Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.594
Peer-review started: August 8, 2021
First decision: October 11, 2021
Revised: October 19, 2021
Accepted: December 7, 2021
Article in press: December 7, 2021
Published online: January 14, 2022
Processing time: 156 Days and 11.8 Hours
Despite the overload of publications on Crohn’s disease (CD), no comprehensive analysis of biologic therapy for CD has been reported.
To determine knowledge gaps and identify areas of interest of biologic therapy for CD.
The top 100 highest-cited original articles were identified from January 1991 to December 2020 in the Clarivate Analytics Web of Science Core Collection database. We conducted a bibliometric analysis of biologic therapy for CD based on total citations, summarized the bibliographic information of the articles related to CD biologic therapy, and explored the research hotspots.
The top 100 highest-cited original articles were identified with total citations ranging from 307 to 2978. The 2000s (Period II, n = 66) yielded the most influential original articles and saw the most dramatic growth. Among the top 10 countries, including 8 European countries and 2 North American countries, the United States (n = 37) and Belgium (n = 20) contributed the most publications. Among the top 10 institutions, the University Hospital Gasthuisberg in Belgium (n = 23), the University of Chicago in the United States (n = 20), and the Mayo Clinic in the United States (n = 17) published the most papers. Regarding authors, Rutgeerts P in Belgium (n = 32), Sandborn WJ in the United States (n = 23), and Feagan BG in Canada (n = 18) published the highest number of studies. The cooperation relationships between the United States and Europe were most frequent. Gastroenterology (impact factor = 22.682) published the most articles on biologic therapy for CD (n = 32) with 17654 total citations. Anti-tumor necrosis factor biologics and monoclonal antibodies were the most studied topics.
The bibliometric analysis emphasized the key contributions to the development of the specialized field. These data would provide useful research insights into biologic therapy for CD for clinicians and researchers.
Core Tip: We conducted a bibliometric analysis from the top 100 highest-cited original articles to determine knowledge gaps and identify areas of interest of biologic therapy for Crohn’s disease (CD). The 2000s yielded the most influential original articles and saw the most dramatic growth. The United States and Europe contributed the most publications, and the cooperation relationships between them were most frequent. Gastroenterology published the most articles on biologic therapy for CD. Anti-tumor necrosis factor (TNF) biologics and monoclonal antibodies were the most studied topics. These data would provide useful research insights into biologic therapy for CD for clinicians and researchers.
- Citation: Shen JL, Zhou Z, Cao JS, Zhang B, Hu JH, Li JY, Liu XM, Juengpanich S, Li MS, Feng X. Biologic therapy for Crohn’s disease over the last 3 decades. World J Clin Cases 2022; 10(2): 594-606
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/594.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.594
Crohn’s disease (CD), the main type of inflammatory bowel disease, is characterized as chronic, refractory, and relapsing transmural inflammation of the digestive tract[1]. Due to the continuous activation of the intestinal immune system, CD patients would suffer chronic abdominal pain, diarrhea, weight loss, malnutrition, and other obstructive symptoms[2]. Previously, the therapeutic strategy for CD was limited to corticosteroids[3], immunomodulators [methotrexate and thiopurines (azathioprine and mercaptopurine)][4-6], and surgery[7,8]. In the past 3 decades, multiple biologics emerged for CD management, including anti-tumor necrosis factor (TNF) agents (infliximab, adalimumab, and certolizumab), anti-integrin agents (vedolizumab and natalizumab), and anti-(IL)-12/23 agent (ustekinumab)[9]. However, it is difficult for researchers to gain critical articles to guide their studies owing to the publication overload of varied scientific quality.
Bibliometrics is an increasingly conducted method for analyzing and summarizing the main characteristics of publications, including the citation count, the cooperative relationships among countries, institutions, and authors, the distribution of journals, and the hotspots in a certain field. By performing bibliometric analysis and creating infographics, researchers can identify and capture the research hotspots and rising patterns. Bibliometric analysis has been broadly performed in various diseases of gastroenterology, such as Helicobacter pylori infection[10], irritable bowel syndrome[11], acute pancreatitis[12], inflammatory bowel disease[13], and so on. Although Connelly et al[14] conducted a bibliometric analysis of the 100 classic articles in ulcerative colitis and offered a reference of highly-citable manuscripts, no bibliometric analysis of biologic therapy for CD has been reported.
In this study, we aimed to analyze the top 100 highest-cited original articles in the field of biologic therapy for CD over the last 3 decades via bibliometric citation analysis based on the total citations (TC), which reflect the direct academic significance of a study. In turn, the analysis would provide clinicians and researchers the meaningful insights into the future directions related to biologic therapy for CD.
A systematic search of literature from January 1991 to December 2020 was performed in the Clarivate Analytics Web of Science Core Collection (WOSCC) database. We used search terms including “biologic therapy,” “Crohn disease,” “anti-tumor necrosis factor,” “infliximab,” “adalimumab,” “certolizumab,” “anti-integrin,” “vedolizumab,” “natalizumab,” “anti-IL-12/23,” “ustekinumab,” and their synonyms. The search strategy was shown in Supplementary Table 1. Original articles whose main topic was biologic therapy for CD were included. Literature that was not related to biologic therapy for CD was excluded, and reviews, commentary, case reports, editorials, consensus statements, and guidelines were also excluded. Two reviewers (J.L.S. and Z.Z.) independently identified the top 100 highest-cited original articles based on TC, and a third reviewer (J.S.C.) was recruited for discussion until any disagreement was settled.
After identifying the top 100 highest-cited original articles, the records with all available information were downloaded from the WOSCC database. Then, the bibliographic information of the top 100 highest-cited studies was converted and analyzed automatically by R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria) with the “bibliometric” package[15]. We further extracted and analyzed the information, including title, author, institution, country, TC, publication year, journal, 2020 Journal Citation Reports impact factor (IF), and keywords, using the “bibliometric” package.
All collected data were entered in a spreadsheet and manipulated using Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, United States). Graphs and figures were created by using R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria). Microsoft Excel 2019 Power Map (Microsoft Corp., Redmond, WA, United States) was utilized for a global map of countries’ publications of the top 100 highest-cited original articles. We used the VOS viewer (Version 1.6.10) to produce author cooperation network map, institution cooperation network map, keyword clustering map, and so on. The cooperation network map among all countries and the tree map of keywords were created on an online platform of bibliometric analysis (https://bibliometric.com/). Finally, 2 researchers (Z.Z. and J.S.C) verified the collected data and further analysis independently.
