Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7459
Peer-review started: November 3, 2021
First decision: December 27, 2021
Revised: January 8, 2022
Accepted: June 4, 2022
Article in press: June 4, 2022
Published online: July 26, 2022
Processing time: 250 Days and 1.4 Hours
Anaplastic carcinoma mural nodules in ovarian mucinous tumors are very rare. This study aimed to report the morphological characteristics, molecular detection results, clinical treatment and prognosis of three ovarian mucinous tumors with mural nodules of anaplastic carcinoma.
The pathomorphological features, molecular detection results, clinical treatment and prognosis of anaplastic carcinoma mural nodules were described in three cases. In case 1, sarcoma-like mural nodules (SLMNs) coexisted with anaplastic carcinoma mural nodules. No mutation was found in mucinous tumors. KRAS mutation was found in anaplastic carcinoma nodules and heterotypic cells were found in SLMNs. In case 2, KRAS mutation occurred in the mucinous epithelium and BRAF mutation occurred in mural nodules. In case 3, both mural nodules and mucinous tumors had the same KRAS mutation and a morphological transition between them was observed. All three patients died within 2 years, whether receiving chemotherapy or not.
Anaplastic carcinoma mural nodules may develop from dedifferentiation of mucinous tumors or are unrelated to mucinous tumors.
Core Tip: Anaplastic carcinoma mural nodules in ovarian mucinous tumors are rare. The pathomorphological features, molecular detection results, clinical treatment and prognosis of anaplastic carcinoma mural nodules were described in three cases. After limited molecular detection, it is inferred that the mural nodules of anaplastic carcinoma may arise from (1) dedifferentiation of mucinous tumors or (2) a tumor unrelated to mucinous tumors and the KRAS signal pathway may be involved in the formation of this tumor and is unrelated to mucinous tumors. The KRAS signal pathway may be involved in the formation of mural nodules of anaplastic carcinoma. The mural nodules of anaplastic carcinoma may promote the progression of borderline mucinous ovarian tumors.
- Citation: Wang XJ, Wang CY, Xi YF, Bu P, Wang P. Ovarian mucinous tumor with mural nodules of anaplastic carcinoma: Three case reports. World J Clin Cases 2022; 10(21): 7459-7466
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7459.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7459
Primary ovarian mucinous tumors, whether benign, borderline or malignant may be associated with mural nodules of various types including true sarcomas, sarcoma-like mural nodules[1], anaplastic carcinomas, carcinosarcomas, mixed nodules and leiomyomas[2]. However, cases of mucinous tumors of the ovary with mural nodules are rare. Also, the histogenesis of the mural nodules is unclear.
This study described the morphological characteristics, molecular detection results, clinical treatment and prognosis of three ovarian mucinous tumors with mural nodules of anaplastic carcinoma so as to supplement the clinicopathological features of this rare case.
Case 1: The patient complained of a conscious abdominal circumference increase for 2 wk and then came to our hospital for further examination.
Case 2: The patient felt lower abdominal distension for more than 2 wk and came to our hospital for further examination.
Case 3: The patient felt dull abdominal pain for 12 years and as it became more frequent and severe, she came to our hospital to relieve her symptoms.
Case 1: The patient complained of a conscious abdominal circumference increase.
Case 2: The patient felt lower abdominal distension.
Case 3: The patient felt dull abdominal pain.
Cases 1-3: Patients' past physical health.
Cases 1 and 2: The patients have no family genetic predisposition.
Case 3: The patient underwent laparoscopic cholecystectomy for gallstones in 2013 and had no family genetic tendency.
Case 1: The patient has normal development, moderate nutrition, clear mind, cooperative physical examination, no obvious enlargement of superficial lymph nodes in the whole body, clear respiratory sounds on auscultation of both lungs, no dry or wet rales, strong heart sound with a uniform rate and no pathological murmur in each valve area. Abdominal swelling, no gastrointestinal type and peristaltic wave, tough abdominal texture, no rebound pain and muscle tension, can touch a package, about 20 cm × 10 cm, mobile voiced negative. No swelling of both lower limbs, normal bowel sounds. There is no deformity of the spine, the limbs move freely, the physiological reflex exists and the pathological reflex is not drawn out.
Case 2: The patient has normal development, moderate nutrition, clear mind, cooperative physical examination, no obvious enlargement of superficial lymph nodes in the whole body, clear respiratory sounds on auscultation of both lungs, no dry or wet rales, strong heart sounds with a uniform rate and no pathological murmur in each valve area. Abdominal swelling, no gastrointestinal type and peristaltic wave, tough abdominal texture, no rebound pain and muscle tension, can touch a package, about 15 cm × 9 cm, mobile voiced negative. No swelling of both lower limbs, normal bowel sounds. There is no deformity of the spine, the limbs move freely, the physiological reflex exists and the pathological reflex is not drawn out.
Case 3: The patient has normal development, moderate nutrition, clear mind, cooperative physical examination, no obvious enlargement of superficial lymph nodes in the whole body, clear respiratory sounds on auscultation of both lungs, no dry or wet rales, strong heart sounds with a uniform rate and no pathological murmur in each valve area. The abdomen is slightly swollen, without gastrointestinal type and peristaltic wave. The abdomen is tough, without rebound pain and muscle tension. A lump can be touched, about 10 cm × 7 cm, mobile voiced negative. No swelling of both lower limbs, normal bowel sounds. There is no deformity of the spine, the limbs move freely, the physiological reflex exists and the pathological reflex is not drawn out.
Cases 1 and 2: The serum CEA, CA125, CA199 and AFP levels were within the normal range.
Case 3: The serum level of CA125 and CA199 was 142.07 U/mL (normal range < 30 U/mL) and 524.60 U/mL (normal range < 37 U/mL), respectively.
