Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8186
Peer-review started: January 24, 2022
First decision: March 8, 2022
Revised: May 12, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 16, 2022
Processing time: 189 Days and 0.4 Hours
Acute-on-chronic liver failure (ACLF) is the abrupt exacerbation of declined hepatic function in patients with chronic liver disease.
To explore the independent predictors of short-term prognosis in patients with hepatitis B virus (HBV)-related ACLF and to establish a predictive short-term prognosis model for HBV-related ACLF.
From January 2016 to December 2019, 207 patients with HBV-related ACLF attending the 910th Hospital of Chinese People's Liberation Army were conti
There were 157 and 50 patients in the survival and death categories, respectively. Univariate analysis revealed significant differences in age, PLT, Tbil, BUN, NLR, HBsAg, AFP, GP73, INR, stage of liver failure, classification of liver failure, and incidence of complications (pulmonary infection, hepatic encephalopathy, spontaneous bacterial peritonitis, and upper gastrointestinal bleeding) between the two groups (P < 0.05). GP73 [hazard ratio (HR): 1.009, 95% confidence interval (CI): 1.005-1.013, P = 0.000], middle stage of liver failure (HR: 5.056, 95%CI: 1.792-14.269, P = 0.002), late stage of liver failure (HR: 22.335, 95%CI: 8.544-58.388, P = 0.000), pulmonary infection (HR: 2.056, 95%CI: 1.145-3.690, P = 0.016), hepatorenal syndrome (HR: 6.847, 95%CI: 1.930-24.291, P = 0.003), and HBsAg (HR: 0.690, 95%CI: 0.524-0.908, P = 0.008) were independent risk factors for short-term prognosis in patients with HBV-related ACLF. Following binary logistics regression analysis, we arrived at the following formula for predicting short-term prognosis: Logit(P) = Ln(P/1-P) = 0.013 × (GP73 ng/mL) + 1.907 × (middle stage of liver failure) + 4.146 × (late stage of liver failure) + 0.734 × (pulmonary infection) + 22.320 × (hepatorenal syndrome) - 0.529 × (HBsAg) - 5.224. The predictive efficacy of the GP73-ACLF score was significantly better than that of the Model for End-Stage Liver Disease (MELD) and MELD-Na score models (P < 0.05).
The stage of liver failure, presence of GP73, pulmonary infection, hepatorenal syndrome, and HBsAg are independent predictors of short-term prognosis in patients with HBV-related ACLF, and the GP73-ACLF model has good predictive value among these patients.
Core Tip: The stage of liver failure, presence of Golgi protein 73 (GP73), pulmonary infection, hepatorenal syndrome, and HBsAg are independent predictors of short-term prognosis in patients with hepatitis B virus-related acute-on-chronic liver failure (ACLF). In addition, the GP73-ACLF model has good predictive value, in the short term, among these patients.
- Citation: Ye QX, Huang JF, Xu ZJ, Yan YY, Yan Y, Liu LG. Short-term prognostic factors for hepatitis B virus-related acute-on-chronic liver failure. World J Clin Cases 2022; 10(23): 8186-8195
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8186.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8186
Acute-on-chronic liver failure (ACLF) is a consequence of the sudden exacerbation of chronic liver disease. The syndrome manifests through worsening of acute jaundice and coagulopathy that can cause complications including hepatic encephalopathy, ascites, electrolyte imbalance, and extrahepatic organ failure[1]. Both hepatic and extrahepatic precipitating events have been implicated in the causation of ACLF[2]. The short-term mortality rate of ACLF is estimated to be in the range of 50%-90%[3]. It is critical to avert a drastic decline in hepatic function and general patient health due to a complexity of factors early and assess the severity and prognosis of ACLF accurately. This can facilitate proper timing of liver transplantation with the potential to improve the survival rate of ACLF patients.