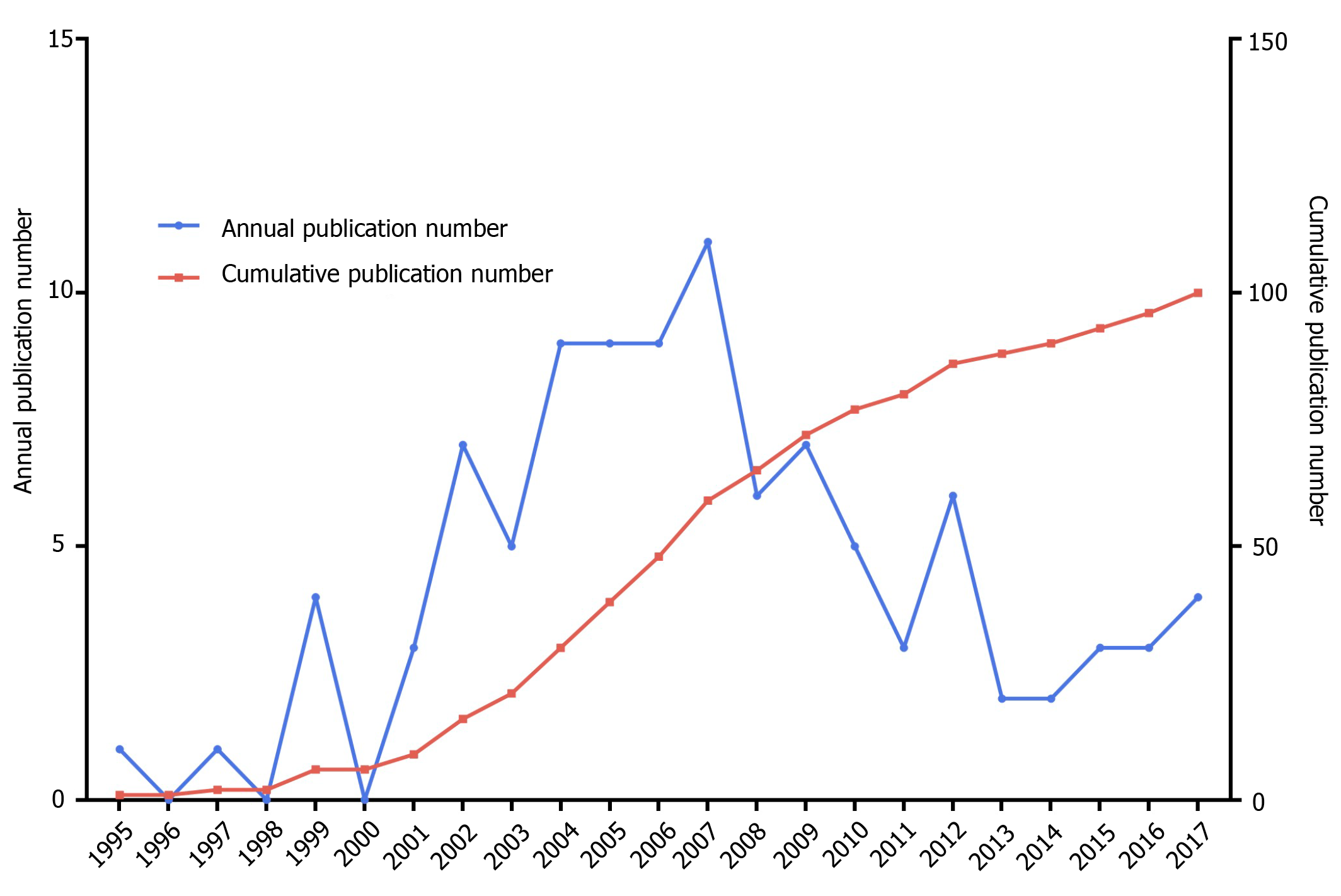
A total of 5489 original articles focusing on biologic therapy for CD were identified from the WOSCC database from January 1991 to December 2020. The top 100 highest-cited original articles were listed in Supplementary Table 2 according to the descending order of TC, and the TC ranged from 2978[16] to 307[17]. The earliest influential original article, which focused on treating CD with anti-TNF and gained TC of 926, was published in 1995[18]. The latest original articles were 4 studies published in 2017 that focused on biologic therapy for CD, including infliximab, adalimumab, and vedolizumab. Both the annual and the cumulative number of publications over the last 3 decades were presented in Figure 1. Interestingly, the 2000s (Period II, n = 66) yielded the most influential original articles and saw the most dramatic growth of them, followed by the 2010s (Period III, n = 28) and the 1990s (Period I, n = 6). Notably, the annual number of publications reached a peak of 11 in the year 2007 in Period II.
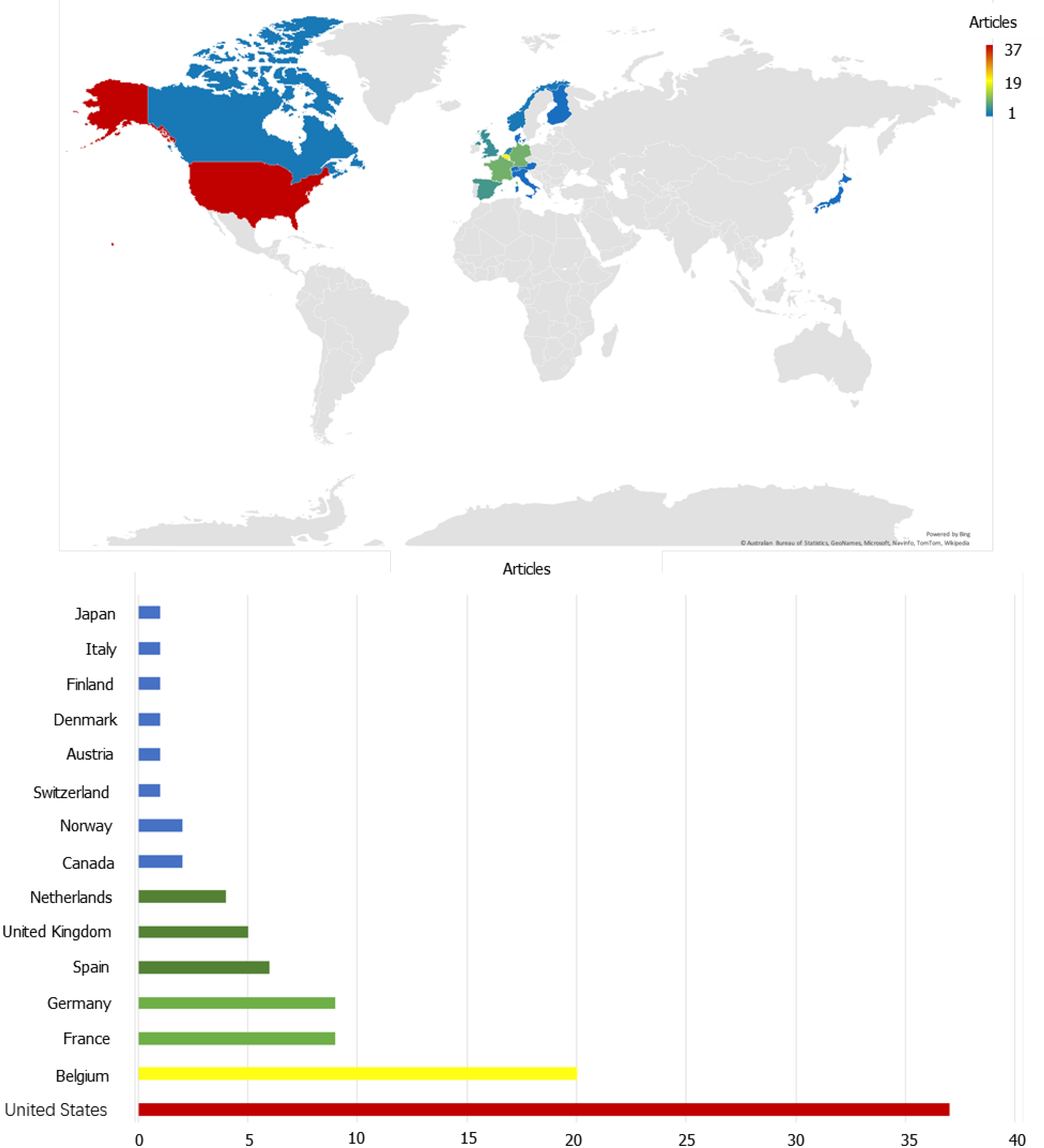
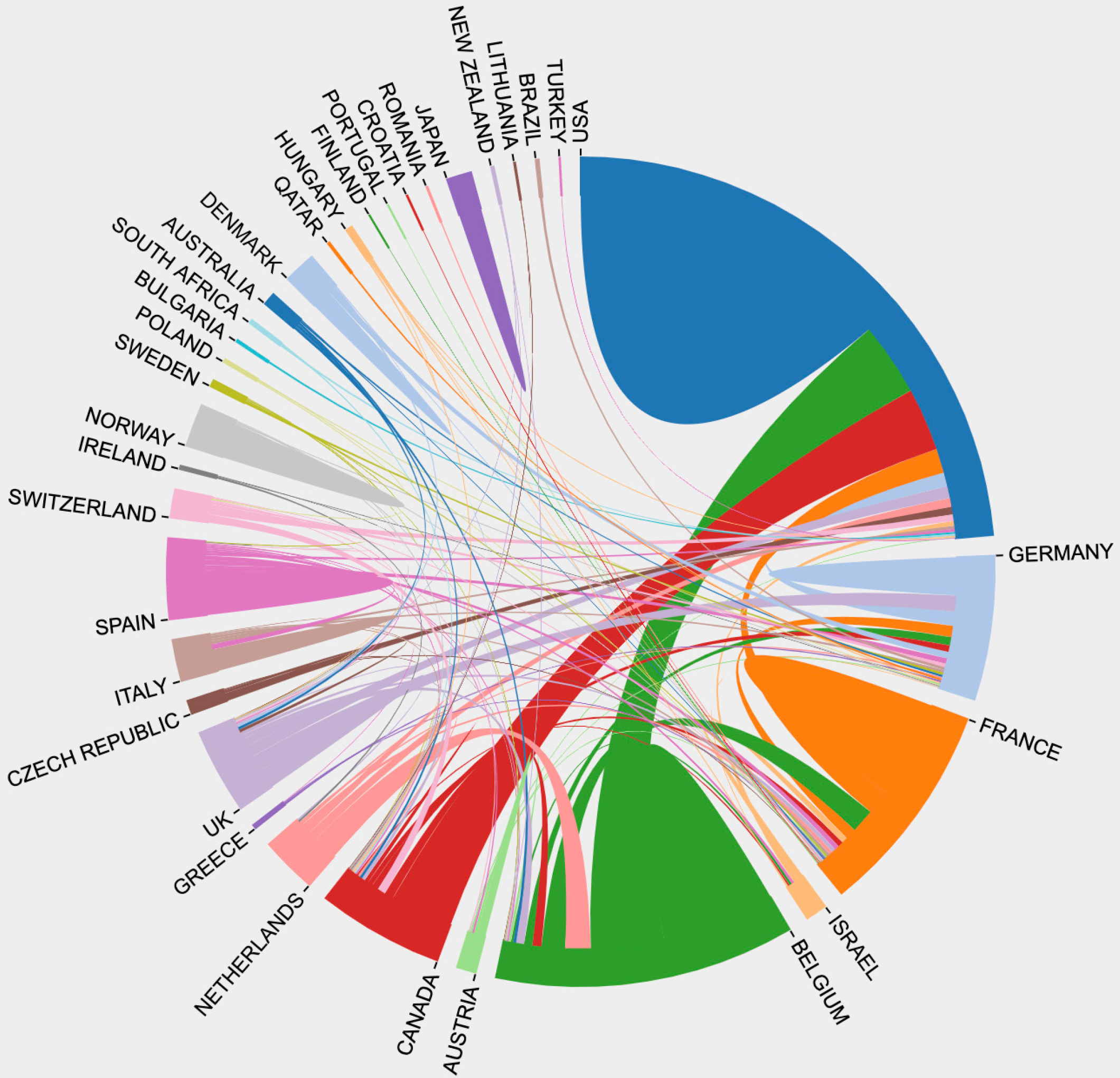
In analyzing the countries to identify the high-impact countries in this field, the top 100 highest-cited original articles originated from 15 countries (Figure 2). The top 10 countries with the most publications were listed in Table 1, including 8 European countries and 2 North American countries. Among the top 100 highest-cited original articles, the United States published the most articles (n = 37), followed by Belgium (n = 20), France (n = 9), Germany (n = 9), Spain (n = 6), United Kingdom (n = 5), Netherlands (n = 4), Norway (n = 2), Canada (n = 2), and Switzerland (n = 1). Notably, the United States has contributed the most studies and TC in the field of biologic therapy for CD, publishing 37 influential articles and 26179 citations. Meanwhile, as the top high-yield country in Europe, Belgium has published 20 articles with a TC of 13325. The ratio of TC to publication represented the average number of citations of each article, namely the average influence of each study. Although Switzerland ranked tenth in the number of original articles, contributing merely 1 article, it had the highest TC/Publication of 872. To be specific, Hueber W et al[19] from Switzerland conducted a randomized, double-blind placebo-controlled trial to explore the effect of a human anti-IL-17A monoclonal antibody, namely secukinumab, for moderate to severe CD, and they failed that blockade of IL-17A was ineffective and caused higher rates of adverse events. Thus, the scientific quality of the research in Switzerland may be generally