Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8242
Peer-review started: December 28, 2021
First decision: April 8, 2022
Revised: April 19, 2022
Accepted: June 27, 2022
Article in press: June 27, 2022
Published online: August 16, 2022
Processing time: 215 Days and 19.9 Hours
ABSTRACT
BACKGROUND
Hepatitis-associated aplastic anemia (HAAA) is a rare condition. Patients with HAAA usually present with acute hepatitis, jaundice and significantly increased transaminase. After 1–2 mo, hepatitis gradually improves, but progressive hemocytopenia, bone marrow hematopoietic failure, and severe or extremely severe aplastic anemia are manifest. Most cases of HAAA are fulminant and usually lethal if left untreated. The literature on Epstein–Barr virus (EBV)-associated HAAA is sparse.
CASE SUMMARY
We report a 30-year-old man who was admitted to our hospital because of pale yellow urine and skin with a simultaneous decrease in peripheral blood ternary cells. We made a diagnosis of EBV-associated HAAA. The treatment strategy for this patient included eltrombopag, an immunosuppressive regimen of rabbit anti-human thymocyte immunoglobulin, cyclosporine, and supportive care. The patient was discharged in normal physical condition after five months. A hemogram performed on follow-up revealed that he had achieved a complete response.
CONCLUSION
Eltrombopag plus anti-thymocyte globubin and cyclosporine may be a therapeutic option for EBV-associated HAAA.Larger studies are warranted to confirm.
Core tip: We found an unexpected association between Epstein–Barr virus (EBV) and hepatitis-associated aplastic anemia (HAAA). Reports on EBV-associated HAAA are sparse. HAAA is a type of severe AA, and the treatment strategy pursued was in accordance with the 2016 British guidelines for AA. The patient achieved a complete response after immunosuppressive therapy. This current study highlights the importance of early diagnosis and timely therapy for EBV-associated HAAA.
- Citation: Zhang WJ, Wu LQ, Wang J, Lin SY, Wang B. Epstein–Barr-virus-associated hepatitis with aplastic anemia: A case report. World J Clin Cases 2022; 10(23): 8242-8248
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8242.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8242
Hepatitis-associated aplastic anemia (HAAA) was first reported by Lorenz and Quaiser in 1955[1]. This subtype of AA results in bone marrow failure because of the progression of hepatitis and usually appears 2–3 mo after an acute attack[2]. In most cases, HAAA is fulminant and usually lethal if left untreated[3,4]. Furthermore, it is most commonly caused by non-A, non-B, and non-C hepatitis, such as those due to Epstein–Barr virus (EBV), cytomegalovirus (CMV), human immunodeficiency virus, and parvovirus B19[4,5]. Specific viruses are not detected in most HAAA cases; however, studies have shown that the destruction of hematopoietic stem cells caused by abnormal T lymphocyte immunity is a potential pathogenic mechanism for HAAA[3]. In the current study, we report a case of a patient with EBV-associated HAAA who received antithymocyte globulin (ATG), cyclosporine (CsA), and eltrombopag. Effective recovery was observed after the treatment.
A 30-year-old man was admitted to our hospital because of pale yellow urine and skin for approximately 2 mo with a simultaneous decrease in peripheral blood ternary cells for nearly 1 mo.
The patient presented with pale yellow urine and skin, fatigue, nausea and vomiting in June 2019. Biochemical tests showed elevated liver enzymes and bilirubin, negative test results for hepatitis and CMV DNA, and a high EBV-DNA load. A liver biopsy revealed hepatitis lesions (unknown viruses). Treatment was aimed at protecting his liver by lowering the transaminase regimen and performing artificial liver therapy. His symptoms improved after the treatment.
In August 2019, a routine blood examination revealed thrombocytopenia; however, it was ignored. On 16 August 2019, he developed fever and scattered hemorrhagic points on the skin all over his body. A routine blood examination showed a decrease in peripheral blood ternary cells, and a routine bone marrow examination indicated a hyperplastic marrow with active granulocytes and erythrocytes. The distribution of megakaryocytes was reduced, and platelets were scattered. On 3 September 2019, he was transferred to the hematology department of our hospital for further treatment.
The patient had no previous medical history.
The patient had no specific personal or family history.
His temperature was 38.5°C. The patient had anemia and scattered hemorrhagic spots on the skin all over his body. There was no yellow staining of the sclera, and the liver and spleen were not palpable under ribs.
Bone marrow aspiration revealed a hyperplastic marrow with 70% granulocytes and 16% erythrocytes. A routine blood test indicated a white blood cell (WBC) count of 1100 cells/mm3, absolute neutrophil count (ANC) of 800 cells/mm3, hemoglobin level of 6.4 g/dL, platelet count of 37 × 109/L, and reticulocyte percentage (Ret%) of 1.80%. Additionally, biochemical tests showed a total bilirubin count of 21.6 μmol/L, direct bilirubin count of 9.2 μmol/L, indirect bilirubin of 12.4 μmol/L, aspartate aminotransferase level of 35 U/L, alanine aminotransferase level of 133 U/L, and γ-glutamyl transpeptidase level of 68 U/L. The percentage of CD3+CD45+ cells was 91.90%, with 65.63% CD3+CD8+ cells, 17.74% CD3+CD4+ cells, 4.62% CD19+ cells, 0.5% natural killer cells (CD3-/CD16+CD56+), and a 0.27 CD4/CD8 ratio. On quantitative detection, HBV DNA was lower than the minimum detection limit. The EBV-DNA count was 1.91 × 104 copies/mL, and the test result for CMV-DNA was negative. The patient was diagnosed with HAAA and EBV infection.
Imaging examination showed no obvious abnormality.
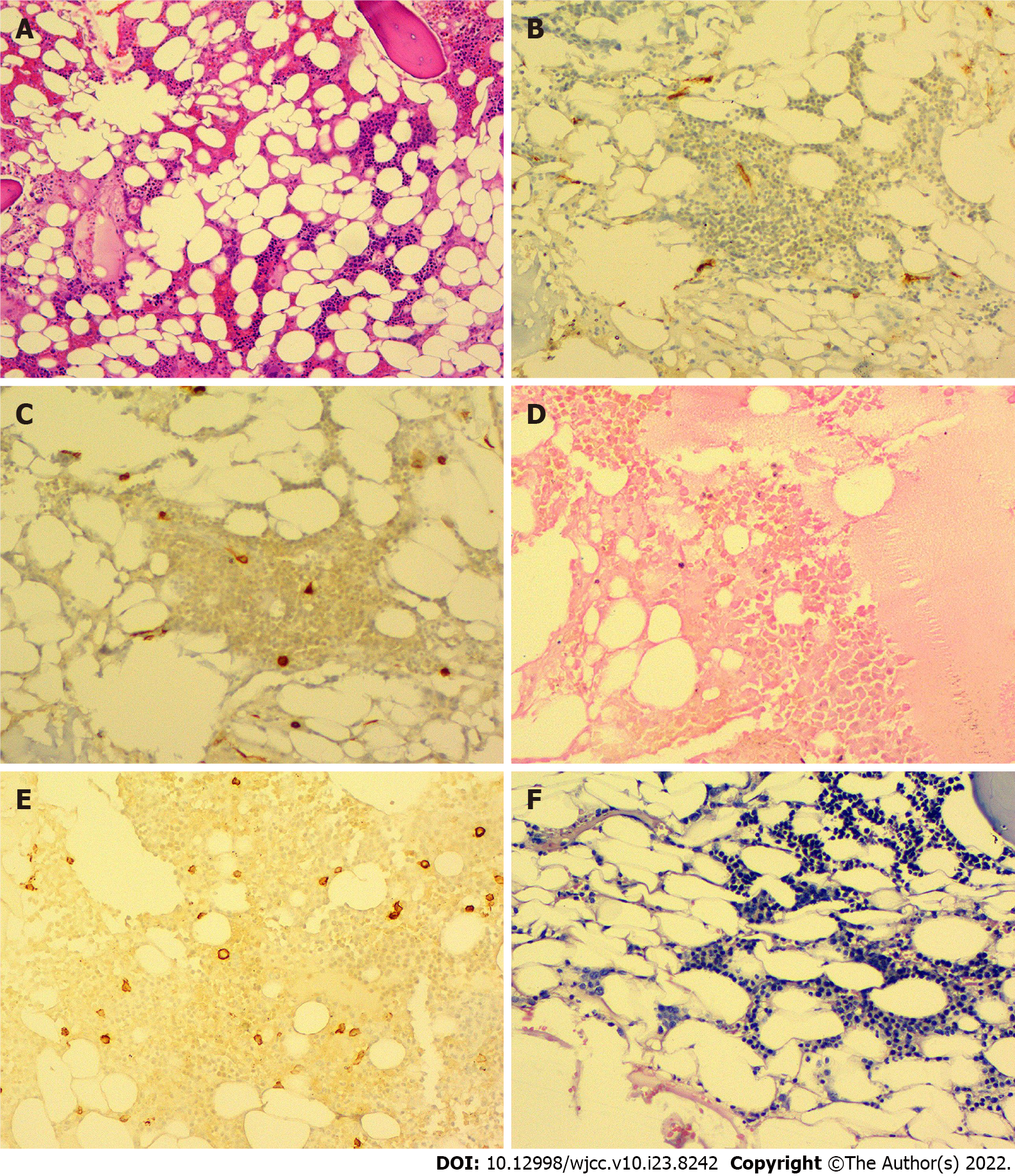
A previous liver biopsy specimen was tested again for EBV and showed negative EBV DNA in the liver tissue. On 16 September 2019, a bone marrow biopsy was performed, which showed significantly low myeloid hyperplasia. Immunohistochemical staining of the bone marrow showed CD34 (-), CD117 (minority +), MPO (partial +), CD235a (partial +), CD31 (minority +), CD20 (-), CD3 (-), and CD138 (minority +). Special staining revealed reticular fibers (-) and Perls (-) (Figure 1).
The patient was diagnosed with HAAA and EBV infection.
The patient received symptomatic treatment for liver protection, granulocyte colony-stimulating factor, and blood transfusion. On 21 September 2019, he was treated with rabbit ATG (d1–d2: 225 mg/d; d3–d5: 250 mg/d), 100 mg CsA twice daily, 50 mg eltrombopag twice daily, and cord blood. On 8 October 2019, the CMV-DNA count was 2.53 × 103 copies/mL, and gammaglobulin was administered. The EBV-DNA copy number continued to increase during treatment and reached 3.08 × 105 copies/mL on 4 November 2019 with 3.34 × 103 copies/mL of CMV DNA. CsA administration was then stopped. Thereafter, the EBV-DNA copy number showed a decreasing trend but remained high, and the test for CMV DNA became negative. On 3 December 2019, he received anti-EBV therapy with 700 mg (375 mg/m2) rituximab. During regular follow-up after treatment, tests for both EBV DNA and CMV DNA were negative, and CsA was administered again on 25 December 2019. During another follow-up, liver enzymes and bilirubin levels gradually decreased to normal levels.
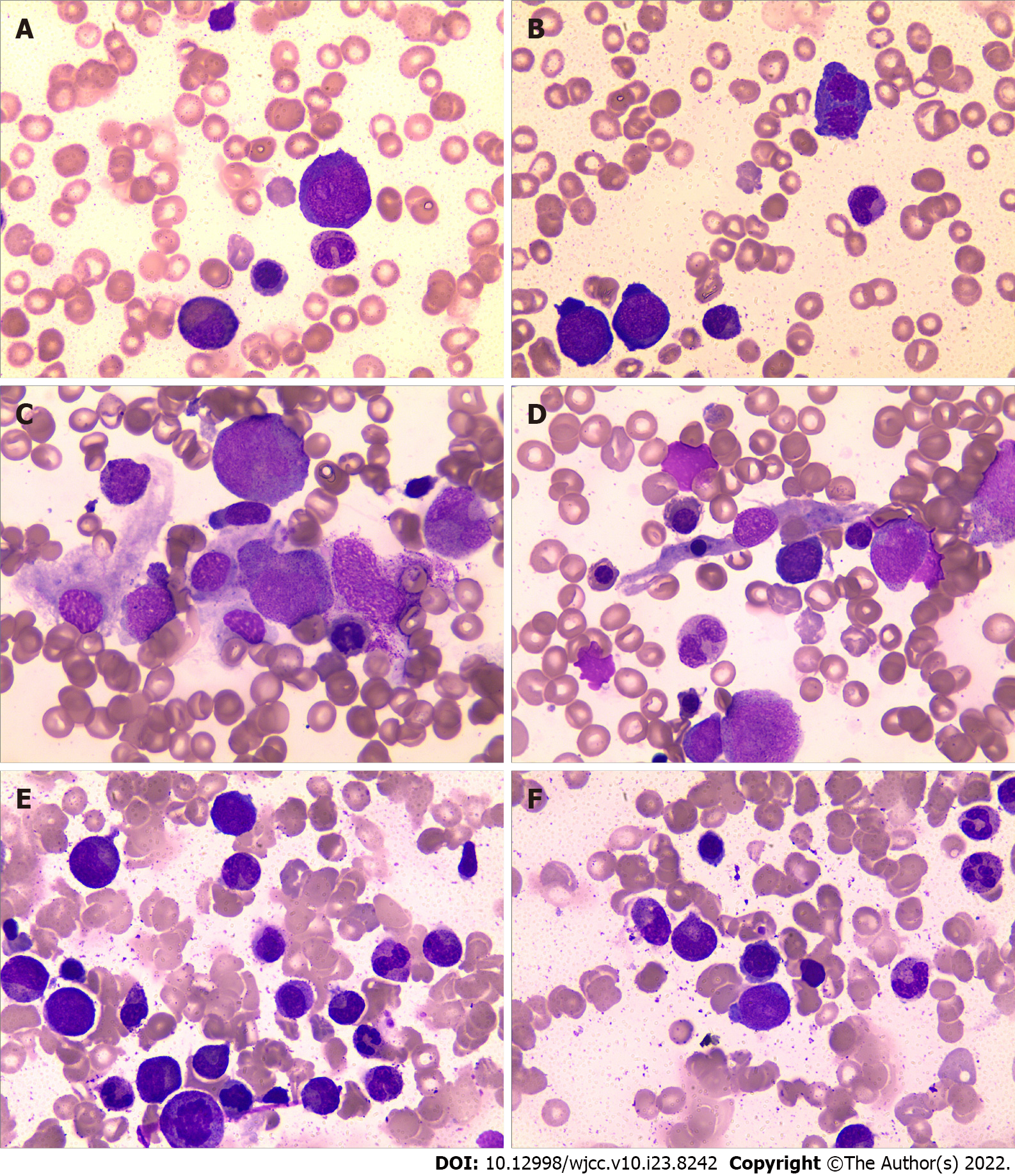
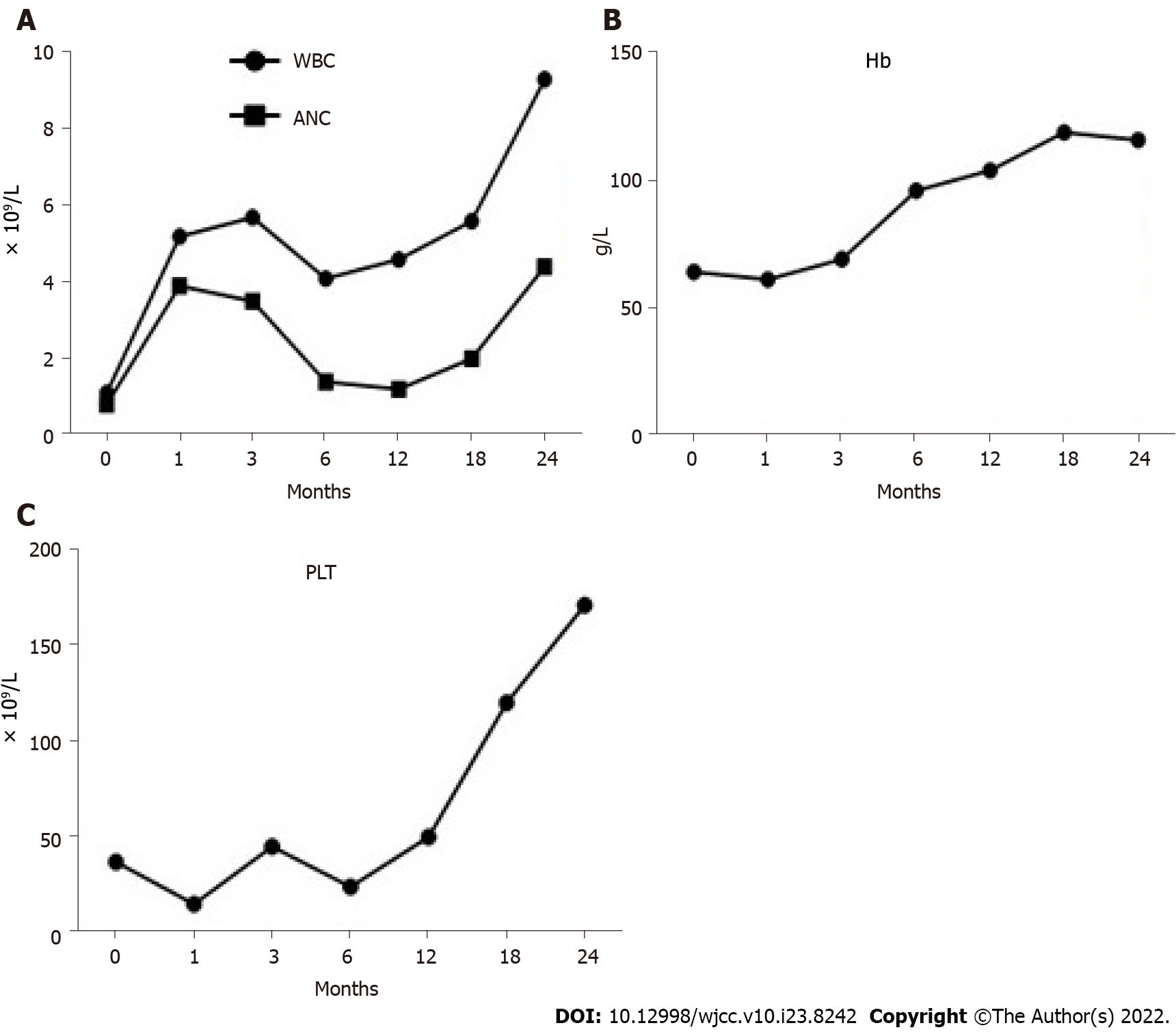
After immunosuppressive therapy, transfusion was gradually terminated, and the patient’s condition progressively improved. A bone marrow morphology test was performed again (Figures 2C–F), and the results of the complete blood count changed as the treatment progressed (Figure 3). At the follow-up, the patient’s hemogram revealed that he achieved a complete response and returned to normal. On 30 September 2021, a routine blood test showed a WBC count of 7600 cells/mm3, ANC of 2400 cells/mm3, hemoglobin level of 11.6 g/dL, platelet count of 187 × 109/L, and Ret% of 1.07%.
Liver injury due to EBV infection is common, with 80%–90% of patients exhibiting mild to moderate transient liver function abnormalities. Although it is a self-limiting disease with a good prognosis, it affects the hematological system and can cause hematopoietic dysfunction in the bone marrow, which eventually manifests as AA [6,7]. In the current study, the EBV-DNA load in the peripheral blood increased during treatment, and liver biopsy indicated hepatitis. However, tests for hepatitis and other common viruses were negative. The patient was definitively diagnosed with HAAA by peripheral blood and liver enzyme tests, bone marrow biopsy, and other related assessments. It was speculated that the patient might have suffered liver and bone marrow damage because of his persistently high EBV copy number.
The pathogenesis of HAAA is complex. HAAA patients have a decreased CD4+/CD8+ lymphocyte ratio and a high proportion of CD8+ lymphocytes, which can produce cytotoxicity and inhibit bone marrow hematopoiesis[8]. Worth et al[9] found that hepatitis-related symptoms and manifestations after EBV infection were more closely related to CD8+ and CD4+ lymphocyte proliferation than to EBV-DNA load. In our case, the detection of T lymphocyte subsets in the peripheral blood showed that CD4+ lymphocytes and the ratio of CD4+/CD8+ decreased, but CD8+ cells increased; this finding was consistent with those of previous studies[10,11]. The imbalance in the proportion of T cells leads to the enhancement of immunosuppression, which is conducive to virus replication and ultimately prevents the effective elimination of the virus. Therefore, it is speculated that the patient may have had HAAA because of abnormal T cell functioning and an immune disorder caused by EBV infection. In this case, EBV may not have been detected in the liver tissue because of the difference in detection concentrations between the peripheral blood and tissue or because the liver biopsy of the patient did not puncture the tissue site infected with EBV. Eventually, the immune disorder caused by EBV-DNA replication and liver damage led to HAAA.
HAAA is a type of severe aplastic anemia (SAA) that is characterized by high mortality and rapid progression, thus necessitating early diagnosis and treatment. Guidelines for SAA patients with a matched sibling donor recommend early hematopoietic stem cell transplantation[12]. In the current study, the patient was treated with eltrombopag and an immunosuppressive regimen of rabbit ATG and CsA to achieve a complete response. Eltrombopag is a low-molecular-weight, synthetic, nonpeptide, oral thrombopoietin receptor agonist approved by the US Food and Drug Administration in 2008 for the treatment of patients with SAA with poor response to immunosuppressive therapy[13]. Schifferli et al[14] suggested that eltrombopag might improve the hematopoietic microenvironment and promote hematopoiesis by increasing regulatory T and B cells, secreting transforming growth factor-β, impeding dendritic cell differentiation, and reducing the release of interferon-γ and tumor necrosis factor .
Previous studies have shown that the use of eltrombopag in AA did not show a significant elevation in abnormal cell clones compared with the use of IST alone[15]. Townsley et al[16] showed that the early combination of immunosuppressive therapy with eltrombopag significantly improved the overall serological response rate, hematological complete response rate, and timely rescue and expansion of residual HSPCs in SAA patients, thus accelerating the speed and quality of hematopoietic recovery.
The patient was critically ill and received eltrombopag with ATG and CsA, which led to a favorable result. It can be speculated that earlier treatment with eltrombopag leads to a better outcome. However, the optimal timing of eltrombopag for SAA with IST requires further confirmation in studies with large sample sizes.
| 1. | Lorenz E, Quaiser K. [Panmyelopathy following epidemic hepatitis]. Wien Med Wochenschr. 1955;105:19-22. [PubMed] |
| 2. | Rauff B, Idrees M, Shah SA, Butt S, Butt AM, Ali L, Hussain A, Irshad-Ur-Rehman, Ali M. Hepatitis associated aplastic anemia: a review. Virol J. 2011;8:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Alshaibani A, Dufour C, Risitano A, de Latour R, Aljurf M. Hepatitis-associated aplastic anemia. Hematol Oncol Stem Cell Ther. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Brown KE, Tisdale J, Barrett AJ, Dunbar CE, Young NS. Hepatitis-associated aplastic anemia. N Engl J Med. 1997;336:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 207] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Khurana A, Dasanu CA. Hepatitis associated aplastic anemia: case report and discussion. Conn Med. 2014;78:493-495. [PubMed] |
| 6. | Kofteridis DP, Koulentaki M, Valachis A, Christofaki M, Mazokopakis E, Papazoglou G, Samonis G. Epstein Barr virus hepatitis. Eur J Intern Med. 2011;22:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Ontanilla Clavijo G, Praena Segovia J, Giráldez Gallego Á, Cordero Matía ME, Sousa Martín JM. Severe acute hepatitis and cold agglutinin-related hemolytic anemia secondary to prime infection with Epstein-Barr virus. Rev Esp Enferm Dig. 2017;109:388-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Cengiz C, Turhan N, Yolcu OF, Yilmaz S. Hepatitis associated with aplastic anemia: do CD8(+) kupffer cells have a role in the pathogenesis? Dig Dis Sci. 2007;52:2438-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Worth AJ, Houldcroft CJ, Booth C. Severe Epstein-Barr virus infection in primary immunodeficiency and the normal host. Br J Haematol. 2016;175:559-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Tu MF, Shao ZH, Liu H, He GS, Bai J, Shi J, Cao YR, Wang HQ, Xing LM, Cui ZZ, Sun J, Chen HS, Xue YP, Yang CL. [The clinical features of hepatitis associated aplastic anemia]. Zhonghua Xue Ye Xue Za Zhi. 2005;26:239-242. [PubMed] |
| 11. | Ikeda T, Morimoto A, Nakamura S, Yokoyama K, Hayase T, Oh Y, Kashii Y, Yotsumoto S, Okamoto H, Y Momoi M. A marked decrease in CD4-positive lymphocytes at the onset of hepatitis in a patient with hepatitis-associated aplastic anemia. J Pediatr Hematol Oncol. 2012;34:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti G, Snowden JA, Samarasinghe S, Wood A, Marsh JC; British Society for Standards in Haematology. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 547] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 13. | Drexler B, Passweg J. Current evidence and the emerging role of eltrombopag in severe aplastic anemia. Ther Adv Hematol. 2021;12:2040620721998126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Schifferli A, Kühne T. Thrombopoietin receptor agonists: a new immune modulatory strategy in immune thrombocytopenia? Semin Hematol. 2016;53 Suppl 1:S31-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Scheinberg P. Activity of eltrombopag in severe aplastic anemia. Hematology Am Soc Hematol Educ Program. 2018;2018:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, Weinstein B, Valdez J, Lotter J, Feng X, Desierto M, Leuva H, Bevans M, Wu C, Larochelle A, Calvo KR, Dunbar CE, Young NS. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med. 2017;376:1540-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Silva LD, Brazil S-Editor: Wang JL L-Editor: Kerr C P-Editor: Wang JL