Recent studies found that several indicators including age, hepatic encephalopathy, total bilirubin (Tbil), prothrombin or international normalized ratio (INR), and alpha-fetoprotein (AFP) have prognostic value in evaluating liver failure. These parameters have been included in classical prognostic models of liver failure such as Child-Turcotte-Pugh (CTP) score, Model for End-Stage Liver Disease (MELD) score, MELD-sodium (Na) score, and the King’s College Hospital (KCH) criteria[1,4-7]. Since the classical prognostic model of liver failure encompasses several types of ACLF, the sensitivity and specificity of the classical prognostic models for ACLF due to a specific cause are lacking. In China, the main cause of cirrhosis in patients with ACLF is hepatitis B virus (HBV) infection.
While Kladney et al[8] found high expression of Golgi protein 73 (GP73) in hepatocytes of giant cell hepatitis, Iftikhar et al[9] found that GP73 is a novel marker for the evaluation of advanced liver disease and hepatocellular carcinoma (HCC)[9]. Some studies have shown GP73 levels to gradually increase with advancing liver inflammation in patients with HBV infection[10]. It has also been reported that serum GP73 has higher sensitivity and specificity than bilirubin in predicting the short-term prognosis of patients with HBV-related ACLF[11]. The revised Guidelines for the Prevention and Treatment of Liver Failure, in 2018, specified new clinical types and clinical stages for patients with HBV-related ACLF[1]. Therefore, the study of mentioned indicators could be helpful to establish a prognostic model for HBV-related ACLF.
The objective of this study was to explore the independent predictors of short-term prognosis in patients with HBV-related ACLF and to establish a predictive short-term prognosis model for HBV-related ACLF.
From January 2016 to December 2019, 207 patients with HBV-related ACLF attending the 910th Hospital of Chinese People's Liberation Army were continuously included in this retrospective study. According to the survival status 3 mo after diagnosis, the patients were divided into either a survival or a death group. All patients underwent venous blood examination and color Doppler ultrasound and received antiviral therapy within 24 h after admission. The antiviral treatment of choice was entecavir or tenofovir dipivoxil. The study protocol was formulated in accordance with the requirements of the Declaration of Helsinki of the World Medical Association and received approval of the Ethics Committee of the 910th Hospital of Chinese People's Liberation Army (No. 32). Written informed consent was obtained from each subject prior to participation.
Patients were allowed to participate in the study if they met the clinical diagnostic criteria for ACLF[1] and were willing to provide informed consent as a prerequisite to take part in the study.
We did not permit patients into the study for any one or a combination of the following reasons: Hepatitis A, C, D, or E virus infection; autoimmune liver disease; drug-induced liver injury; alcoholic liver disease; Wilson's disease; malignancy including HBV-related HCC; obstructive jaundice; history of liver transplantation; use of anticoagulants. Similarly, those who did not grant informed consent to participate in the study were excluded.
The diagnosis of HBV-related ACLF was determined based on a positive HBsAg test over a 6-mo period; coagulopathy (an INR of ≥ 1.5 or prothrombin activity < 40%); Tbil ≥ 10 upper limit of normal, or Tbil increase > 1 mg/dL daily[1].
We stratified the HBV-related ACLF as follows: (1) Type A: ACLF based on chronic non-cirrhotic liver disease; (2) Type B: ACLF based on compensatory cirrhosis, usually within 4 wk; and (3) Type C: ACLF based on decompensated cirrhosis.
HBV-related ACLF was further staged into early, middle, or late stage as described previously[1]. Briefly, for early stage, the prothrombin time activity (PTA) was between 30%-40% or 1.5 ≤ INR < 1.9 and there was not any complications or other extrahepatic organ failure. For the middle stage, the PTA was between 20%-30% or 1.9 ≤ INR < 2.6 together with one complication and/or failure of one extrahepatic organ. The late stage was determined by a PTA less than 20% or INR ≥ 2.6 together with two complications and/or failure of two or more extrahepatic organs.
General demographics regarding gender and age were obtained alongside coagulation function parameters including prothrombin time and INR.
Routine blood test and coagulation function were analyzed using the Sysmex XN (Sysmex, Kobe, Japan) automatic analyzer with Sysmex kit reagents. The indicators included neutrophil-to-lymphocyte ratio (NLR) and platelet count (PLT).