high. Figure 3 showed the cooperation relationships among countries that contributed to the top 100 highest-cited original articles. The United States, Belgium, France, and Germany were intuitively observed to be involved in the close partner
| Country | Publication | TC | TC/Publication |
| United States | 37 | 26179 | 708 |
| Belgium | 20 | 13325 | 666 |
| France | 9 | 6791 | 755 |
| Germany | 9 | 4768 | 530 |
| Spain | 6 | 2916 | 486 |
| United Kingdom | 5 | 1980 | 396 |
| Netherlands | 4 | 2408 | 602 |
| Norway | 2 | 1163 | 582 |
| Canada | 2 | 816 | 408 |
| Switzerland | 1 | 872 | 872 |
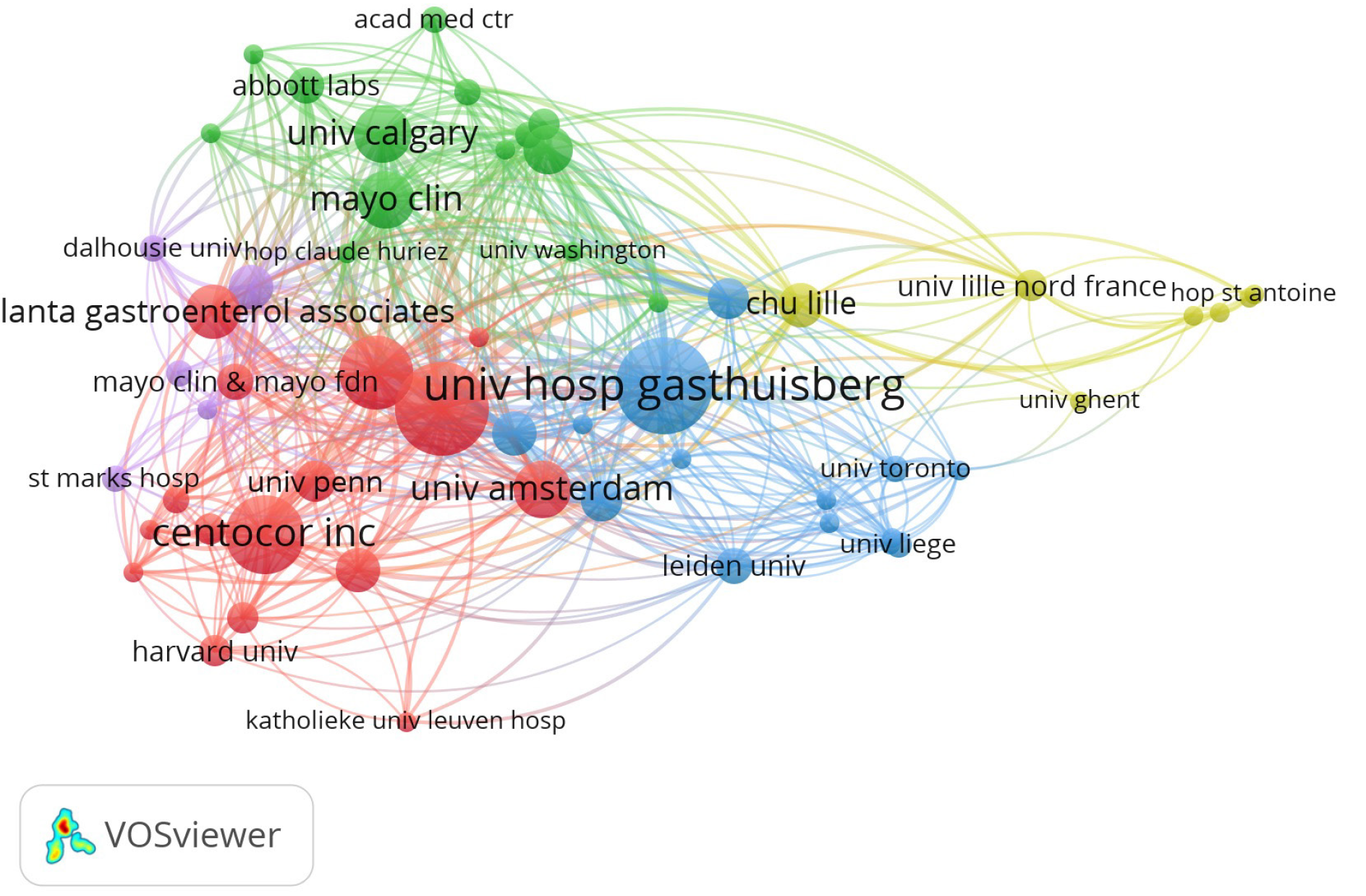
The top 10 institutions with the most publications were listed in Table 2, including the University Hospital Gasthuisberg in Belgium, the University of Chicago in the United States, and the Mayo Clinic in the United States with 23, 20, and 17 papers, respec
| Institutions | Publication | TC | TC/Publication |
| University Hosp Gasthuisberg | 23 | 17529 | 762 |
| University of Chicago | 20 | 19342 | 967 |
| Mayo Clinic | 17 | 14879 | 875 |
| University of Western Ontario | 15 | 14284 | 952 |
| University of Amsterdam | 12 | 9082 | 757 |
| University of Calgary | 11 | 7255 | 660 |
| University of Pennsylvania | 10 | 9086 | 909 |
| University of Kiel | 10 | 9066 | 907 |
| University of California San Diego | 9 | 4450 | 494 |
| Abbott Labs | 6 | 4614 | 769 |
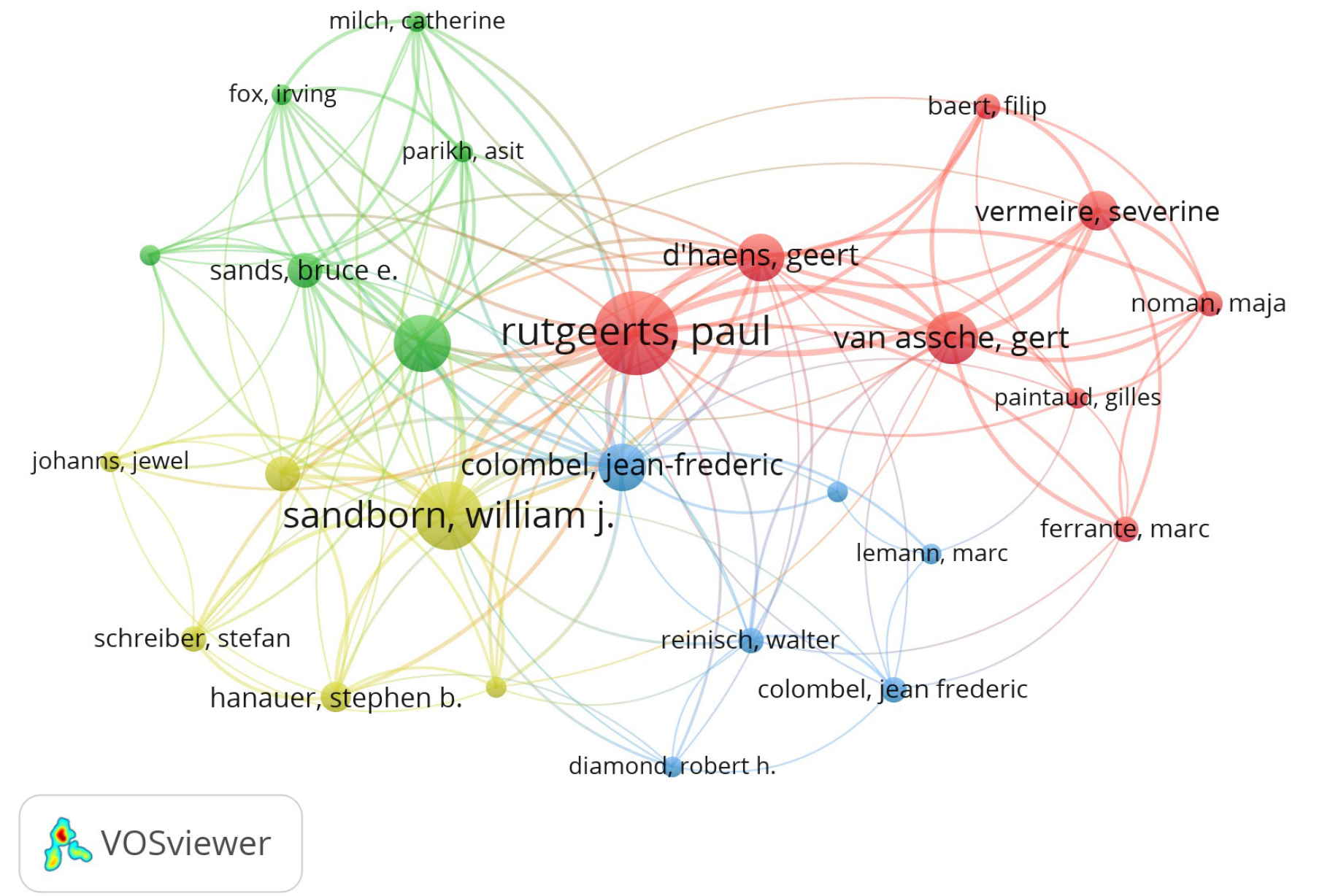
The top 10 most influential authors with the most publications were listed in Table 3, including Rutgeerts P in Belgium, Sandborn WJ in the United States, and Feagan BG in Canada with 32, 23, and 18 papers, respectively, and with 26039 citations, 18034 citations, and 16127 citations, respectively. Notably, Hanauer SB in the United States had the highest TC/Publication with merely 14 publications, which meant that his studies were of high scientific quality. The partnership among authors that have co-published more than 3 top-cited articles was shown in Figure 5.
| Authors | Publication | TC | TC/Publication |
| Rutgeerts P | 32 | 26039 | 814 |
| Sandborn WJ | 23 | 18034 | 784 |
| Feagan BG | 18 | 16127 | 896 |
| Colombel JF | 18 | 14724 | 818 |
| Hanauer SB | 14 | 13785 | 985 |
| D’haens G | 13 | 8951 | 689 |
| Van Assche G | 13 | 8286 | 637 |
| Vermeire S | 12 | 6902 | 575 |
| Sands BE | 9 | 6988 | 776 |
| Panaccione R | 9 | 5976 | 664 |
Based on the descending order of the number of the top 100 most influential original articles, the top 10 journals were listed in Table 4. Over the last 3 decades, Gastroenterology (IF = 22.682) has published the extremely most articles on biologic therapy for CD, including 32 publications and 17654 TC. Among the top 10 most influential journals, 5 journals had TC/Publication exceeding 500, in which The New England Journal of Medicine had the highest IF of 91.245, the highest TC of 18379, and the highest TC/Publication of 1225, exceeding 90, 18000, and 1225, respectively. The rest were The Lancet (IF = 79.321, TC/Publication = 855), Journal of Crohn’s and Colitis (IF = 9.071, TC/Publication = 799), Annals of Internal Medicine (IF = 25.391, TC/Publication = 668), and Gastroenterology (IF = 22.682, TC/Publication = 552).
| Journal | Publication | 2020 IF | TC | TC/Publication |
| Gastroenterology | 32 | 22.682 | 17654 | 552 |
| New England Journal of Medicine | 15 | 91.245 | 18379 | 1225 |
| Gut | 10 | 23.059 | 4693 | 469 |
| Lancet | 7 | 79.321 | 5988 | 855 |
| Clinical Gastroenterology and Hepatology | 6 | 11.382 | 2860 | 477 |
| American Journal of Gastroenterology | 5 | 10.864 | 1854 | 371 |
| Journal of Crohn’s and Colitis | 3 | 9.071 | 2397 | 799 |
| Inflammatory Bowel Diseases | 3 | 5.325 | 1127 | 376 |
| Arthritis and Rheumatology | 2 | 10.995 | 810 | 405 |
| Annals of Internal Medicine | 1 | 25.391 | 668 | 668 |
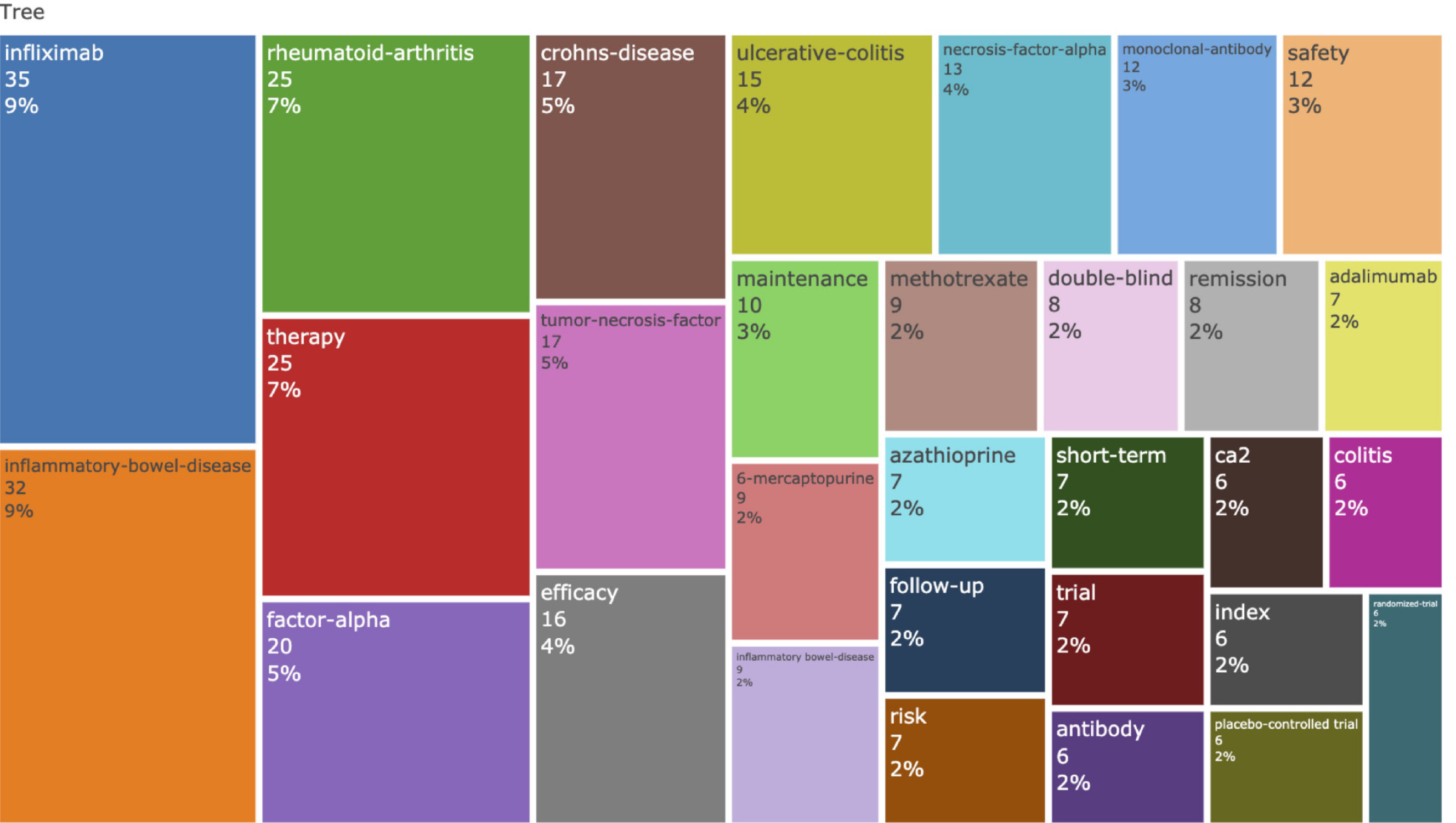
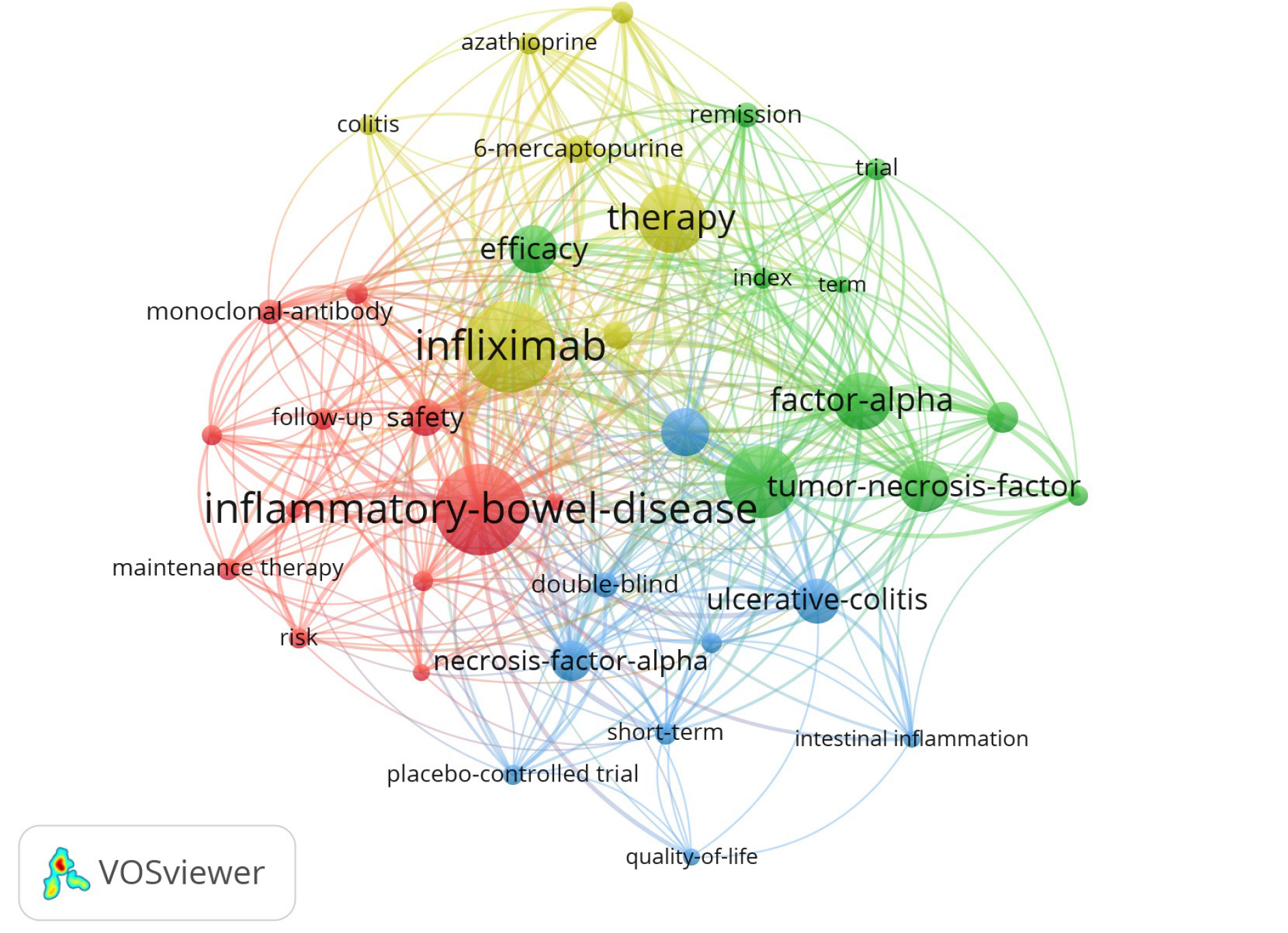
The research hotspots in the field of biologic therapy for CD were explored and demonstrated in the treemap (Figure 6). Infliximab, tumor necrosis factor, monoclonal antibody, and adalimumab accounted for 9%, 5%, 3%, and 2% of keywords, respectively. The cluster analysis of keywords that appeared more than seven times was also conducted to validate the results (Figure 7).
Due to the lack of a systematic approach to identifying the important information, it is challenging for clinicians and researchers to review the development of biological therapy for CD over the past 3 decades. In the study, we identified and ranked the top 100 highest-cited original articles by TC according to the WOSCC database. Through the bibliometric analysis, we summarized the basic characteristics of these original articles, such as publication, citation, countries, institutions, authors, journals, and keywords. In addition, we could identify research hotspots of biologic therapy for CD.
The analysis of annual and cumulative publications in different publication periods (Period I, Period II, and Period III) enabled clinicians and researchers to understand the development of biologic therapy for CD intuitively. In the 1990s (Period I), the number of most influential articles grew slowly. Notably, there was an explosive growth of the number of studies in the 2000s (Period II) because the first biologic agent, infliximab, was approved for CD treatment by the Food and Drug Administration[20]. Meanwhile, an increasing number of clinical trials were designed and conducted during Period II, including the ACCENT I trial (maintenance infliximab for CD)[16], the CLASSIC I and II trials (maintenance adalimumab for CD)[21,22], the CHARM trial (maintenance adalimumab for clinical response and remission of CD)[23], and other trials (ustekinumab, natalizumab, and certolizumab treatment for CD)[24-26]. Especially in the year of 2007, with the appearance and clinical use of adalimumab, certolizumab, and natalizumab, the annual publications peaked. However, less high-cited articles were published since the 2010s (Period III). A possible explanation was that the emerging biologics were relatively novel drugs, and the TC of related articles could not be accumulated within the limited time. A study conducted by Azer et al[27] further confirmed that the year of publication would be relative to TC, and therefore the TC of recently published articles was low.
The contributions of countries, institutions, and authors to biologic therapy for CD were identified in the study. The top 10 countries with the most publications were 2 North American countries and 8 European countries because the highest incidence rates have been shown in the United States and Europe[28]. This phenomenon may be attributed to the confounding factors of genetics and the environment[29], and the latter, including diet, pollution, microbial exposure, and sanitation, were implicated in the development of CD[30]. Notably, the United States, Belgium, France, and Germany occupied the leading positions and had the most cooperation among them in biologic therapy for CD, thus generating the most high-cited original articles with the highest TC in this field. Rutgeerts P from Belgium and Sandborn WJ from the United States have published the most influential studies and made excellent contributions to biologic therapy for CD, which was worth remembering. More attention should be paid to international cooperation, but it is not limited to the United States and Europe. Further multicenter clinical trials among different countries should be performed to offer evidence for biologic therapy for CD in the future.
Various medical journals were engaged in promoting the development of biologic therapy for CD. In terms of influence, the top 10 journals with the most publications were Gastroenterology, The New England Journal of Medicine, and Gut, with a total of 57 articles. The others were The Lancet, Clinical Gastroenterology and Hepatology, American Journal of Gastroenterology, Journal of Crohn’s and Colitis, Inflammatory Bowel Disease, Arthritis Rheumatology, and Annals of Internal Medicine, making a total of 27 publications. The top 10 journals were mostly in the field of digestive diseases, while some of them were comprehensive journals, namely The New England Journal of Medicine and The Lancet. Both journals have relatively high IF of 91.245 and 79.321, respectively, with high TC/Publication of 1225 and 855, respectively. The high scientific level of clinical trials in the top-cited original articles could contribute a lot to the higher citations per paper. One of the significant clinical trials, which was called the ACCENT I rando
The research hotspots in the top 100 highest-cited original articles over the past 3 decades were infliximab, tumor necrosis factor, monoclonal antibody, and adali
The study had several limitations that needed to be discussed. First, the current study may not include all influential articles in the field of biologic therapy for CD merely based on the WOSCC database. Although we did utilize broad search terms to search all related articles, it is possible that the search strategy may have missed some crucial literature. Further bibliometric analysis would be conducted with a precise search strategy from WOSCC, PubMed, and PMC databases. Second, the potential citation biases may affect the list of the top 100 highest-cited original articles and subsequently generate inaccurate results. In particular, the latest articles may have insufficient time to accumulate TC. Inappropriate citations, including self-citations, institutional biases, powerful author biases, and language biases, may also be inevitable and further affect the results of the analysis potentially.
In summary, the top 100 highest-cited original articles of biologic therapy for CD over the last 3 decades were identified and entered a bibliometric analysis to provide useful insights for clinicians and researchers. Moreover, the study offered an overview of countries, institutions, authors, and journals that had contributed significantly to the development of the specialized field. We focused on study keywords to explore the current and future research hotspots of biologic therapy for CD. Undoubtedly, studies and innovation of the field will continue to evolve and become an area of interest in the future.
There is an overloading amount of publications on biologic therapy for Crohn’s disease (CD).
No comprehensive analysis of biologic therapy for CD has been reported.
To determine knowledge gaps and identify areas of interest of biologic therapy for CD.
We conducted a bibliometric analysis of biologic therapy for CD based on the top 100 highest-cited original articles, summarized the bibliographic information, and explored the research hotspots.
The 2000s yielded the most influential original articles and saw the most dramatic growth. The United States and Europe contributed the most publications, and the cooperation relationships between them were most frequent. Gastroenterology published the most articles on biologic therapy for CD. Anti-tumor necrosis factor biologics and monoclonal antibodies were the most studied topics.
The bibliometric analysis emphasized the key contributions made to the development of the specialized field.
These data would provide useful research insights into biologic therapy for CD for clinicians and researchers.
We thank Yu TN, Department of General Surgery, Sir Run-Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, Zhejiang Province, China, for revising our manuscript. We also thank Topatana W, an international graduate and English native speaker in the Department of General Surgery, Sir Shaw RR Hospital, Zhejiang University School of Medicine, Hangzhou 310016, Zhejiang Province, China, for polishing our manuscript. We are grateful to our colleagues for their assistance in checking the data of the studies.
| 1. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1968] [Article Influence: 218.7] [Reference Citation Analysis (113)] |
| 2. | Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010;105:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 789] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 3. | Blackwell J, Selinger C, Raine T, Parkes G, Smith MA, Pollok R. Steroid use and misuse: a key performance indicator in the management of IBD. Frontline Gastroenterol. 2021;12:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2015;CD000067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | McDonald JW, Wang Y, Tsoulis DJ, MacDonald JK, Feagan BG. Methotrexate for induction of remission in refractory Crohn's disease. Cochrane Database Syst Rev. 2014;CD003459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Patel V, Wang Y, MacDonald JK, McDonald JW, Chande N. Methotrexate for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2014;CD006884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Spinelli A, Allocca M, Jovani M, Danese S. Review article: optimal preparation for surgery in Crohn's disease. Aliment Pharmacol Ther. 2014;40:1009-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Holder-Murray J, Marsicovetere P, Holubar SD. Minimally invasive surgery for inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1443-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Cushing K, Higgins PDR. Management of Crohn Disease-Reply. JAMA. 2021;325:1794-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Ouyang Y, Zhu Z, Huang L, Zeng C, Zhang L, Wu WK, Lu N, Xie C. Research Trends on Clinical Helicobacter pylori Eradication: A Bibliometric Analysis from 1983 to 2020. Helicobacter. 2021;26:e12835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Zyoud SH, Smale S, Waring WS, Sweileh W, Al-Jabi SW. Global research trends in the microbiome related to irritable bowel syndrome: A bibliometric and visualized study. World J Gastroenterol. 2021;27:1341-1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Sun W, Huang P, Song H, Feng D. Bibliometric analysis of acute pancreatitis in Web of Science database based on CiteSpace software. Medicine (Baltimore). 2020;99:e23208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Chen X, Yang K, Xu Y, Li K. Top-100 highest-cited original articles in inflammatory bowel disease: A bibliometric analysis. Medicine (Baltimore). 2019;98:e15718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Connelly TM, Devane L, Kelly JC, Wrafter P, Messaris E. The 100 classic papers in ulcerative colitis: a bibliometric analysis. Expert Rev Gastroenterol Hepatol. 2016;10:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Aria M, Cuccurullo C. bibliometrix: An R-tool for comprehensive science mapping analysis. Journal of Informetrics 2017; 11: 959-975. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1736] [Cited by in RCA: 2613] [Article Influence: 290.3] [Reference Citation Analysis (1)] |
| 16. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P; ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3101] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 17. | Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422-G430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 361] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 18. | van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology. 1995;109:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 732] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 19. | Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, Karczewski J, Pezous N, Bek S, Bruin G, Mellgard B, Berger C, Londei M, Bertolino AP, Tougas G, Travis SP; Secukinumab in Crohn's Disease Study Group. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1183] [Cited by in RCA: 1236] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 20. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2446] [Article Influence: 152.9] [Reference Citation Analysis (1)] |
| 21. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-33; quiz 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1211] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 22. | Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B, Li J, Pollack PF. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007;56:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 783] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 23. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1650] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 24. | Adedokun OJ, Xu Z, Marano C, O'Brien C, Szapary P, Zhang H, Johanns J, Leong RW, Hisamatsu T, Van Assche G, Danese S, Abreu MT, Sands BE, Sandborn WJ. Ustekinumab Pharmacokinetics and Exposure Response in a Phase 3 Randomized Trial of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;18:2244-2255.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Kane SV, Horst S, Sandborn WJ, Becker B, Neis B, Moscandrew M, Hanson KA, Tremaine WJ, Bruining DH, Faubion WA, Pardi DS, Harmsen WS, Zinsmeister AR, Loftus EV. Natalizumab for moderate to severe Crohn's disease in clinical practice: the Mayo Clinic Rochester experience. Inflamm Bowel Dis. 2012;18:2203-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Tatla D, Mountian I, Szegvari B, VanLunen B, Schiff M. A multicenter, open-label study to evaluate the safe and effective use of a new electromechanical auto-injection device for self-injection of certolizumab pegol. Expert Opin Drug Deliv. 2020;17:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Azer SA, Azer S. What can we learn from top-cited articles in inflammatory bowel disease? BMJ Open. 2018;8:e021233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4464] [Article Influence: 496.0] [Reference Citation Analysis (111)] |
| 29. | Shivashankar R, Lewis JD. The Role of Diet in Inflammatory Bowel Disease. Curr Gastroenterol Rep. 2017;19:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2010;6:339-346. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fan KS, Romano M S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL