Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7397
Peer-review started: June 25, 2021
First decision: September 1, 2021
Revised: September 16, 2021
Accepted: June 17, 2022
Article in press: June 17, 2022
Published online: July 26, 2022
Processing time: 381 Days and 6.2 Hours
Mannosyl-oligosaccharide glucosidase (MOGS) deficiency is an extremely rare type of congenital disorder of glycosylation (CDG), with only 12 reported cases. Its clinical, genetic, and glycomic features are still expanding. Our aim is to update the novel clinical and glycosylation features of 2 previously reported patients with MOGS-CDG.
We collected comprehensive clinical information, and conducted the immunoglobulin G1 glycosylation assay using nano-electrospray ionization source quadruple time-of-flight mass spectrometry. Novel dysmorphic features included an enlarged tongue, forwardly rotated earlobes, a birth mark, overlapped toes, and abnormal fat distribution. Novel imaging findings included pericardial effusion, a deep interarytenoid groove, mild congenital subglottic stenosis, and laryngomalacia. Novel laboratory findings included peripheral leukocytosis with neutrophil predominance, elevated C-reactive protein and creatine kinase, dyslipidemia, coagulopathy, complement 3 and complement 4 deficiencies, decreased proportions of T lymphocytes and natural killer cells, and increased serum interleukin 6. Glycosylation studies showed a significant increase of hypermannosylated glycopeptides (Glc3Man7GlcNAc2/N2H10 and Man5GlcNAc2/N2H5) and hypersialylated glycopeptides. A compensatory glycosylation pathway leading to an increase in Man5GlcNAc2/N2H5 was indicated with the glycosylation profile.
We confirmed abnormal glycomics in 1 patient, expanding the clinical and glycomic spectrum of MOGS-CDG. We also postulated a compensatory glycosylation pathway, leading to a possible serum biomarker for future diagnosis.
Core Tip: We updated the clinical and glycosylation features of 2 previously published patients with mannosyl-oligosaccharide glucosidase-congenital disorders of glycosylation (MOGS-CDG) by confirming abnormal glycomics and expanding the phenotypical and glycomic spectrum of MOGS-CDG. We also postulated a compensatory glycosylation pathway, leading to a possible serum biomarker for future diagnosis.
- Citation: Abuduxikuer K, Wang L, Zou L, Cao CY, Yu L, Guo HM, Liang XM, Wang JS, Chen L. Updated clinical and glycomic features of mannosyl-oligosaccharide glucosidase deficiency: Two case reports. World J Clin Cases 2022; 10(21): 7397-7408
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7397.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7397
Protein glycosylation is a process of glycoprotein or glycolipid biosynthesis and modification. Congenital disorders of glycosylation (CDGs) influence the properties and functions of glycoproteins or glycolipids, leading to multiple organ/system disorders[1]. The mannosyl-oligosaccharide glucosidase (MOGS) gene (OMIM*601336) encodes the first enzyme in the N-linked oligosaccharide processing pathway, namely MOG (also known as glucosidase I). This enzyme is responsible for removing distal alpha-1,2-linked glucose from Glc3Man9GlcNac2 (N2H12) (N, N-acetylglucosamine or N-acetylga
The N-glycan profile of immunoglobulin G (IgG) from patients show an increased level of Glc3Man7GlcNAc2 (N2H10), whereas serum or skin fibroblast samples have increased levels of Glc3Man7GlcNAc2 (N2H10), Glc3Man8GlcNAc2 (N2H11), and Glc3Man9GlcNAc2 (N2H12)[5,12]. Urine analyses from patients have shown accumulation of tetrasaccharide Glc(a1-2)Glc(a1-3)Glc(a1-3)Man (Glc3Man1, H4)[4,9,10]. Isoelectric focusing of serum transferrin is suggestive of a normal[4,9,10] or type II pattern[6] with increased trisialotransferrin but normal mono-sialotransferrin or asialotransferin.
MOGS-CDG is an extremely rare disease with only 12 reported cases to date[4-11]. Its genetic, glycomic, and clinical features of MOGS-CDG are still expanding. In this study, we updated the clinical and glycosylation features of two patients with MOGS-CDG who were previously reported without solid functional or glycomic evidence[7].
Case 1: A newborn infant (the younger sister) had difficulties feeding and breathing.
Case 2: A newborn infant (the elder sister) had birth asphyxia, severe pneumonia, stridor, gastroesophageal reflux, and respiratory failure.
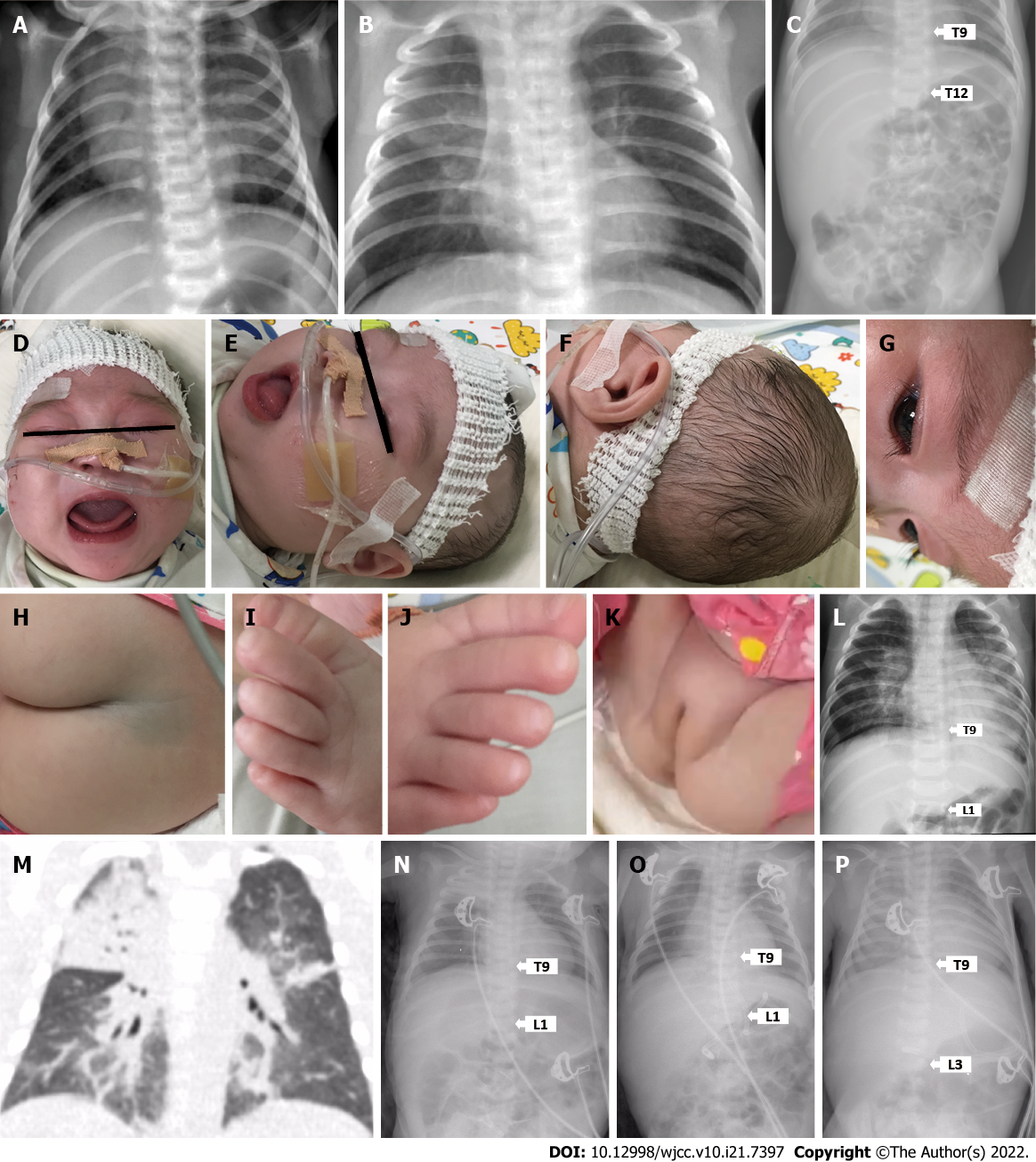
Case 1: The younger sister was born after 39 wk of gestation, with a birth weight of 3300 g. Apgar score was 7 at 1 min and 9 at 5 min after birth. She was found to have difficulties feeding and breathing during admission, and continued to be treated for persistent pneumonia (Figure 1A-C). At 5.6 mo and still on nasogastric tube feeding, she was admitted for breathing difficulties with recurrent seizures. However, parents decided to leave the hospital with oral antibiotics and home-based oxygen therapy.
Case 2: The elder sister was born after 40.1 wk of uncomplicated pregnancy with a normal birth weight (3450 g), but was immediately hospitalized for birth asphyxia, severe pneumonia, stridor, gastroesophageal reflux, and respiratory failure. At the age of 1.4 mo, she was admitted for severe pneumonia with frequent choking on oral feeding, and transferred to intensive care unit for respiratory failure. At the age of 1.8 mo, she was admitted to a regional pediatric hospital for pneumonia and recurrent seizure while on nasogastric tube feeding. After intensive antibiotic and ganciclovir therapies during more than 2 mo of in-patient stay, the parents took the baby to home care with nasogastric tube feeding and nasal oxygen therapy.
Both patients started having symptoms since birth.
Both patients started having symptomes since birth, and non-consanguineous parents are healthy.
Case 1 (the younger sister): At 5.6 mo, vital signs, which included body temperature and blood pressure, were within their normal ranges. But respiration and heart rate were abnormal. Dysmorphic features, such as microcephaly, narrow forehead, hypertelorism, retrognathia, enlarged tongue, large ear lobes with forward rotation, hirsutism with long eyelashes, a birthmark, abnormal fat distribution surrounding the genital area, and overlapped toes on both feet, were noted on physical examination (Figure 1D-K). Liver was palpated 4 cm below the right costal margin and 3 cm below the xiphoid process.
Case 2 (the elder sister): At the age of 1.4 mo, she had slight hepatomegaly (liver was palpable 2 cm below the right costal margin) and failure to thrive (3500 g body weight, less than the 1st percentile by World Health Organization standards). At the age of 1.8 mo, significant umbilical and inguinal hernias, hepatomegaly (3 cm below the right costal margin), and malnutrition were noted on physical examination. Parents recalled she had similar facial features and a head as small as her younger sister, but a larger tongue and clenched fingers.
Case 1: At the age of 3.5 mo, the younger sister was found to have high percentage of peripheral blood reticulocytes (5.58%) and slightly higher level of serum lactate levels (4 mmol/L, normal range: 0-2 mmol/L). At 5.6 mo and during admission for breathing difficulties with recurrent seizures, complete blood count showed significantly elevated white blood cell (WBC) count with a higher neutrophil percentage (62.9%, normal range: 20%-50%) and elevated C-reactive protein (CRP) level. Lactic acid level (2.9 mmol/L, normal range: < 2.0 mmol/L) and serum transaminase levels were slightly elevated (Table 1).
| Patient | Case 1 (younger sister) | Case 2 (elder sister) | ||||||
| Age in mo | 1 | 1.5 | 3.5 | 5.6 | 1.4 | 1.8 | 3.9 | |
| Complete blood count | White blood cell (4-10 × 109/L) | 24.4 | 21.8 | 27.0 | 32.2 | 20.9 | 23.9 | 8.8 |
| Lymphocyte (45%-75%) | 50.6 | 44.5 | 65.5 | 32 | 37.8 | 25.5 | 47.8 | |
| Neutrophil (20%-50%) | 35 | 42.8 | 28.5 | 62.9 | 75.0 | 72.8 | 43.8 | |
| Red blood cell (4.0-5.5 × 1012/L) | 4.3 | 3.8 | - | 4.0 | 4.1 | - | 3.0 | |
| Hemoglobin (110-160 g/L) | 143 | 124 | 100 | 110 | 125 | 108 | 84 | |
| Platelet count (100-300 × 109/L) | 506 | 509 | 303 | 308 | 418 | 357 | 464 | |
| C-reactive protein (< 8 mg/L) | < 8 | < 8 | 10.6 | 50 | 15 | 14 | 62 | |
| Serum biochemistry | Albumin (35-55 g/L) | 37 | - | 39.5 | 39 | 41.8 | 35.8 | 38.7 |
| Globulin (20-30 g/L) | 20.7 | - | 20.4 | 20 | 20.4 | 26.4 | 19.5 | |
| Alanine aminotransferase (0-40 IU/L) | 14 | - | 81 | 51 | 17 | 56 | 29 | |
| Aspartate aminotransferase (0-40 IU/L) | 46 | - | 154 | 93 | 53 | 121 | 49 | |
| Total bilirubin (5.1-17.1 μmol/L) | 8.5 | - | - | 4.8 | - | 5.0 | 3.0 | |
| Direct bilirubin (0-6 μmol/L) | 2.4 | - | - | 0.2 | - | 1.1 | 0.8 | |
| γ-Glutamyl transpeptidase (7-50 IU/L) | 60 | - | - | 14 | - | - | 16 | |
| Total bile acid (0-10 μmol/L) | 9.6 | - | - | 10.2 | - | - | - | |
| Alkaline phosphatase (42-383 IU/L) | 265 | - | - | 259 | - | - | 113 | |
| Creatine kinase (25-200 IU/L) | 142 | - | 175 | 140 | 141 | 813 | 72 | |
| Creatine kinase-MB (< 25 IU/L) | 73 | - | 69 | 38 | 34 | 302 | 24 | |
| α-Hydroxybutyrate dehydrogenase (72-182 IU/L) | 452 | - | 342 | 297 | - | - | 274 | |
| Lactate dehydrogenase (180-430 IU/L) | 556 | - | 500 | 330 | - | 922 | 296 | |
| Glucose (3.9-5.8 mmol/L) | 4.3 | - | 4.9 | - | - | - | - | |
| Creatinine (31-52 μmol/L) | 27 | - | 23.9 | 24 | 19 | 15 | 17 | |
| Urea (2.5-6.5 mmol/L) | 5.0 | - | 6.6 | 7.1 | 2.3 | 1.4 | 3.6 | |
| Uric acid (90-420 μmol/L) | 168 | - | 338 | 301 | - | 281 | 167 | |
| Total cholesterol (3.1-5.2 mmol/L) | 5.0 | - | - | - | - | 4.4 | 4.3 | |
| Triglyceride (0.6-1.7) | 1.0 | - | - | - | - | 2.1 | 2.1 | |
| Blood coagulation profiles | Activated partial thromboplastin time (28.0-44.5 s) | 35.8 | - | - | 37.1 | - | - | - |
| D-dimer (0-0.3 mg/L) | 0.54 | - | - | 1.51 | - | - | - | |
| Fibrinogen (2-4 g/L) | 3.43 | - | - | 3.26 | - | - | - | |
| Fibrinogen degradation products (0-5 μg/mL) | 1.16 | - | - | 4.4 | - | - | - | |
| Thrombin time (14-21 s) | 18.5 | - | - | 17.9 | - | - | - | |
| International normalized ratio (0.8-1.2) | 0.99 | - | - | 1.37 | - | - | - | |
| Prothrombin time (12.0-14.8 s) | 13 | - | - | 17 | - | - | - | |
| Prothrombin time activity (80%-100%) | 103 | - | - | 62 | - | - | - | |
| Immune function profiles | IgG (3.70–8.30 g/L) | - | 7.6 | - | - | 12.31 | 13.91 | - |
| IgM (0.33–1.25 g/L) | - | 0.52 | - | - | 0.63 | 0.61 | - | |
| IgA (0.14–0.50 g/L) | - | 0.05 | - | - | 0.10 | 0.12 | - | |
| Complement 3 (0.67-1.76 g/L) | - | - | - | - | 0.45 | 0.49 | - | |
| Complement 4 (0.10-0.40 g/L) | - | - | - | - | < 0.06 | 0.10 | - | |
| Total T cells (CD3) (53%-84%) | - | - | - | - | 41.1 | 38.9 | - | |
| Helper T cells (CD4) (35%-64%) | - | - | - | - | 34.7 | 29.8 | - | |
| Cytotoxic T cells (CD8) (12%-28%) | - | - | - | - | 15.9 | 10.9 | - | |
| Natural killer cells (CD16 + CD56) (4%-18%) | - | - | - | - | 2.3 | 9.9 | - | |
| B cells (CD19) (6%-32%) | - | - | - | - | 36.3 | 43.6 | - | |
| Procalcitonin (< 0.05 ng/mL) | - | - | - | 0.6 | 0.2 | 0.2 | - | |
| Interleukin-6 (< 7 pg/mL) | - | - | 14.8 | - | - | - | ||
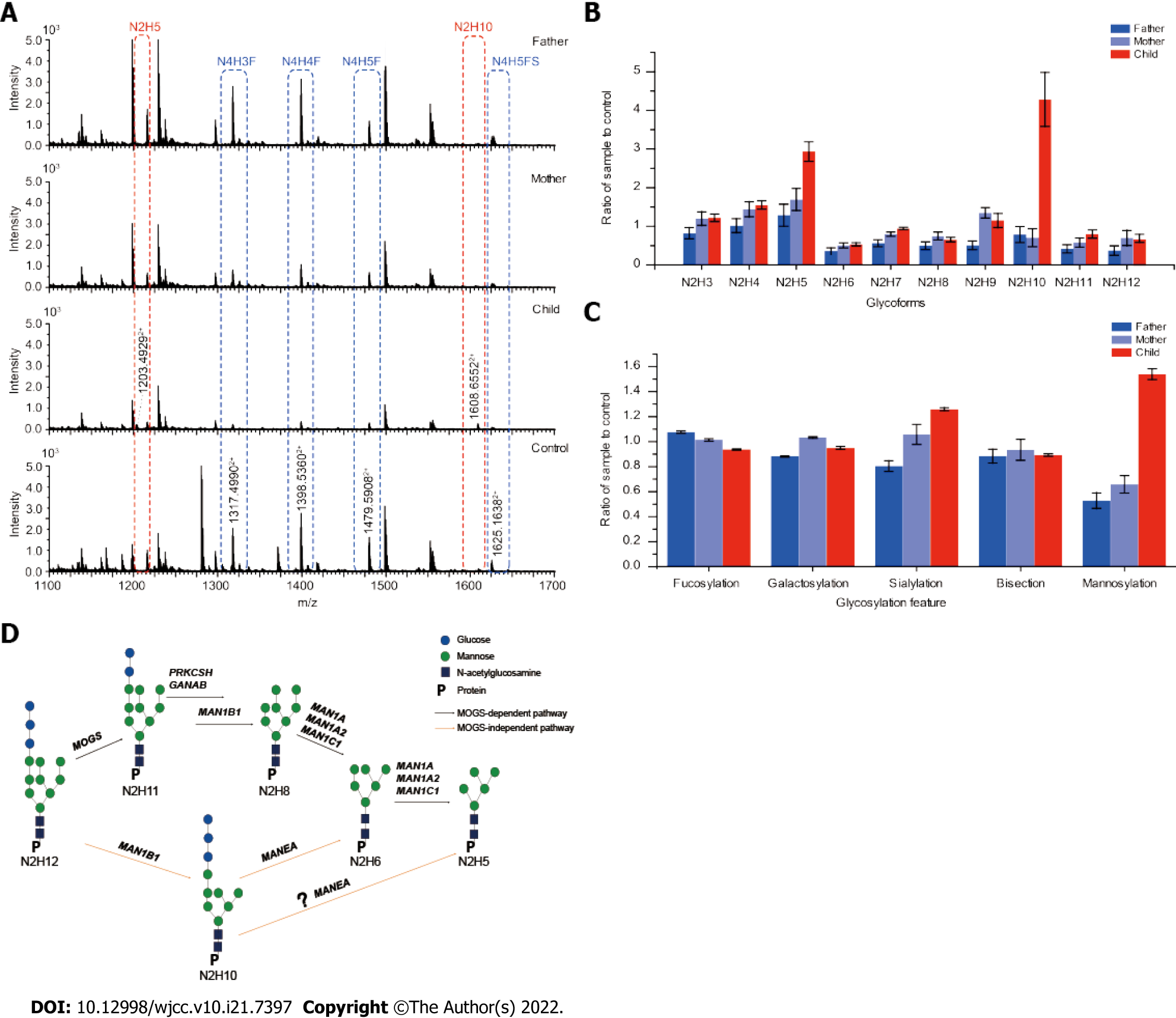
Blood samples were collected from the patient and parents for glycosylation analyses after written informed consent was obtained. Using the Waters Alliance 2695-2489 HPLC system (Waters Co., Waters, Manchester, United Kingdom) equipped with Waters Empower software[13], plasma samples collected from the younger sister and both parents were subjected to a standard peptidoglycan assay to examine the profile of conjugated oligosaccharides on IgG1. Compared to the pooled healthy controls, the profiles of conjugated oligosaccharides of the mother and the father were normal. However, an abnormal peak of N2H10 (Glc3Man7GlcNAc2) was detected in the patient’s sample (Figure 2A), and this result was further confirmed by normalizing N2 (GlcNAc2) serial oligosaccharides to controls (Figure 2B). The level of N2H10 (Glc3Man7GlcNAc2) in the younger sister was five-times higher than that in the pooled healthy controls and parents. Detection of abnormal levels of N2H10 (Glc3Man7GlcNAc2) was consistent with two previously reported cases of MOGS[4,5], and provided glycomic evidence for the diagnosis of MOGS-CDG. In addition to N2H10 (Glc3Man7GlcNAc2), a significantly high level of N2H5 (Man5GlcNAc2) compared to normal control was also detected (Figure 2A and B). To examine the collective impact of the mutations on the glycosylation process, we pooled all detected glycopeptides and calculated the fractions of fucosylated, galactosylated, sialylated, mannosylated, and bisected forms. The ratios of the family were normalized to those of the pooled healthy control (Figure 2C). The results indicated that, in addition to hypermannosylation, sialylation level was also significantly higher in the patient’s sample compared to the parents. Available blood test results throughout the disease course are provided in Table 1.
Case 2: The elder sister was tested positive for serum cytomegalovirus IgM and cytomegalovirus DNA (in serum, sputum, and alveolar lavage fluid) at the age of 1.8 mo. Blood test results revealed significant elevations of peripheral WBCs with neutrophil predominance, significant elevations of creatine kinase/creatine kinase-MB/Lactate dehydrogenase, and abnormal immune function parameters (Table 1). At the age of 3.4 mo, alveolar lavage fluid culture was positive for multiple drug-resistant Acinetobacter baumannii. Parents recalled she had low levels of immunoglobulin requiring frequent use of intravenous immunoglobulin (IVIG), but the exact dates and dosages of IVIG therapy were unknown. Available blood test results of both patients throughout the disease course are provided in Table 1.
Case 1: The younger sister had a serial X-rays showed persistent pneumonia (Figure 1A-C), and echocardiography showed patent foramen ovale (1.6 mm) with slight pericardial effusion (2.5-2.7 mm). At 5.6 mo, X-ray showed pneumonia with enlarged heart size and increased liver span (Figure 1L).
Case 2: The elder sister was admitted to a regional pediatric hospital for pneumonia at the age of 1.8 mo (Figure 1M). Serial X-rays afterwards showed recurrent exacerbations of pneumonia, progressive hepatomegaly, and enlarged heart size (Figure 1N-P).
Updated clinical (Figure 1 and Table 2), laboratory (Table 1), genetic (Table 2), glycomic (Figure 2 and Table 2) findings, and characteristics of all previously reported patients (Table 2) are summarized in the accompanying tables and figures within the manuscript.
| Patients in the order of publication date | 1[4] | 2/31[5] | 4[6] | 52[7] and present report | 62[7] and present report | 7[8] | 8[9] | 9[10] | 103[10] | 113[10] | 12[11] |
| Sex | Female | Male/Female | Male | Female (younger) | Female (elder) | Female | Male | Male | Female | Male | Female |
| Age of onset | Neonatal | Early in life | Neonatal | Neonatal | Neonatal | Neonatal | Neonatal | Neonatal | Neonatal | Neonatal | Neonatal |
| Neurologic symptoms | |||||||||||
| Microcephaly | + | + | + | + | + | ND | + | + | + | + | + |
| Seizures | EIEE | + | ND | + | ND | Infantile spasm | + | EIEE | EIEE | EIEE | + |
| Psychomotor disturbance | ND | Profound | ND | Profound | Profound | + | ND | Profound | Profound | Profound | Profound |
| Hypotonia | + | + | + | - | - | + | + | + | + | + | + |
| Cerebral abnormality | - | Small corpus callosum, optic-nerve atrophy | - | ND | Frontal gyrus stenosis, high T1W1 signal in anterior pituitary, and thin corpus callosum | Thin corpus callosum, wide cerebral sulcus | Agenesis of corpus callosum, septo-optic dysplasia | Loss of white matter volume, delayed myelination | Loss of white matter volume | Loss of white matter volume | Delayed myelination, cortical and subcortical atrophy |
| Dysmorphic features | + (ND) | + (ND) | |||||||||
| Broad nose | + | + | + | + | + | + | + | + | - | ||
| High-arched palate | + | + | + | ND | ND | + | + | + | ND | ||
| Retrognathia | + | + | + | + | ND | + | + | + | + | ||
| Short palpebral fissure | + | + | + | + | + | + | + | + | - | ||
| Enlarged ears | + | ND | + | + | ND | + | + | + | ND | ||
| Overlapping finger/toe | + | + | + | ND | ND | + | + | + | ND | ||
| Arthrogryposis | + | + | ND | ND | ND | ND | + | + | + | ND | |
| Hypertrichosis | + | + | + | + | ND | + | + | + | + | + | |
| Hypoplastic genitalia | + | + | ND | Abnormal fat distribution | - | - | Hypogonadism | + | ND | ND | ND |
| Cardiac involvement | - | - | ASD, LVH | ASD, PFO, heart failure, and pericardial effusion | PFO, heart failure | PFO, ASD | Dilated cardiomyopathy | + | - | - | ND |
| Elevated AST/ALT (IU/L) | +(80/34) | ND | ND | + (46-154/14-81) | + (49-121/17-56) | + | + (30-1547/9- 1132) | + (35-144/23-83) | + (36-226/50.5-164) | ND | |
| Cirrhosis | ND | ND | ND | ND | ND | ND | ND | - | + | + | ND |
| Hepatomegaly | + | ND | + | + | + | + | + | + | + | + | ND |
| Hypogammaglobulimia | Low IgA | Low IgG, IgA, IgM | Low IgA, IgM | Low IgA | Low IgA, Normal IgG and IgM after IVIG | Low IgG, IgA, IgM | Low IgA | Low IgA | Low IgA | Low IgA and IgG | Low IgG2 |
| Recurrent infections | + | - | + | + | + | + | + | + | + | + | + |
| Endocrine abnormality | ND | ND | SIADH | ND | Hypoglycemia, electrolyte disturbance, and central hypothyroidism | ND | ND | Hyponatremia | ND | ND | Hypoglycemia; elevated cortisol, progesterone, and androstenedione levels |
| Edema | + | ND | + | + | + | + | ND | + | ND | ND | - |
| Hearing impairment | Flat with ABR | Sensorineural hearing loss | Abnormal ABR | Hearing impairment | ND | - | ND | No wave with ABR | Only I wave with ABR | Only I wave with ABR | Hypoacusia |
| EEG | Suppression burst pattern | ND | ND | ND | ND | Atypical hypsarrhythmia | ND | Suppression burst pattern | Suppression burst pattern | Suppression burst pattern | Hypsarrhythmia |
| Isoelectric focusing of transferrin | Normal | ND | Increased trisialotransferrin | ND | ND | Normal | Normal | Normal | Normal | Normal | Normal |
| Urinary oligosaccharide | Abnormal | ND | ND | ND | ND | ND | Abnormal | Abnormal | ND | ND | ND |
| IgG or serum glycan analysis | ND | Increased N2H10 in IgG; Increased N2H10, N2H11 and N2H12 in serum | ND | Increased N2H10 and N2H5 in IgG | ND | ND | ND | ND | ND | ND | ND |
| MOGS gene mutations | p.Arg486Thr and p.Phe652Leu | p.Ala22Glu, p.Arg110His, and p.Gln124Ter | p.Thr802Ileand p.Arg535Ter | p.Asp414Leufs*17, p.Gly182Arg, and p.Asp566Glu | p.Asp414Leufs*17, p.Gly182Arg, and p.Asp566Glu | p.Arg565Gln and p.Arg540His | p.Arg495Ter and p.Gly752Asp | p.Gln505del and p.Arg495Ter | ND | p.Gln505del and p.Arg535Ter | p.Pro513Ser and p.Gly824Asp |
| Prognosis | Died (74 d) | Alive (11 yr/6 yr) | Died (4 mo) | Died (9 mo) | Died (10 mo) | Alive (2 yr 1 mo) | Died (1 yr) | Alive (13 yr) | Died | Died | Alive (19 yr) |
Clinical exome (which includes more than 4000 known disease-causing genes) sequencing and whole exome sequencing of the parents and both sisters were positive for three variants in the MOGS gene (c.1698C>A/p.Asp566Glu, c.544G>A/p.Gly182Arg, and c.1239-1240insCTTCTACGGACAAGGGCTGGTATTGCCA/p.Asp414Leufs*17). Details of genetic testing and Sanger sequencing confirmation in family members were published by Li et al[7].
Case 1 (the younger sister): Nasogastric tube feeding was started at the age of 5 d. Stridor was improved after laryngotracheoplasty, but feeding difficulty and choking were not improved even after 3 wk of intensive oral motor training. At 5.6 mo, seizure attacks were stopped and breathing difficulty was improved after treatment with antibiotics, nasal oxygen, and nasogastric tube feeding.
Case 2 (the elder sister): Supportive treatments such as mechanical ventilation, antibiotics, nasogastric tube feeding, intravenous immunoglobulin, ganciclovir, and anti-seizure medications (not specified).
Case 1 (the younger sister): At the age of 7.5 mo, parents stopped nasogastric tube feeding and refused to follow our medical advice on intensive anti-microbial therapy with immune-supportive measures to contain the infection. The patient later died of multiple organ (respiratory, heart, and renal) failure at a local hospital at the age of 9 mo.
Case 2 (the elder sister): At the age of 10 mo, she died of multiple organ failure at a local hospital.
Since the first report of MOGS-CDG in 2000[4], 12 patients have been reported to date[4,11]. Previously reported dysmorphic features include prominent occiput, short palpebral fissures, long eyelashes, broad nose, retrognathia, high-arched palate, generalized edema, hypoplastic genitalia, clenched hands with overlapped fingers, alopecia, and thoracic scoliosis. Updated dysmorphic features in our patients included enlarged tongue, large and forwardly rotated earlobes, birthmark, abnormal fat distribution around external genitalia, and overlapped toes. Contrary to previous findings of hypoplastic genitalia, we observed abnormal fat distribution surrounding genital area suggestive of hyperplastic genitalia in the younger sister. We observed persistent respiratory infection with progressive hepatomegaly and cardiomegaly during the course of disease (Figure 1). Updated laboratory findings included significantly elevated peripheral WBC count with neutrophil predominance, elevated CRP, significant but transient elevations of creatine kinase and lactate dehydrogenase, elevation of serum triglyceride, and coagulopathy. As observed in previous studies in MOGS-CDG[4-11], immune profiles were abnormal with low levels of IgM and IgA, and with increased percentage of B lymphocytes. We observed additional evidence of immune deficiency or dysregulation such as complement 3 and complement 4 deficiencies, decreased proportions of T cell subtypes and natural killer cells, as well as elevated levels of serum interleukin 6. Our findings indicated abnormalities in both innate and adaptive immune responses. Since glycans expressed on the surface of immune cells are essential for cell development and function[14], abnormal glycosylation caused by MOGS gene mutation may have caused immune deficiency leading to persistent pneumonia in these siblings. Contrary to previous findings of resistance to infection by glycosylation-dependent enveloped viruses[5,15], the elder sister in our report had evidence of cytomegalovirus (CMV, also an enveloped virus) infection. Although some glycosylated proteins (i.e., gpUL37 and UL55) are involved during CMV replication[16,17], our case seems to suggest CMV replication was not affected by MOGS gene mutation. Further studies are needed to confirm whether CMV replication is dependent on MOGS-mediated glycosylation.
Sadat et al[5] first reported the presence of Glc3Man7GlcNAc2 (N2H10) when analyzing N-glycans in IgG from 2 siblings with MOGS-CDG. Using RapiFluor mass spectrometry, Messina et al[12] recently confirmed the presence of N2H10 in the IgG of an unspecified patient with MOGS-CDG. Consistent with the previous glycan analyses on MOGS-CDG[5,12], an abnormal peak of 12-saccharide (N2H10, Glc3Man7GlcNAc2) was detected in IgG1 of the younger sister. In addition, abnormal accumulation of N2H5 (Man5GlcNAc2) was detected in the serum sample, which could be another biomarker for MOGS-CDG. Endo-α-1,2-mannosidase might be hyperactivated in MOGS-CDG to partially compensate for MOGS deficiency. Messina et al[12] also reported the presence of fucosylated and unfucosylated hybrid N-glycans in serum IgG, but we did not observe similar changes in our analyses.
The presence of N2H10 detected in MOGS-CDG suggests the activation of a distal α-1,2-mannosidase. The detection of N2H5 (Man5GlcNAc2) but not N2H6 (Man6GlcNAc2) indicated that in addition to H4 (Glc3Man1), endo-α-1,2 mannosidase might be able to remove H5 (Glc3Man2) and from N2H10 (Glc3Man7GlcNAc2) as well. Based upon all of the collected glycomic results of MOGS-CDG by De Praeter et al[4], Sadat et al[5], Messina et al[12], and our cases, a MOGS-independent pathway through N2H10 (Glc3Man7GlcNAc2) to N2H5 (Man5GlcNAc2) is proposed (Figure 2D). Several groups[4,9,10] have detected an H4 (Glc3Man1) oligosaccharide in urine samples collected from patients with MOGS-CDG, but urine samples were not available from our patients.
When the saccharide-specific profile of glycopeptides in the younger sister was compared to parents and healthy controls, fractions of fucosylation, galactosylation, and bisection were similar. However, fractions of mannosylation (> 1.5 times the normal controls) and sialylation (about 1.2 times the normal controls) were increased compared to parents (Figure 2C). Although N2H10 (Glc3Man7GlcNAc2) accumulation has been previously reported[5,12], higher levels of N2H5 (Man5GlcNAc2) glycans and hypersialylation of IgG1 have not been reported in MOGS-CDG. The hypermannosylation of proteins may have secondary effects on inflammation or infection through mannose-recognizing C-type lectin receptors and mannose-recognizing antibodies[18]. However, hypersialyation is related to neurodegeneration[19] and abnormal immune modulation[20] that could partly explain developmental delay, seizure, and immune deficiency in patients with MOGS-CDG. Our findings may not only provide a potential serum biomarker but may also shed some light on the pathogenesis and subsequent treatment of MOGS deficiency.
In conclusion, we confirmed the presence of glycomic abnormality in a patient, expanded the clinical and glycomics spectrum of MOGS-CDG, and postulated a compensatory glycosylation pathway in MOGS deficiency. Updated clinical and glycomic features may provide a better understanding of MOGS-CDG, leading to better identification and management of future patients.
| 1. | Ng BG, Freeze HH. Perspectives on Glycosylation and Its Congenital Disorders. Trends Genet. 2018;34:466-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 2. | Kalz-Füller B, Bieberich E, Bause E. Cloning and expression of glucosidase I from human hippocampus. Eur J Biochem. 1995;231:344-351. [DOI] [Full Text] |
| 3. | Nairn AV, Moremen KW. Mannosyl-Oligosaccharide Glucosidase (Glucosidase I, MOGS). In: Taniguchi N., Honke K., Fukuda M., Narimatsu H., Yamaguchi Y., Angata T. (eds) Handbook of Glycosyltransferases and Related Genes. Springer, Tokyo 2014. [DOI] [Full Text] |
| 4. | De Praeter CM, Gerwig GJ, Bause E, Nuytinck LK, Vliegenthart JF, Breuer W, Kamerling JP, Espeel MF, Martin JJ, De Paepe AM, Chan NW, Dacremont GA, Van Coster RN. A novel disorder caused by defective biosynthesis of N-linked oligosaccharides due to glucosidase I deficiency. Am J Hum Genet. 2000;66:1744-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 132] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Sadat MA, Moir S, Chun TW, Lusso P, Kaplan G, Wolfe L, Memoli MJ, He M, Vega H, Kim LJY, Huang Y, Hussein N, Nievas E, Mitchell R, Garofalo M, Louie A, Ireland DC, Grunes C, Cimbro R, Patel V, Holzapfel G, Salahuddin D, Bristol T, Adams D, Marciano BE, Hegde M, Li Y, Calvo KR, Stoddard J, Justement JS, Jacques J, Priel DAL, Murray D, Sun P, Kuhns DB, Boerkoel CF, Chiorini JA, Di Pasquale G, Verthelyi D, Rosenzweig SD. Glycosylation, hypogammaglobulinemia, and resistance to viral infections. N Engl J Med. 2014;370:1615-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Kim YM, Seo GH, Jung E, Jang JH, Kim SZ, Lee BH. Characteristic dysmorphic features in congenital disorders of glycosylation type IIb. J Hum Genet. 2018;63:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Li M, Xu Y, Wang Y, Yang XA, Jin D. Compound heterozygous variants in MOGS inducing congenital disorders of glycosylation (CDG) IIb. J Hum Genet. 2019;64:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Peiwei Zhao, Peng X, Luo S, Huang Y, Tan L, Shao J, He X. Identification and characterization of novel mutations in MOGS in a Chinese patient with infantile spams. Neurogenetics. 2020;21:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Ota M, Miyahara J, Itano A, Sugiura H, Ohki S. Mannosyl-oligosaccharide glucosidase - congenital disorder of glycosylation: A patient with novel variants. Pediatr Int. 2020;62:417-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Anzai R, Tsuji M, Yamashita S, Wada Y, Okamoto N, Saitsu H, Matsumoto N, Goto T. Congenital disorders of glycosylation type IIb with MOGS mutations cause early infantile epileptic encephalopathy, dysmorphic features, and hepatic dysfunction. Brain Dev. 2021;43:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Lo Barco T, Osanni E, Bordugo A, Rodella G, Iascone M, Tenconi R, Barone R, Dalla Bernardina B, Cantalupo G. Epilepsy and movement disorders in CDG: Report on the oldest-known MOGS-CDG patient. Am J Med Genet A. 2021;185:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Messina A, Palmigiano A, Esposito F, Fiumara A, Bordugo A, Barone R, Sturiale L, Jaeken J, Garozzo D. HILIC-UPLC-MS for high throughput and isomeric N-glycan separation and characterization in Congenital Disorders Glycosylation and human diseases. Glycoconj J. 2021;38:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Guo Z, Wang C, Liang T, Liang X. Polar-copolymerized approach based on horizontal polymerization on silica surface for preparation of polar-modified stationary phases. J Chromatogr A. 2010;1217:4555-4560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Monticelli M, Ferro T, Jaeken J, Dos Reis Ferreira V, Videira PA. Immunological aspects of congenital disorders of glycosylation (CDG): a review. J Inherit Metab Dis. 2016;39:765-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Chang J, Block TM, Guo JT. Viral resistance of MOGS-CDG patients implies a broad-spectrum strategy against acute virus infections. Antivir Ther. 2015;20:257-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Mercorelli B, Sinigalia E, Loregian A, Palù G. Human cytomegalovirus DNA replication: antiviral targets and drugs. Rev Med Virol. 2008;18:177-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Emery VC, Cope AV, Bowen EF, Gor D, Griffiths PD. The dynamics of human cytomegalovirus replication in vivo. J Exp Med. 1999;190:177-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 188] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Loke I, Kolarich D, Packer NH, Thaysen-Andersen M. Emerging roles of protein mannosylation in inflammation and infection. Mol Aspects Med. 2016;51:31-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Nagamine S, Yamazaki T, Makioka K, Fujita Y, Ikeda M, Takatama M, Okamoto K, Yokoo H, Ikeda Y. Hypersialylation is a common feature of neurofibrillary tangles and granulovacuolar degenerations in Alzheimer's disease and tauopathy brains. Neuropathology. 2016;36:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Rodrigues E, Macauley MS. Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Miyoshi E, Japan A-Editor: Susak YM, Ukraine S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