Case 1: Ultrasonic imaging showed a huge, cystic mixed mass with a thick cystic area and septum in the abdominal cavity. The last computed tomography (CT) examination showed that multiple enlarged lymph nodes (the largest one was about 2.1 cm in diameter) in the retroperitoneal space, left internal iliac vascular space, upper mediastinal vascular space, right anterior trachea, subcarinal and right hilum were considered metastatic. Masses in the right accessory area (3.1 cm × 2.7 cm) were considered metastatic. Large amounts of pelvic effusion and bilateral pleural effusion were considered. Adenocarcinoma cells were found in the pleural fluid along with ascites.
Case 2: Ultrasonic imaging showed multilocular cystic solid masses in the pelvic cavity.
Case 3: Ultrasonic imaging showed a cystic solid mass on the right side of the pelvic cavity with a clear boundary. CT results showed solid nodules in S4, S5 and S7 segments of the liver, considering metastasis, and the CA199 level increased continuously.
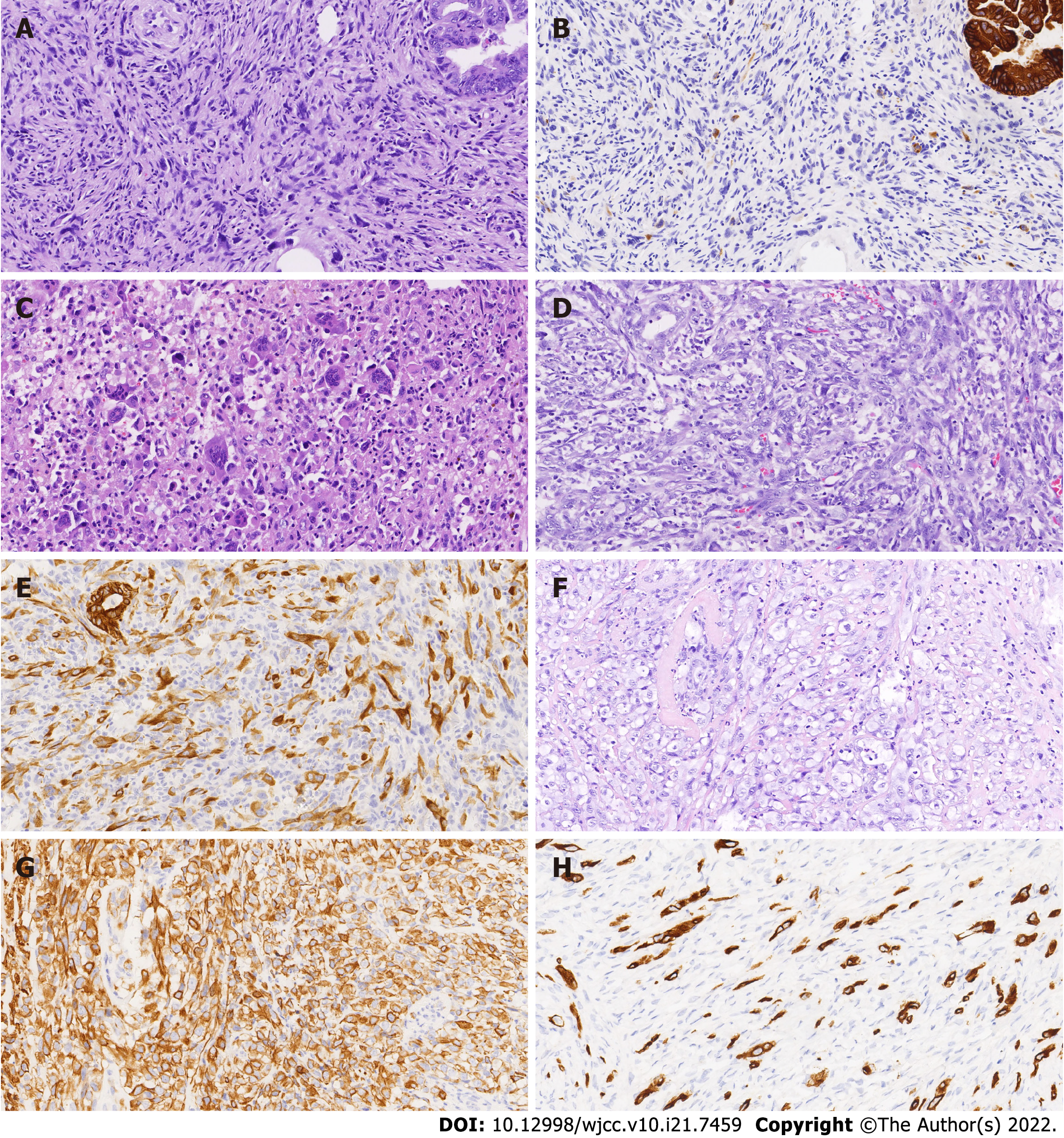
Case 1: The left ovary tumor measured 18 cm × 17 cm × 2 cm. The tumor was cystic and composed of benign, borderline or malignant intestinal mucinous epithelium. Several gray/dark mural nodules (diameter 0.5-1.2 cm) projected into the cyst cavities. Some nodules were composed of AE1/AE3- spindle cells (shown in Figure 1A and B), multinucleated giant cells and histiocytes. Necrosis and hemosiderin deposits were found in these nodules. Multinucleated giant cells with abundant eosinophilic cytoplasm were detected with an osteoclast-like appearance (shown in Figure 1C). Some AE1/AE3+ spindle cells showed invasive growth with nuclear pleomorphism and active mitotic figures (approximately 25/10 per high-power field, HPF) in another nodule (shown in Figure 1D and E). The final histopathological diagnosis was a mucinous adenocarcinoma with SLMNs and anaplastic carcinoma mural nodules, without omentum involvement. Ovarian mucinous adenocarcinoma cells were found in the ascites.
Case 2: The right ovary measured 11 cm × 8 cm × 2 cm with a mural nodule (diameter 3 cm). The AE1/AE3+ rhabdomyoid cells were distributed in the fibrous interstitium of mural nodules (shown in Figure 1F and G). The nuclei were obviously heteromorphic with nucleoli, mitotic figures (> 15/10 HPF) and pathological mitosis. The right ovary was diagnosed with a mucinous borderline tumor having mural nodules of anaplastic carcinoma. No lesions were found in the omentum, lymph nodes and appendix.
Case 3: The size of the right ovary was 12 cm × 10 cm × 2 cm and two gray/white nodules (diameter 0.7-1.1 cm) were observed on the cyst wall. The nodule (diameter approximately 0.7 cm) was infiltrated with atypical mucinous glands and heterotypic AE1/AE3+ spindle cells were seen in another nodule (1.1 cm in diameter) with mitotic figures (approximately 7/10 HPF) (shown in Figure 1H). The final histopathological diagnosis was a mucinous adenocarcinoma with anaplastic carcinoma mural nodules.
The immunohistochemical and molecular results are shown in Table 1.
| Case 1 | Case 2 | Case 3 | ||||
| Epithelium | Nodule | Epithelium | Nodule | Epithelium | Nodule | |
| Immunohistochemical | ||||||
| AE1/CK7 | + | + | + | + | + | + |
| Vim | - | + | - | + | - | + |
| ER/PR | - | - | - | - | - | - |
| Pax8 | Focally+ | - | + | + | + | + |
| TP53 | WT | WT | WT | WT | WT | WT |
| PTEN | + | + | + | + | - | - |
| Hotspot mutations | ||||||
| KRAS | WT | p.G12S/D | p.G12S/D | WT | p.G13D | p.G13D |
| NRAS | WT | WT | WT | WT | WT | WT |
| BRAF | WT | WT | WT | p.V600E/K/R/O | WT | WT |
The final histopathological diagnosis was a mucinous adenocarcinoma with SLMNs and anaplastic carcinoma mural nodules without omentum involvement. Ovarian mucinous adenocarcinoma cells were found in the ascites. The tumor was staged as International Federation of Gynecology and Obstetrics (FIGO 2014) Ic.
The right ovary was diagnosed with a mucinous borderline tumor having mural nodules of anaplastic carcinoma. No lesions were found in the omentum, lymph nodes and appendix. (FIGO 2014) Ia.
The final histopathological diagnosis was a mucinous adenocarcinoma with anaplastic carcinoma mural nodules. (FIGO 2014)IIIc.
The patient refused to receive the subsequent treatment.
The patient received chemotherapy (six courses of paclitaxel and carboplatin) in other hospitals. The specific details were unknown.
After the surgery, paclitaxel + carboplatin systemic chemotherapy was given for six cycles. Each cycle was monitored using ultrasound and blood tumor markers. After four cycles, the CA199 level gradually decreased to 17.05 U/mL. After five cycles, the CA199 level began to increase. At the same time, abdominal CT showed solid nodules in the S5 segment of the liver. After six cycles, the patient received two cycles of systemic chemotherapy of docetaxel + oxaliplatin. The level of blood tumor marker CA199 did not decrease significantly. Gemcitabine chemotherapy was continued for two cycles. At this time, the CT results showed solid nodules in S4, S5, and S7 segments of the liver, considering metastasis, and the CA199 level increased continuously.
Case 1 died of the disease 12 mo following tumor resection. Case 2 died of the disease 18 mo after her surgery. Case 3 died 14 mo after the surgery due to the disease.
The detailed clinicopathological features are shown in Table 2.
| Case | Age in yr | Mucinous tumor | Size of nodule in cm3 | Nodule type | FIGO stage | Prognosis |
| 1 | 25 | MA | 4 × 4 × 4 | SAAC | Ic | DT (12 mo) |
| 2 | 60 | BMC | 3 × 3 × 3 | AC | Ia | DT (18 mo) |
| 3 | 55 | MA | 1 × 1 × 1 | AC | IIIc | DT (14 mo) |
Sporadic reports of malignant mural nodules such as clear cell carcinoma, carcinosarcoma, sarcoma and anaplastic carcinoma have been found[3]. The mural nodules of anaplastic carcinoma can be divided into three types: rhabdomyoid, spindle cell-like and pleomorphic[4]. The anaplastic carcinoma cells in the present report were spindle cell-like and rhabdomyoid.
The differential diagnosis between SLMNs and anaplastic carcinoma mural nodules is generally considered to be particularly important. SLMNs usually occur in young women and are characterized by a small size[5]. Matias et al[6] proposed that cytokeratin immunohistochemical staining should be used to distinguish between SLMNs and anaplastic carcinoma mural nodules. CK staining was negative or focal positive in SLMNs, while strong positive diffuse staining was detected in anaplastic carcinoma nodules. Shao et al[7] mentioned that most benign SLMNs were associated with anaplastic carcinoma mural nodules. Case 1 was a young woman and the diameter of the mural nodules was 0.5-1.2 cm. Some nodules were SLMNs and AE1/AE3 staining was focal positive. However, in other nodules, spindle cells showed invasive growth and diffusely expressed AE1/AE3. The heterogeneous expression of AE1/AE3 suggested the presence of SLMNs and anaplastic carcinoma mural nodules. Case 1 was very young. The cells in the mural nodule of anaplastic carcinoma were spindle shaped. They were difficult to distinguish from the spindle cells in the mural nodule of SLMNs and easy to be ignored during diagnosis. Therefore, enough samples are needed for careful observation when ovarian mucinous tumors are accompanied by mural nodules.
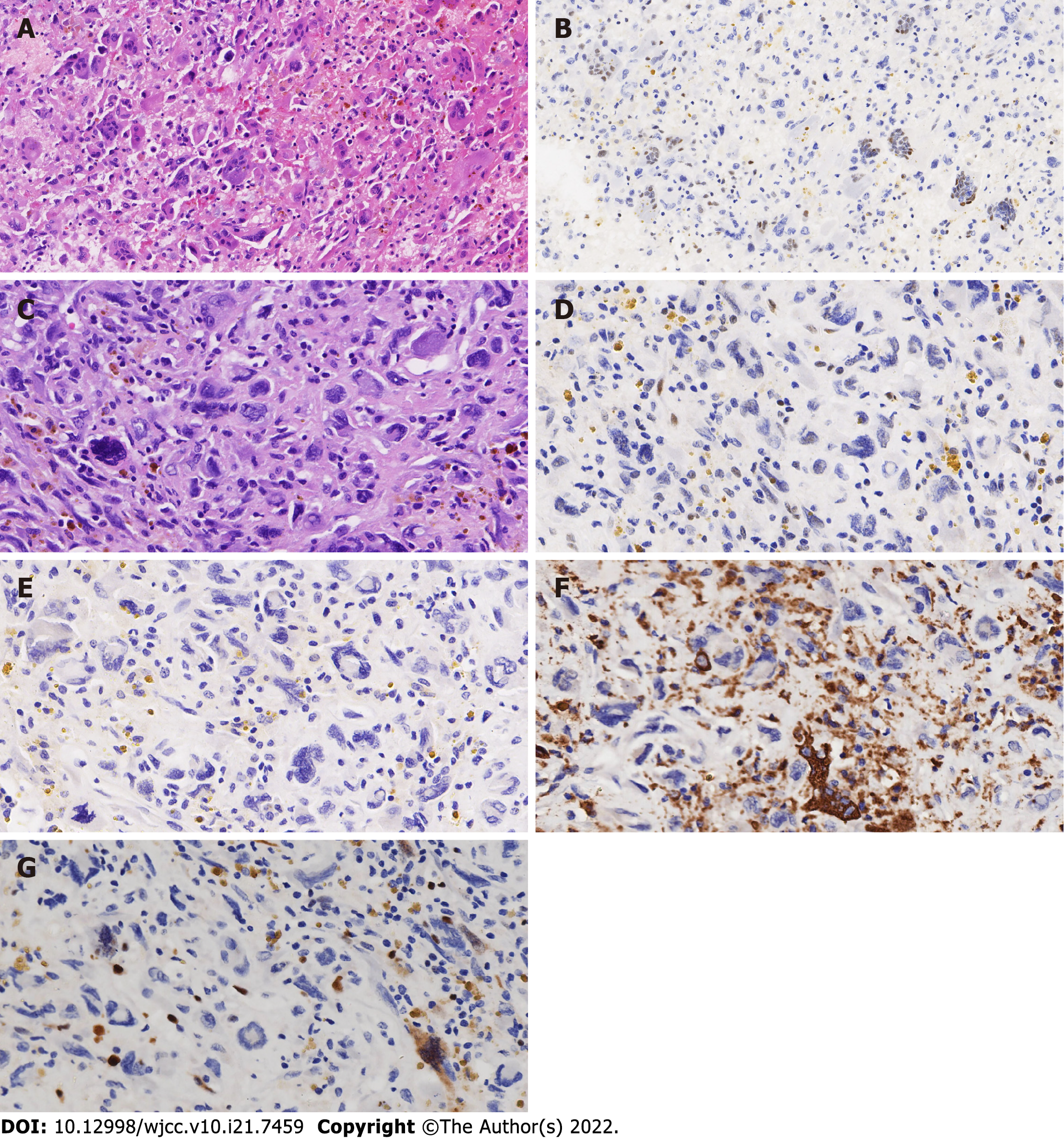
Zheng et al[3] suggested that the formation of SLMNs might result from the differentiation of undifferentiated stromal cells beneath the mucous epithelium under external stimulation, such as intramural hemorrhage or cyst contents. However, the latest research on mural nodules showed tumor cell groups with the abnormal expression of TP53 and methylthioadenosine phosphorylase in SLMNs. It suggested that SLMNs were not benign and reactive and all mural nodules in mucinous ovarian tumors should be regarded as potentially malignant neoplasms. In the SLMNs of Case 1, two types of giant cells were found. The first type was osteoclast-like giant cells with wild-type expression of TP53 (as shown in Figure 2A and B) which expressed CD68. The other types of giant cells were mononuclear or multinuclear. The nucleus was located around the cytoplasm. Furthermore, the cells were heteromorphic with TP53 nonsense mutation (as shown in Figure 2C and D), AE1/AE3 and CD68 were not expressed in these cells (as shown in Figure 2E and F), and the expression of Ki-67 was also low (as shown in Figure 2G). However, pathological mitotic images could be found and the morphology was concerning.
Mesbah et al[2] also speculated that the mural nodules of anaplastic carcinoma might result from the dedifferentiation of mucinous tumors in which TP53 mutation played a key role. Chapel et al[8] reported that only 1 of 13 cases showed a TP53 mutation in the mural nodules but not in the associated differentiated mucinous tumor. In 8 of 13 cases, both tumor components harbored the same TP53 mutation, indicating that TP53 mutation did not play a key role in the occurrence of mural nodules. TP53 had wild-type expression in mucinous tumors and mural nodules in three cases which did not confirm that TP53 played a key role in the formation of mural nodules.
Mesbah et al[2] identified KRAS mutations in the anaplastic carcinoma mural nodules and paired mucinous epithelial neoplasms in six of seven cases. Therefore, it was inferred that the mural nodules of anaplastic carcinoma might originate from tumors with KRAS mutation. Similarly, a concordant KRAS mutation (p.G13D) was identified in both mucinous tumors and mural nodules in Case 3. The tumor was composed of well-differentiated to moderately differentiated invasive mucinous carcinoma. At the same time, the transition of malignant mucinous epithelium from well-differentiated glandular tubular cells to dedifferentiated single cancer cells was observed under the microscope. Based on the immunohistochemical staining and molecular detection results of Case 3, it was also speculated that some anaplastic carcinoma mural nodules might originate from the mucinous epithelium which was the result of epithelial dedifferentiation. This conjecture was consistent with reports by Desouki et al[9] and Zheng et al[3]. Different from Case 3, KRAS (p.G12S/D) missense mutation was found in the mural nodules of Case 1 and BRAF (p.V600E/K/R/O) missense mutation was found in the mural nodules of Case 2 with no such change in the two corresponding mucinous tumors. Therefore, it was speculated that the formation of some mural nodules might not be related to mucinous tumors and the KRAS signal pathway might be involved in the formation of these mural nodules.
All three patients died within 2 years, whether receiving chemotherapy or not. Cases 1 and 3 were mucinous adenocarcinoma and therefore the effect of anaplastic mural nodules on disease progression could not be evaluated. Case 2 was a borderline mucinous tumor with the anaplastic carcinoma mural nodule. The patient still died of the disease 14 mo after the surgery. Therefore, it was speculated that the presence of anaplastic carcinoma nodules might accelerate the progression of the disease.
Three cases of mucinous tumors and associated mural nodules were positive for mismatch repair protein immunohistochemistry indicating that the microsatellite status of these three cases of tumors was stable.
In conclusion, the present study reported three cases of mucinous tumors with mural nodules of anaplastic carcinoma. Worrisome giant cells were found in SLMNs of Case 1. All SLMNs were recommended to be fully sampled to avoid missed diagnoses. Based on the existing literature and the experimental results of this study, it is speculated that the mural nodules of anaplastic carcinoma may have two origins: (1) Dedifferentiation of mucinous tumors; and (2) a tumor unrelated to mucinous tumors and the KRAS signal pathway may be involved in the formation of this tumor. The mural nodules of anaplastic carcinoma may promote the progression of borderline mucinous ovarian tumors.
We thank all medical and ancillary staff of the hospital and the patients for consenting to participate.
| 1. | Suurmeijer AJ. Carcinosarcoma-like mural nodule in an ovarian mucinous tumour. Histopathology. 1991;18:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Mesbah Ardakani N, Giardina T, Amanuel B, Stewart CJ. Molecular Profiling Reveals a Clonal Relationship Between Ovarian Mucinous Tumors and Corresponding Mural Carcinomatous Nodules. Am J Surg Pathol. 2017;41:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Zheng J, Geng M, Li P, Li Y, Cao Y. Ovarian mucinous cystic tumor with sarcoma-like mural nodules and multifocal anaplastic carcinoma: a case report. Int J Clin Exp Pathol. 2013;6:1688-1692. [PubMed] |
| 4. | Provenza C, Young RH, Prat J. Anaplastic carcinoma in mucinous ovarian tumors: a clinicopathologic study of 34 cases emphasizing the crucial impact of stage on prognosis, their histologic spectrum, and overlap with sarcomalike mural nodules. Am J Surg Pathol. 2008;32:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Lage JM. Diagnostic dilemmas in gynecologic and obstetric pathology. Semin Diagn Pathol. 1990;7:146-155. [PubMed] |
| 6. | Matias-Guiu X, Aranda I, Prat J. Immunohistochemical study of sarcoma-like mural nodules in a mucinous cystadenocarcinoma of the ovary. Virchows Arch A Pathol Anat Histopathol. 1991;419:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Shao Y, Liu Q, Shi H, Lu B. Ovarian mucinous tumors with mural nodules: immunohistochemical and molecular analysis of 3 cases. Diagn Pathol. 2020;15:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Chapel DB, Lee EK, Da Silva AFL, Teschan N, Feltmate C, Matulonis UA, Crum CP, Sholl LM, Konstantinopoulos PA, Nucci MR. Mural nodules in mucinous ovarian tumors represent a morphologic spectrum of clonal neoplasms: a morphologic, immunohistochemical, and molecular analysis of 13 cases. Mod Pathol. 2021;34:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Desouki MM, Khabele D, Crispens MA, Fadare O. Ovarian mucinous tumor with malignant mural nodules: dedifferentiation or collision? Int J Gynecol Pathol. 2015;34:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lakshmi Haridas K, India; Ros J, Spain; Samara AA, Greece A-Editor: Liu X, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM