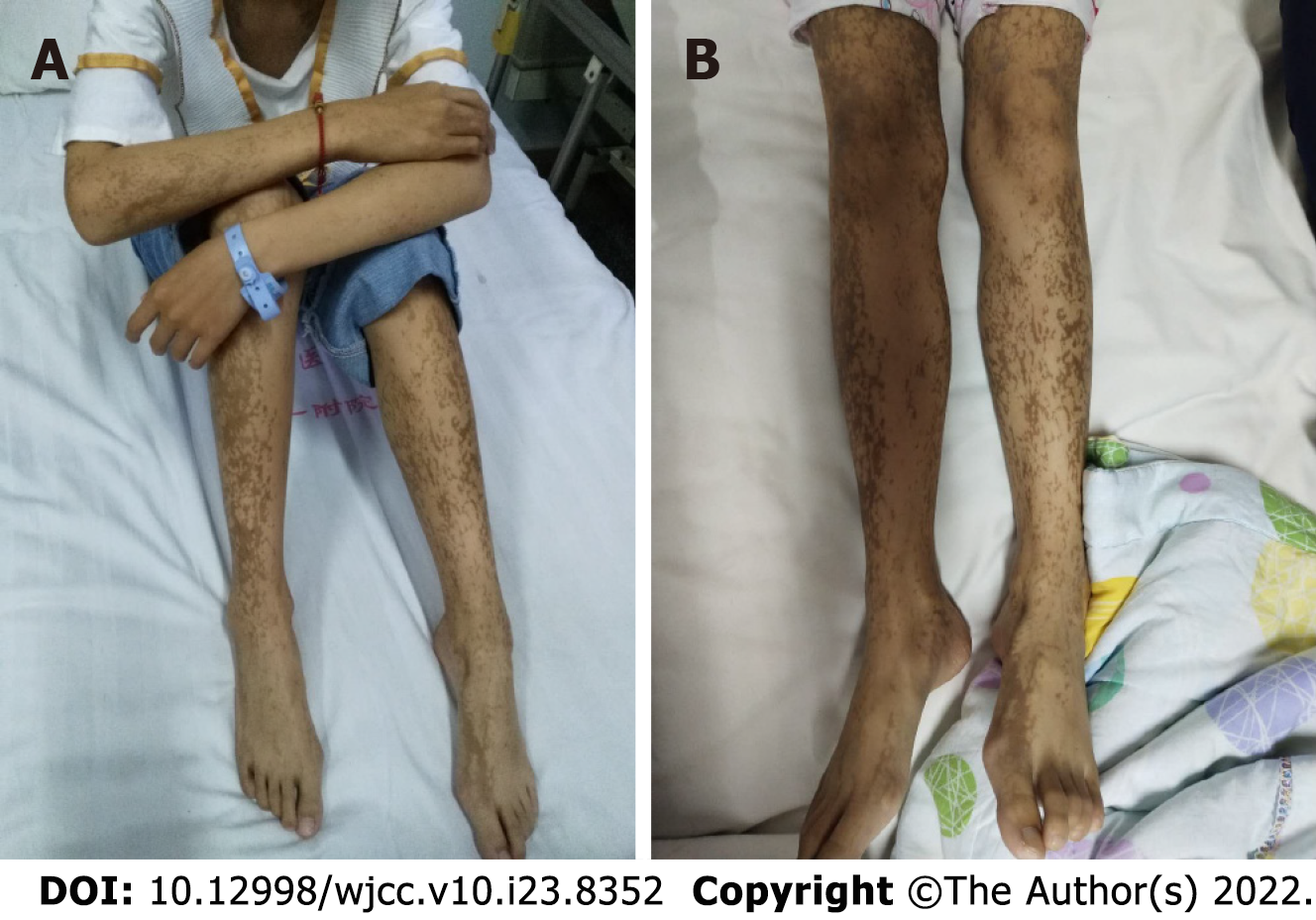
Her vital signs were stable. Consciousness, fluent language, memory, calculation, comprehension, judgment, and orientation tests were basically normal. Limb muscle strength was grade 5, and muscle tone was normal; physiological reflex was present, but pathological reflex was not elicited. Ink-splattered and streak-like brown hyperpigmentation was observed all over the trunk and extremities (Figure 1).
The white blood cell count on routine blood testing was slightly increased (12.2 × 10/L). The results of other routine laboratory biochemical tests showed no abnormalities.
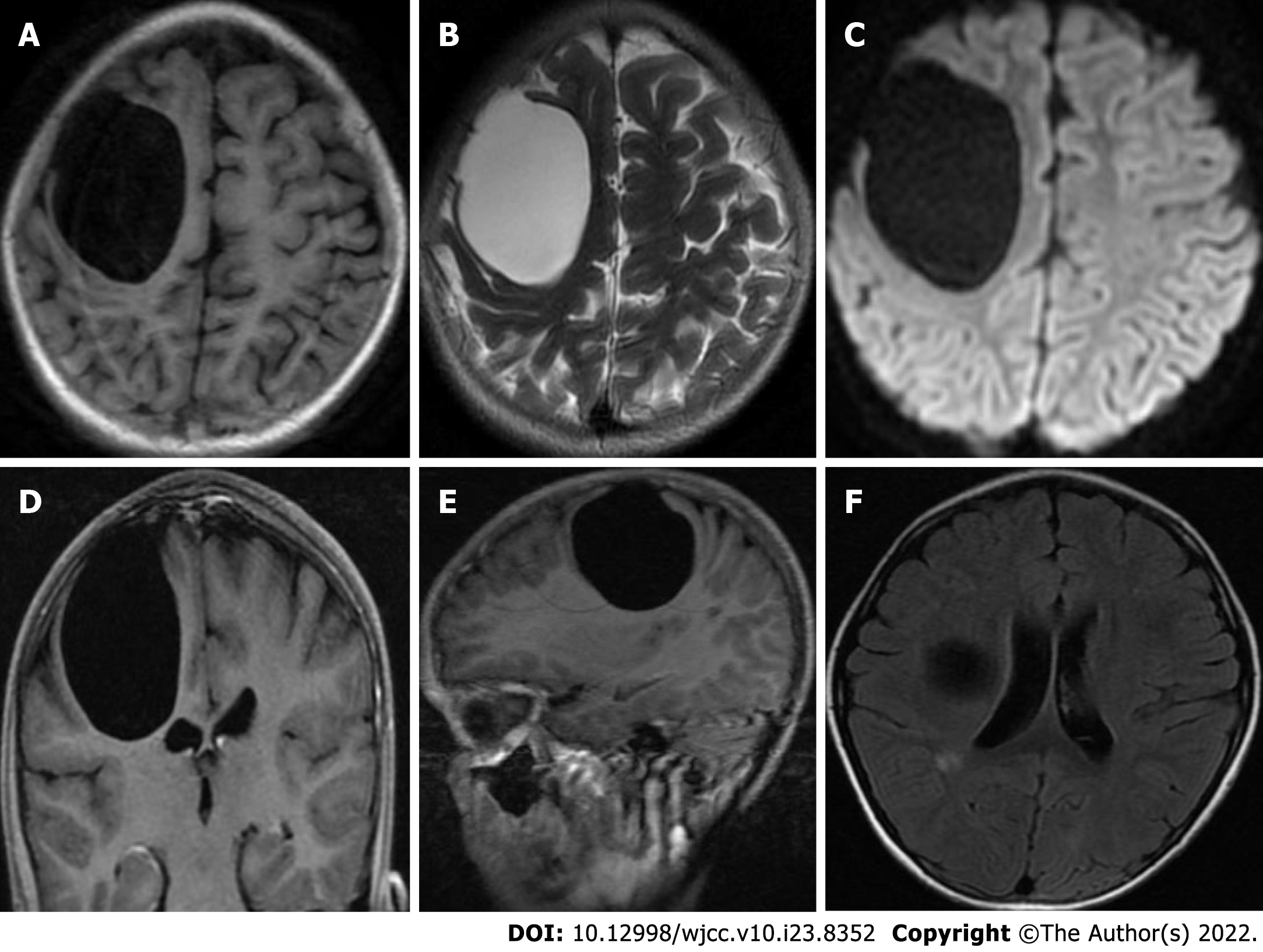
Preoperative magnetic resonance imaging (MRI) revealed a large, circular, homogeneous sac-like signal in the right frontal brain area, approximately 49.3 mm × 58.5 mm × 54.0 mm, with clear boundaries, and the adjacent brain sulci were compressed and narrowed, and the right lateral ventricle was deformed due to compression. Diffusion weighted images showed a low signal, and no obvious enhancement on the enhanced scan. A small patchy long T1-weighted imaging and long T2-weighted imaging signal was observed next to the posterior horn of the right lateral ventricle, and T2 fluid-attenuated inversion recovery showed a slightly high signal, and no obvious enhancement was found on the enhanced scan. Radiological diagnosis of the right frontal lobe revealed a cystic mass, which suggested an IAC; small ischemic foci next to the posterior horn of the right lateral ventricle were noted (Figure 2). Considering that the disease can involve multiple organ systems in the body, we performed chest X-ray and abdominal plain computed tomography (CT) scans before surgery, and no lesions were found.
The patient was diagnosed with IP and IAC.
A multidisciplinary consultation with a pediatrician and a dermatologist was initially conducted to confirm the patient’s diagnosis. After further evaluation of the patient's condition, exclusion of surgical contraindications, and active preoperative preparation, a craniotomy was then performed. During the operation, the skin and subcutaneous tissue were incised along the marked line, the skin flap was turned over, and the bone window size was approximately 5.0 × 6.0 cm. A radial incision of the dura mater revealed an arachnoid cyst with high tension and local arachnoid thickening. The cyst wall was intact and accompanied by hyperplasia of blood vessels. The excised cyst wall was sent to the pathology department for examination. The cyst and subarachnoid space were thoroughly communicated, and the dura mater was sutured and the bone flap was reset after the instruments were checked. The skin was sutured layer by layer. Infusions and epilepsy prevention, and other related treatments were administered after the operation.
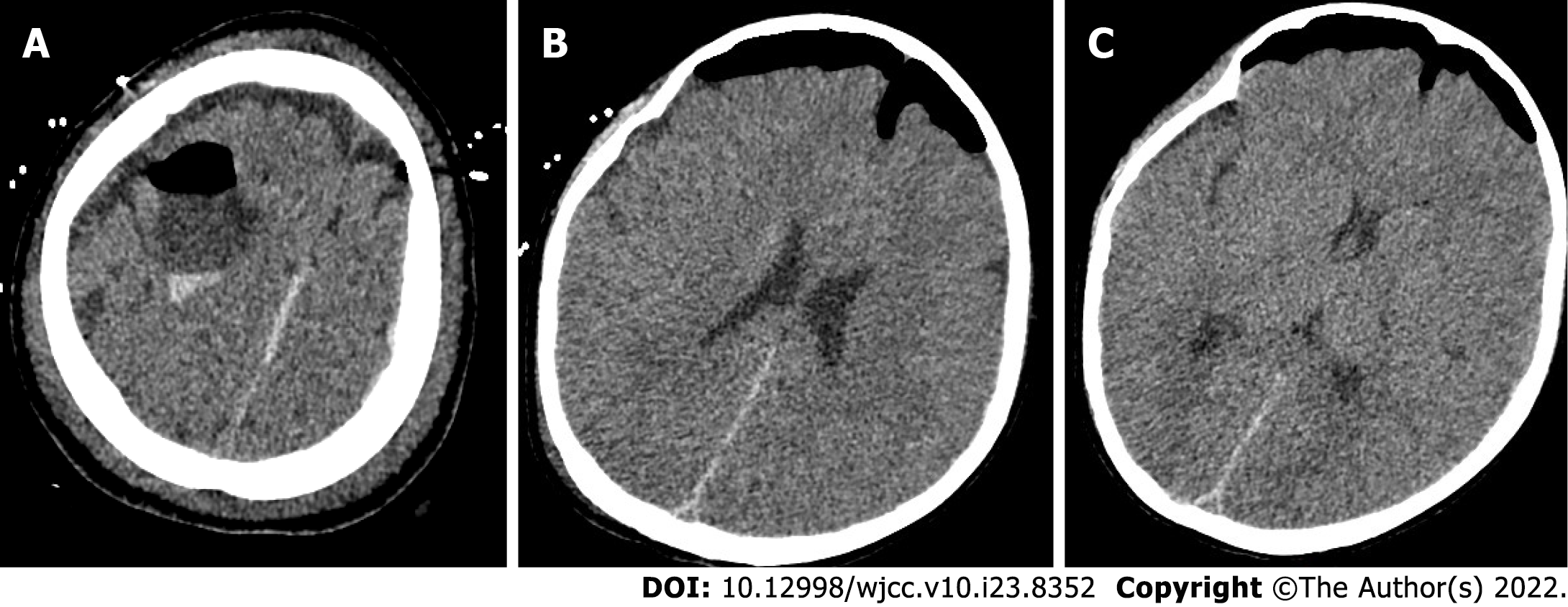
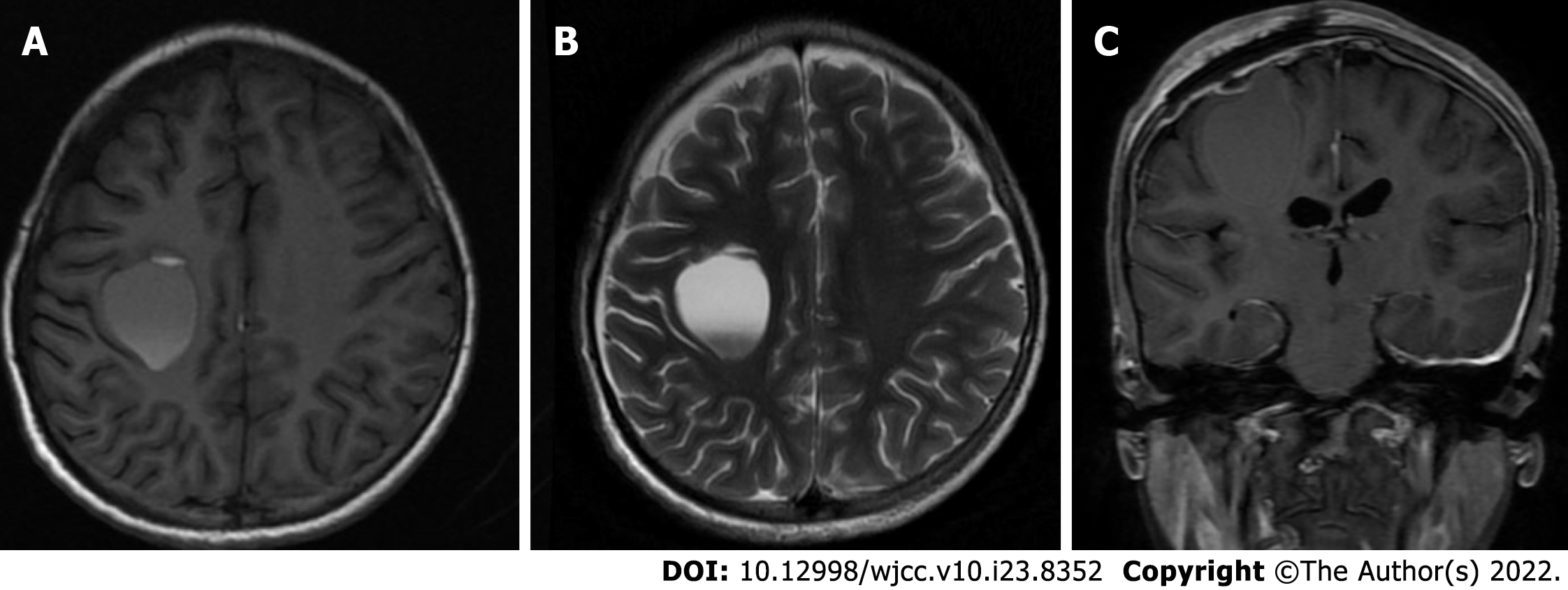
Pathological examination of the tissue was performed. The results showed that gray tissue with a diameter of 0.2 cm was observed. Immunohistochemical results revealed an arachnoid cyst (Figure 3). Postoperative head CT was performed (Figure 4). Head MRI was also performed during follow-up and the local bone of the right frontal area showed postoperative changes, and the cystic shadow in the surgical area was significantly smaller than that before surgery at approximately 23.8 mm × 30.0 mm × 28.5 mm (Figure 5).
Incontinentia pigmenti (IP) is a rare systemic disease involving multiple organ systems. The disease was first reported by Bloch in 1926, and was further described by Sulzberger in 1982; thus, it is also called Bloch-Sulzberger syndrome[2,3]. Recent studies have shown that neonatal IP is caused by mutation of the NEMO gene located on chromosome Xq28, and the most common mutation type is deletion of NEMOΔ4-10. It is also due to the particularity of the genetic mode of the disease itself that male children inherit homozygous IP, so in most cases of male fetuses, miscarriage or stillbirth may occur during pregnancy. Therefore, in clinical practice, the disease is more common in female children, and the hospital visit rate is higher than that of male children[4-6]. Our female patient had IP complicated with intracranial space-occupying lesions. Her mother had a history of miscarriage in the past, and she stated that the possibility of a male fetus was high, but the specific situation could not be verified. The overall situation was consistent with the epidemiology of the disease. We believe that this is the first case of IP with an IAC. This patient was initially misdiagnosed with primary epilepsy. The final diagnosis was IP with IAC based on further investigation of the patient's medical history, physical examination, imaging examination and postoperative pathology.
At present, IP is diagnosed using internationally recognized standards. The main indicators in patients with no previous positive family history are as follows: (1) Typical erythroid blisters in the neonatal period, and the blisters contain eosinophilia; (2) Pigmentation with a linear distribution on the trunk; and (3) Linear skin atrophy and other signs. Secondary indicators include the following aspects: (1) Baldness; (2) Abnormal teeth; (3) Retinopathy; and (4) Abnormal nails. The main diagnostic criteria for those with a positive family history are as follows: (1) Presence of pigmentation; (2) Skin and hair damage; (3) Abnormal teeth; (4) Baldness; (5) Retinopathy; and (6) Evidence of multiple pregnancy and male abortion. The comprehensive clinical diagnostic criteria are as follows: For those with no positive family history, one major diagnostic indicator and one secondary indicator are required to support the diagnosis. If there is a definite positive family history, the diagnosis can be made with only one clinical criterion[7,8].
The characteristic rash is the main clinical manifestation of IP, which often occurs at birth and in infancy. It can be roughly divided into phase I to IV. The first period is also known as lupus erythematosus blister, in which skin lesions usually occur at birth or within 2 wk after birth, and are characterized by pimples, erythema, blisters, rash along the inside of the limbs and the trunk with a linear lateral distribution. No bacteria are present in the blister fluid, but it does contain a large number of eosinophils. Skin histopathological findings have suggested typical eosinophilic sponge edema. The second period is also known as the verrucous hyperplasia period, in which an excrescence rash is formed combined with different degrees of pigmentation. Histological examination results showed stratum spinosum hypertrophy, often occurring 2-6 wk after birth. The third period is pigmentation, in which skin damage occurs more than 12-26 wk after birth. This period is characterized by pigmentation and a splash-ink swirl pattern. Histological examination revealed dermal melanin cell infiltration. The fourth period is also known as atrophic pigment loss, during which skin macular atrophy, pigmentation and atrophy of skin hair loss occur. The skin lesions in the above stages may overlap but may be alleviated to some extent with age[9,10]. In the present case, typical rashes of stages I, II and III were present, but stage III was the main manifestation at presentation, and no obvious overlap was observed.
Damage and deformity of the central nervous system is one of other major clinical manifestations in children with IP. A considerable number of children seek treatment for neurological diseases with head symptoms. In this case, the patient sought treatment due to frequent epileptic seizures, showing consistency. According to previous reports, the incidence of neurological involvement in neonatal IP children is 18%-32%[11,12]. At present, the pathogenic mechanism of central nervous system damage in children with IP is unclear, and the possibility of vasogenic damage and an inflammatory mechanism is reasonably strong. In addition, the occurrence time and disease type of neurological damage in IP children vary, and the clinical manifestations of neurological damage can be epilepsy, acute disseminated encephalomyelitis and ischemic stroke. Epilepsy can be the first symptom of the disease. The head imaging performed during hospitalization showed that the patient had intracranial space-occupying lesions and ischemic foci. The occurrence of these lesions coincides strikingly with the epidemiology of IP.
The rash in some children only appears in the neonatal period and gradually subsides after 1-2 years, which was consistent with the course of disease development of the mother in this case. In recessive children, epilepsy symptoms such as seizures with unknown causes are often misdiagnosed as primary epilepsy. However, routine treatment of such epilepsy symptoms with sodium valproate has no definite clinical effect. Therefore, it is suggested that the possibility of IP should be considered in children with convulsions without obvious cause, especially female children with a history of specific rash. In addition, systemic damage amongst IP children includes the eyes, teeth, hair, bones, finger (toe) and other areas, mainly manifested as strabismus, unexplained visual loss, cataract, optic nerve atrophy, retinal detachment, absence of teeth, tooth deformity, tooth loss, teeth misalignment, short stature, saggy wrists, saddle head and horseshoe inside turned foot[5,6,10-12].
As mentioned earlier, IP is a multi-system disease that presents with typical skin lesions at birth, but the long-term prognosis of this disease depends on the involvement of systems other than the skin, especially the nervous system and ocular lesions. In this case, intracranial space-occupying lesions were present. Combined with her clinical symptoms, signs, and preoperative imaging examinations, it was suggested that an arachnoid cyst was likely. After clear indications for surgery and exclusion of contraindications to surgery, the patient underwent a craniotomy. Combined with the postoperative pathological results, the diagnosis of IAC was confirmed. An IAC is now considered an external brain parenchymal, non-oncologic space-occupying lesion. Most scholars believe that the formation mechanism of IAC is during the early stage of arachnoid development and dura stratification. Cerebrospinal fluid (CSF) flow is damaged, and CSF constantly impinges on the lumen formed through the rupture site. In addition, there are factors secondary to trauma, including inflammation, infection, intracranial hematoma and other factors[13]. What is the probability of developing an IAC in patients with IP? By reviewing previous literature, we do not have a clear answer at this time and further research is required.
A small IAC can have no obvious clinical symptoms. However, with the gradual increase in size of the cyst, increased compression of adjacent brain tissue, blood vessels and nerves can hinder circulation of the CSF and gradually increase corresponding intracranial pressure, cranial enlargement, epilepsy, cranial nerve compression and other symptoms. Since Howship's report in 1819 and Bright's report in 1931, the diagnosis and treatment of IAC has had a history of more than 70 years, but the treatment of arachnoid cyst, especially the choice of surgical method, is controversial in the medical field. Surgical treatment of an arachnoid cyst aims to relieve the symptoms of the cyst itself on adjacent brain tissue, nerves and blood vessels, promote the recovery of brain tissue, improve clinical symptoms and reduce the recurrence rate of IAC as far as possible. At present, the main surgical methods include craniotomy for arachnoid cyst, microscopic resection of the capsule wall with communication in the subarachnoid space, a cyst peritoneal shunt, and endoscopic fistula[14-18].
Surgical intervention in the treatment of IAC is not only confined to restoring brain anatomy, but to also provide an effective treatment, recovery and safeguard normal neural physiological function. On this basis, the lumen of the adjacent subarachnoid communication adequately (pool) circulates the CSF, eventually eliminating symptoms and preventing recurrence. Holst et al[19]pointed out that a cyst-peritoneal shunt can rapidly reduce the size of the cyst and is beneficial to the recovery of compressed brain tissue, making it an effective surgical method.
Traditional craniotomy for arachnoid cyst excision has been widely used in clinical practice, and it has the advantages of full decompression, accurate curative effect and complete hemostasis. However, complete removal of the cyst is difficult due to the close adhesion between the cyst wall and adjacent brain tissues, blood vessels and nerves. Regeneration of the residual cyst wall and re-closure of the cyst wall can significantly increase the rate of IAC recurrence. However, microscopic resection of the cyst wall and the opening of the arachnoid cistern around the cyst can be performed. On the basis of partial resection of the IAC, the adjacent arachnoid cistern is communicated. Compared with the simple arachnoid cyst-peritoneal shunt, it is more in line with the physiological circulation of CSF. Moreover, because it has the advantage of not relying on the shunt device, it avoids the complications related to the postoperative shunt tube. Compared with traditional craniotomy cyst excision, neurosurgeons can more clearly explore the relationship between the IAC and surrounding tissues during microscopic surgery and the cyst can be excised more safely and effectively. In addition, the entire cyst wall does not need to be removed, thereby reducing the damage caused by separation of the cyst wall.
Finally, we chose this surgical plan based on the clinical symptoms, imaging manifestations and wishes of the child’s family members. Intraoperative neuroelectrophysiological monitoring was performed to further avoid related neurological function damage.
In summary, IP is a systemic multi-system disease characterized by skin lesions, and the long-term prognosis of this disease depends on the involvement of other systems aside from the skin, especially nervous system and ocular involvement. In terms of treatment, there is currently no cure for the skin lesions of it. If the lesions inof other systems other than the skin system are cured, there is usually no discomfort. Thuserefore, for patients with IP incontinentia pigmenti, cliniciansal doctors generally focus on symptomatic treatment, and give prioritizey to the treatment of critical symptoms. The patient recovered well after surgery and did not develop recurrent epilepsy symptoms.
| 1. | Smahi A, Courtois G, Vabres P, Yamaoka S, Heuertz S, Munnich A, Israël A, Heiss NS, Klauck SM, Kioschis P, Wiemann S, Poustka A, Esposito T, Bardaro T, Gianfrancesco F, Ciccodicola A, D'Urso M, Woffendin H, Jakins T, Donnai D, Stewart H, Kenwrick SJ, Aradhya S, Yamagata T, Levy M, Lewis RA, Nelson DL. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature. 2000;405:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 504] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 2. | Cai YR, Liang Y, Zhong X. Late contralateral recurrence of retinal detachment in incontinentia pigmenti: A case report. World J Clin Cases. 2022;10:4171-4176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (3)] |
| 3. | Nirmalasari DA, Tabri F, Waspodo N, Rimayani S, Adriani A. Incontinentia pigmenti / Bloch-Sulzberger syndrome: a case report. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:39-41. [PubMed] |
| 4. | Chambelland A, Aubert H, Bourrat E, Morice-Picard F, Puzenat E, Lacour JP, Chiaverini C; Société française de dermatologie pédiatrique research group. Incontinentia pigmenti in boys: Causes and consequences. Ann Dermatol Venereol. 2020;147:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Sun S, Li F, Liu Y, Qu H, Wong SW, Zeng L, Yu M, Feng H, Liu H, Han D. A novel inhibitor of nuclear factor kappa-B kinase subunit gamma mutation identified in an incontinentia pigmenti patient with syndromic tooth agenesis. Arch Oral Biol. 2019;101:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Frost M, Tencerova M, Andreasen CM, Andersen TL, Ejersted C, Svaneby D, Qui W, Kassem M, Zarei A, McAlister WH, Veis DJ, Whyte MP, Frederiksen AL. Absence of an osteopetrosis phenotype in IKBKG (NEMO) mutation-positive women: A case-control study. Bone. 2019;121:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Cammarata-Scalisi F, Fusco F, Ursini MV. Incontinentia Pigmenti. Actas Dermosifiliogr (Engl Ed). 2019;110:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Minić S, Trpinac D, Obradović M. Incontinentia pigmenti diagnostic criteria update. Clin Genet. 2014;85:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Habeshian KA, Kirkorian AY. Congenital Pigmentary Anomalies in the Newborn. Neoreviews. 2021;22:e660-e672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Ni Y, Huang X, Ruan L, Xue K, Yu J, Peng J, Zhao P. Intravitreal injection of ranibizumab in severe retinopathy of incontinentia pigmenti. J AAPOS. 2018;22:325-327.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Greene-Roethke C. Incontinentia Pigmenti: A Summary Review of This Rare Ectodermal Dysplasia With Neurologic Manifestations, Including Treatment Protocols. J Pediatr Health Care. 2017;31:e45-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Hadj-Rabia S, Froidevaux D, Bodak N, Hamel-Teillac D, Smahi A, Touil Y, Fraitag S, de Prost Y, Bodemer C. Clinical study of 40 cases of incontinentia pigmenti. Arch Dermatol. 2003;139:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | White ML, M Das J. Arachnoid Cysts. 2022 Mar 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. [PubMed] |
| 14. | Werner C, Mathkour M, Scullen T, Dallapiazza RF, Dumont AS, Maulucci CM. Recurrent arachnoid cysts secondary to spinal adhesive arachnoiditis successfully treated with a ventriculoperitoneal shunt. Clin Neurol Neurosurg. 2020;194:105835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Lim JW, Choi SW, Song SH, Kwon HJ, Koh HS, Youm JY. Is arachnoid cyst a static disease? Childs Nerv Syst. 2019;35:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Chen B, Miao Y, Hu Y, Liao Y, Tu M, Yang X, Qiu Y. Rare Intrasellar Arachnoid Cyst Distinguishing From Other Benign Cystic Lesions and its Surgical Strategies. J Craniofac Surg. 2019;30:e400-e402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Kim KH, Lee JY, Phi JH, Cho BK, Shin MS, Kim SK. Neurocognitive profile in children with arachnoid cysts before and after surgical intervention. Childs Nerv Syst. 2019;35:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Hayes MJ, TerMaath SC, Crook TR, Killeffer JA. A Review on the Effectiveness of Surgical Intervention for Symptomatic Intracranial Arachnoid Cysts in Adults. World Neurosurg. 2019;123:e259-e272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Holst AV, Danielsen PL, Juhler M. Treatment options for intracranial arachnoid cysts: a retrospective study of 69 patients. Acta Neurochir Suppl. 2012;114:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Surani S, United States S-Editor: Wang LL L-Editor: A P-Editor: Wang LL