Blood biochemistry captured alanine aminotransferase, aspartate aminotransferase, Tbil, albumin, cholinesterase, blood urea nitrogen (BUN), creatinine (Cr), blood glucose, and Na. The TBA120FR automatic biochemical analyzer (Toshiba, Japan) was used for analyses. The kit was purchased from Beijing Kangda Taike Medical Technology Co., LTD.
The tumor marker AFP was analyzed using the Cobas E601 biochemical immunoanalyzer (roche Diagnostics, Germany). The kit was purchased from Roche Diagnostics (Shanghai) Co., LTD. GP73 was detected by ELISA, which was provided by Beijing Reking Biotechnology Co., LTD.
For virological indicators, HBV-DNA was determined by fluorescence quantitative polymerase chain reaction (PCR). Both Taq enzyme, deoxyribonucleoside-triphosphates, and uracil glycosylation enzyme were purchased from Shanghai Huamei Biological Engineering Company. Standard substance, negative and positive control substance, and PCR buffer were purchased from Shanghai Fosun Industrial Company. Primers were synthesized by Shanghai Shenyou Co., LTD. Fluorescence quantitative gene amplifiers were produced by Roche Light Cycler Co., LTD. HBV markers were measured by electrochemiluminescence assay using Cobas 6000 biochemical immunoassay [Roche Diagnostics (Shanghai) Co., LTD.] The kit was purchased from Roche Diagnostics (Shanghai) Co., LTD.
Information regarding existence of any of the following complications were also noted: Hepatic encephalopathy, hepatorenal syndrome, spontaneous peritonitis, gastrointestinal bleeding, and pulmonary infection.
We referred to previously defined models to compare the results from the current study. These are: (1) MELD score = 3.78 × ln [bilirubin (mg/dL)] + 11.24 × ln (INR) + 9.57 Ln [Cr (mg/dL)] + 6.43[4]; and (2) MELD-Na score = MELD + 1.59 × [135-Na (mmol/L)][5].
All study data were analyzed using SPSS 19.0 software and MedCalc statistical software. The normality of continuous variables was tested by the Kolmogorov-Smirnov test. Normally distributed measurement data are expressed as the mean ± SD, while non-normally distributed measurement data are expressed as median (P25, P75), and the comparisons were examined by Student’s t-test and Mann-Whitney test (non-parametric distribution). Categorical data are expressed as n (%), and the differences between two groups were examined by the chi-square test or Fisher's exact test. The risk factors affecting short-term prognosis of HBV-related ACLF were examined by Cox regression analysis. The establishment of a diagnostic model for HBV-related ACLF was based on Logistic analysis. The predictive short-term prognosis model for HBV-related ACLF was evaluated by receiver operating characteristic (ROC) analysis. Delongs method in MedCalc software was used for ROC curve analysis and comparison of various diagnostic criteria. The statistical significance level was set at 0.05 for a two-sided test.
This study recruited 207 patients with HBV-related ACLF, of whom 157 were in the survival group. The proportion of male patients was higher in the survival group (130, 82.8%) compared to the group in which patients died (27, 54.0%). On the other hand, patients who survived were on average 10 years younger compared to those who died. Specific patient demographic and clinical characteristics are shown in Table 1.
| Survival group (n = 157) | Death group (n = 50) | P value | |
| Age (yr) | 40 (32, 48) | 51(39, 55) | 0.000 |
| Gender (male, %) | 130/157, 82.8 | 42/50, 84.0 | 0.844 |
| Classification of liver failure (%) | 0.006 | ||
| A | 116/157, 73.89 | 29/50, 58.00 | |
| B | 92/157, 58.60 | 16/50, 32.00 | |
| C | 2/157, 1.27 | 5/50, 10.00 | |
| Stage of liver failure (%) | 0.000 | ||
| Early | 101/157, 64.33 | 5/50, 10.00 | |
| Middle | 42/157, 26.75 | 13/50, 26.00 | |
| Late | 14/157, 8.92 | 32/50, 64.00 | |
| Complications (%) | |||
| Pulmonary infection | 39/157, 24.84 | 24/50, 48.00 | 0.003 |
| Hepatic encephalopathy | 10/157, 6.37 | 23/50, 46.00 | 0.000 |
| Spontaneous bacterial peritonitis | 10/157, 6.37 | 11/50, 22.00 | 0.005 |
| Hepatorenal syndrome | 0/157, 0 | 4/50, 8.00 | 0.003 |
| Upper gastrointestinal hemorrhage | 2/157, 1.27 | 6/50, 12.00 | 0.003 |
| PLT (109/L) | 128 (102, 154.50) | 103 (87.50, 153) | 0.044 |
| ALB (g/dL) | 3.28 ± 0.50 | 3.21 ± 0.53 | 0.381 |
| Tbil (mg/dL) | 13.55 (9.59, 19.88) | 17.19 (12.36, 25.65) | 0.011 |
| ALT (U/L) | 948.50 (400, 1984.7) | 838.8 (331.40, 1705.78) | 0.626 |
| AST (U/L) | 687.90 (273.85, 1258.50) | 721.50 (252.50, 1554.35) | 0.800 |
| CHE (U/L) | 4359 (3288, 5429) | 3898 (2786.75, 5029.50) | 0.197 |
| Glu (mmol/L) | 4.26 (3.65, 5.23) | 4.64 (3.73, 5.75) | 0.276 |
| BUN (mg/dL) | 9.08 (7.21, 12.30) | 10.97 (8.42, 15.16) | 0.014 |
| Cr (mg/dL) | 0.73 (0.61, 0.84) | 0.76 (0.62, 0.94) | 0.309 |
| Na (mmol/L) | 136.30 (134.30, 138.00) | 135.22 (132.48, 138.33) | 0.172 |
| NLR | 2.66 (1.89, 4.05) | 4.00 (2.42, 6.43) | 0.000 |
| INR | 1.83 (1.65, 2.13) | 2.23 (1.93, 3.18) | 0.000 |
| HBsAg (log10IU/L) | 3.70 (3.31, 3.91) | 3.23 (2.65, 3.74) | 0.001 |
| HBeAg positive (%) | 89/157 (56.70) | 16/50 (32.00) | 0.002 |
| HBV-DNA (log10IU/L) | 6.34 (5.14, 7.46) | 6.39 (4.87, 7.82) | 0.698 |
| AFP (ng/mL) | 63.15 (15.35, 204) | 16.18 (6.79, 48.33) | 0.000 |
| GP73 (ng/mL) | 237.92 (208.84, 309.79) | 305.83 (215.32, 366.49) | 0.003 |
Univariate analysis revealed significant differences in several parameters, including age, disease condition, and biochemical profile as summarized in Table 1. PLT, Tbil, BUN, NLR, HBsAg, HBeAg, AFP, GP73, INR, stage of liver failure, classification of liver failure, and incidence of complications (pulmonary infection, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome, and upper gastrointestinal bleeding) differed significantly between the two groups (P < 0.05) (Table 1).
Independent variables were included in the collinearity analysis. The tolerance of each variable was greater than 0.1, and the variance inflation factor was less than 10, showing no obvious multicollinearity amongst the variables. Amongst factors included in the Cox analysis, GP73, stage of liver disease, pulmonary infection, hepatorenal syndrome, and HBsAg were independent risk factors for short-term prognosis in patients with HBV-related ACLF (P < 0.05) (Table 2).
| B | SE | Wald | HR | P value | 95%CI | |
| GP73 (ng/mL) | 0.009 | 0.002 | 23.872 | 1.009 | 0.000 | 1.005-1.013 |
| Stage of liver failure (middle) | 1.621 | 0.529 | 9.373 | 5.056 | 0.002 | 1.792-14.269 |
| Stage of liver failure (late) | 3.106 | 0.490 | 401.135 | 22.335 | 0.000 | 8.544-58.388 |
| Pulmonary infection | 0.721 | 0.298 | 5.832 | 2.056 | 0.016 | 1.145-3.690 |
| Hepatorenal syndrome | 1.924 | 0.646 | 8.867 | 6.847 | 0.003 | 1.930-24.291 |
| HBsAg | -0.371 | 0.140 | 6.998 | 0.690 | 0.008 | 0.524-0.908 |
The formula for calculating the predicted survival 3 mo after diagnosis, derived from binary logistics regression, was Logit(P) = Ln(P/1-P) = 0.013 × (GP73 ng/mL) + 1.907 × (middle stage of liver failure) + 4.146 × (late stage of liver failure) + 0.734 × (pulmonary infection) + 22.320 × (hepatorenal syndrome) - 0.529 × (HBsAg) - 5.224. In this case, P represents the survival probability of patients after 3 mo. A score of 1, in the case of 1-P, indicates that the patient has advanced stage of liver disease, pulmonary infection, and hepatorenal syndrome. If other factors, as mentioned previously, were existent, a score of 0 (as to read “0-P”) was applied instead. This multifactor model displayed good fitting of data based on the Hosmer-Lemeshow test (P = 0.467).
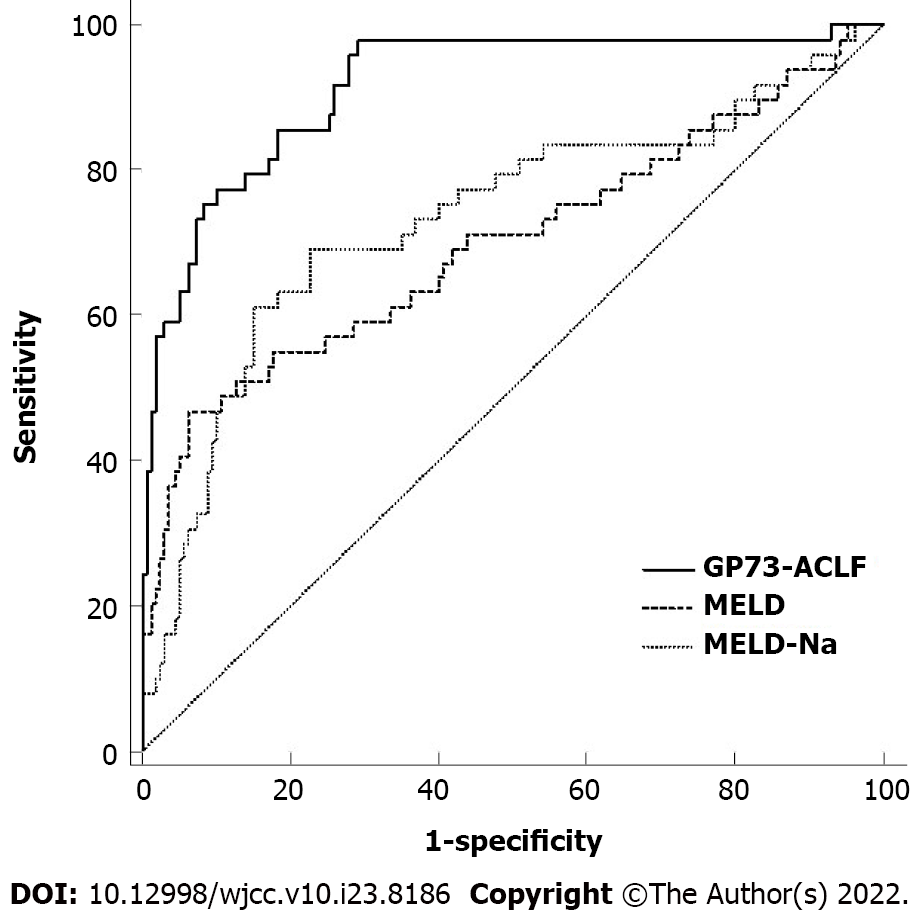
Results from the GP73-ACLF prognostic model were compared with those obtained using MELD and MELD-Na scores (Figure 1). The specific ROC curve analysis values for the models are shown in Table 3. The predictive efficacy of the GP73-ACLF score was significantly better than those of MELD and MELD-Na scores in patients with HBV-related ACLF (P < 0.05) (Table 4).
| Prognostic model | Area under curve | 95%CI | Sensitivity | Specificity | Youden’s index | Positive predictive value | Negative predictive value |
| MELD | 0.699 | 0.631-0.761 | 0.652 | 0.543 | 0.196 | 31.24 | 82.80 |
| MELD-Na | 0.734 | 0.668-0.793 | 0.684 | 0.554 | 0.238 | 32.81 | 84.63 |
| GP73-ACLF | 0.916 | 0.870-0.950 | 0.816 | 0.597 | 0.413 | 39.20 | 91.06 |
| Prognostic model | Difference in area | 95%CI | Z | P value |
| GP73-ACLF vs MELD | 0.217 | 0.122-0.312 | 4.487 | < 0.0001 |
| GP73-ACLF vs MELD-Na | 0.183 | 0.087-0.278 | 3.746 | 0.0002 |
| MELD vs MELD-Na | 0.035 | -0.018 to 0.087 | 1.286 | 0.198 |
The current treatment of HBV-related ACLF mainly relies on use of drugs, artificial liver support therapy, and liver transplantation. Liver transplantation remains the only effective approach to manage HBV-related ACLF[1]. In China, the scarcity of liver donors, and concerns due to post-surgical complications and continuous postoperative immunotherapy present hurdles in the application of liver transplantation to varying degrees. Therefore, early and accurate judgment of the severity and prognosis of HBV-related ACLF patients is essential for the development of a suitable clinical treatment plan.
The development of HBV-related ACLF involves various cytokines. The study of these cytokines is helpful in understanding the pathogenesis of HBV-related ACLF. In this study, univariate analysis showed that age, PLT, Tbil, BUN, NLR, HBsAg, HBeAg, AFP, GP73, INR, stage of liver failure, classification of liver failure, and incidence of complications were associated with survival outcomes 3 mo after diagnosis. Tbil and INR have been recognized as prognostic indicators of viral hepatitis-associated liver failure[3]. As liver damage worsens, the liver's ability to clear endotoxins decreases. The accumulation of endotoxin in turn induces platelet aggregation and their subsequent activation and damage, resulting in a decrease in platelet count. On the other hand, hypersplenism secondary to cirrhosis can also cause a decrease in platelet count. Therefore, platelet level can reflect liver function and the degree of cirrhosis, to a certain extent. The results of this study suggest that platelets may play a role in predicting the prognosis of chronic subacute liver failure.
Similar findings were found in studies on NLR, which was significantly higher in ACLF patients than in patients with chronic hepatitis B at admission. Furthermore, NLR was associated with the severity of the disease and 3-mo mortality[12]. The increase in NLR in patients in the death group was mainly due to a decrease in the number of lymphocytes. NLR reflected the severity of inflammation, and in patients with malignancies, NLR was markedly increased in peripheral blood and significantly decreased after receiving treatment[13]. In patients with HBV-related ACLF, changes in NLR during treatment may be used as an indicator to determine the prognosis.
This study showed that age and hepatic encephalopathy were independent predictors of short-term prognosis in patients with HBV-related ACLF, consistent with previous studies[14,15]. GP73 is expressed in the hilar bile duct epithelial cells, with little or no expression in normal liver cells, and increased in autoimmune hepatitis, hepatitis B, or C virus infection[16]. Iftikhar et al[9] found that GP73 was mainly derived from hepatocytes and activated hepatic stellate cells, suggesting that serum GP73 could better reflect the pathological changes of the liver[9]. An increasing number of current studies have confirmed that GP73, as a liver cancer marker, has increased cellular expression levels in acute or chronic liver disease, and that serum GP73 levels gradually increased in patients with aggravated inflammation[17]. In the current study, GP73 was also confirmed as an independent predictor of short-term prognosis in HBV-related ACLF. High GP73 expression by hepatocytes has been associated with liver inflammation resulting from an HBV-induced immune response[18]. However, this association coexisted in viral and non-viral liver diseases[9]. In patients with chronic hepatitis B, serum GP73 levels were not associated with HBeAg status or HBV-DNA levels[17]. Although the exact mechanism of GP73 on liver injury is not clear, studies have shown that GP73-deficient mice are more likely to develop severe liver cell injury, suggesting that GP73 levels have a certain role in predicting the severity of liver injury[19].
Current prognostic evaluation models for liver failure include CTP score, MELD score, MELD-Na score, and KCH criteria. In one study, the evaluation of ascites and hepatic encephalopathy in CTP score was subjective, and the prognosis of patients in the same grade varied greatly. This limits the application of the model predicting prognosis among patients with liver failure[20]. MELD score was first used for the short-term prognostic assessment of patients undergoing transjugular portosystemic shunting and was modified to rely on objective experimental parameters to distinguish the severity of the patient's condition[21]. In recent years, MELD score has been widely used to predict the mortality of patients with end-stage liver disease, and many studies have used it to assess the prognosis of HBV-related ACLF[22]. However, MELD score did not take liver failure-related complications, such as hepatic encephalopathy and gastrointestinal bleeding, into account. At present, the most widely used prognostic model for liver failure, KCH criteria, had the strongest predictive power and high specificity, and can also be used to evaluate patients undergoing liver transplantation, but the sensitivity was relatively poor[6]. In this study, the area under the ROC curve of the GP73-ACLF model for prognostic prediction of HBV-related ACLF patients reached 0.916, with a sensitivity of 81%, specificity of 60%, positive predictive value of 39%, and negative predictive value of 91%, which was higher than that of the MELD score and MELD-Na score. Moreover, the model could accurately determine whether patients with HBV-related ACLF require liver transplantation as a treatment approach in the short term. This can be reassessed based on disease progression.
Our study, despite offering promising insights, suffers some limitations. First, this was a single-center retrospective study with a small sample size that may not be generalized. Second, the specificity (60%) of the GP73-ACLF model was only modest; this can negatively affect the predictive power of the model. Notably, however, the specificity of the GP73-ACLF model was significantly improved by combining it with the KCH criteria.
Overall, GP73, stage of liver disease, pulmonary infection, hepatorenal syndrome, and HBsAg are independent risk factors for short-term prognosis in patients with HBV-related ACLF. In addition, the GP73-ACLF model displays good predictive value for clinical outcomes in the short term.
Acute-on-chronic liver failure (ACLF) refers to a syndrome precipitated by sudden worsening of chronic liver disease, seen as severe acute jaundice, coagulopathy, and other manifestations of liver failure. The short-term mortality rate of ACLF is 50%-90%.
Early and accurate assessment of disease severity and short-term prognosis in patients with ACLF can help determine the timing of liver transplantation, which can significantly improve the survival rate of patients with ACLF.
To explore the independent predictors of short-term prognosis in patients with hepatitis B virus (HBV)-related ACLF and to establish a predictive short-term prognosis model for HBV-related ACLF.
Patients were divided into either a survival group or a death group according to their survival 3 mo after diagnosis. Data of relevant observation indicators of patients were retrospectively collected and analyzed. After determining the influencing factors of short-term prognosis, a prognostic model was established based on binary logistics regression and the prediction value of this model was tested by comparing with selected classical prognostic models.
Univariate analysis showed significant differences in age, platelet count, total bilirubin, blood urea nitrogen, neutrophil-to-lymphocyte ratio, HBsAg, alpha-fetoprotein, Golgi protein 73 (GP73), international normalized ratio, stage of liver failure, classification of liver failure, and incidence of complications between the groups. In addition, GP73, stage of liver failure, pulmonary infection, hepatorenal syndrome, and HBsAg were independent risk factors for short-term prognosis in patients with HBV-related ACLF. The predictive efficacy of the GP73-ACLF score prognostic model was significantly better than those of both the Model for End-Stage Liver Disease (MELD) and MELD-Na score models.
The GP73-ACLF model has good predictive value, while GP73, stage of liver disease, pulmonary infection, hepatorenal syndrome, and HBsAg are independent risk factors for short-term prognosis in patients with HBV-related ACLF.
Combined with the King’s College Hospital Criteria, the low specificity of GP73-ACLF prognostic model can be greatly enhanced; this is worth verifying in subsequent studies.
| 1. | Liver Failure and Artificial Liver Group; Chinese Society of Infectious Diseases; Chinese Medical Association. Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. [Guideline for diagnosis and treatment of liver failure]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 57] [Reference Citation Analysis (1)] |
| 2. | Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (2)] |
| 3. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2276] [Article Influence: 175.1] [Reference Citation Analysis (6)] |
| 4. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2107] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 5. | Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R, Kamath P, Klintmalm G. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 572] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 6. | O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1517] [Cited by in RCA: 1335] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 7. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5823] [Article Influence: 109.9] [Reference Citation Analysis (2)] |
| 8. | Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, Simon DJ, Koutoubi Z, Fimmel CJ. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene. 2000;249:53-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 212] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Iftikhar R, Kladney RD, Havlioglu N, Schmitt-Gräff A, Gusmirovic I, Solomon H, Luxon BA, Bacon BR, Fimmel CJ. Disease- and cell-specific expression of GP73 in human liver disease. Am J Gastroenterol. 2004;99:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Xu Z, Shen J, Pan X, Wei M, Liu L, Wei K, Yang H, Huang J. Predictive value of serum Golgi protein 73 for prominent hepatic necroinflammation in chronic HBV infection. J Med Virol. 2018;90:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (3)] |
| 11. | Wei H, Zhang J, Li H, Ren H, Hao X, Huang Y. GP73, a new marker for diagnosing HBV-ACLF in population with chronic HBV infections. Diagn Microbiol Infect Dis. 2014;79:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 12. | Zheng MH, Shi KQ, Fan YC, Li H, Ye C, Chen QQ, Chen YP. A model to determine 3-month mortality risk in patients with acute-on-chronic hepatitis B liver failure. Clin Gastroenterol Hepatol. 2011;9:351-356.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Ietomi K. [A study on the role of granulocytes in carcinoma-bearing hosts--G/L ratio as a new host indicator]. Nihon Gan Chiryo Gakkai Shi. 1990;25:662-671. [PubMed] |
| 14. | Xiang DD, Zhang S, Wang YM, Chen YK. Analysis of Prognostic Factors in 477 Patients with Severe Viral Hepatitis. Disan Junyi Daxue Xuebao. 2001;23:2. |
| 15. | Khot AA, Somani P, Rathi P, Amarapurkar A. Prognostic factors in acute-on-chronic liver failure: a prospective study from western India. Indian J Gastroenterol. 2014;33:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Ba MC, Long H, Tang YQ, Cui SZ. GP73 expression and its significance in the diagnosis of hepatocellular carcinoma: a review. Int J Clin Exp Pathol. 2012;5:874-881. [PubMed] |
| 17. | Xu Z, Liu L, Pan X, Wei K, Wei M, Yang H, Liu Q. Serum Golgi protein 73 (GP73) is a diagnostic and prognostic marker of chronic HBV liver disease. Medicine (Baltimore). 2015;94:e659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (3)] |
| 18. | Block TM, Comunale MA, Lowman M, Steel LF, Romano PR, Fimmel C, Tennant BC, London WT, Evans AA, Blumberg BS, Dwek RA, Mattu TS, Mehta AS. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc Natl Acad Sci U S A. 2005;102:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 304] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 19. | Wright LM, Yong S, Picken MM, Rockey D, Fimmel CJ. Decreased survival and hepato-renal pathology in mice with C-terminally truncated GP73 (GOLPH2). Int J Clin Exp Pathol. 2009;2:34-47. [PubMed] |
| 20. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh vs MELD. J Hepatol. 2005;42 Suppl:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 443] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 21. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3778] [Article Influence: 151.1] [Reference Citation Analysis (2)] |
| 22. | McPhail MJ, Farne H, Senvar N, Wendon JA, Bernal W. Ability of King's College Criteria and Model for End-Stage Liver Disease Scores to Predict Mortality of Patients With Acute Liver Failure: A Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:516-525.e5; quiz e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gerlich W, Germany; Leowattana W, Thailand S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR