Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6307
Peer-review started: December 31, 2021
First decision: February 21, 2022
Revised: March 16, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 26, 2022
Processing time: 168 Days and 0.2 Hours
Thyroid follicular renal cell carcinoma is a special type of renal cell carcinoma newly recognized in recent years. It has attracted attention because of its unique histology, immunophenotype, and clinical characteristics. It has a very low incidence, and the number of case reports available for review is limited. Moreover, a thyroid mass with type of tumour is rare.
We report a case of a renal mass with a bilateral thyroid mass that was acci
This is the third published case in which thyroid tumour biopsy was performed to confirm that thyroid follicular renal cell carcinoma is not thyroid related.
Core Tip: This is only the third published report combined with a thyroid tumor biopsy to confirm that thyroid follicular renal cell carcinoma is not thyroid related. In addition to the typical pathologic features of this tumor, our patient had radiographic features that were different from those previously reported.
- Citation: Wu SC, Li XY, Liao BJ, Xie K, Chen WM. Thyroid follicular renal cell carcinoma excluding thyroid metastases: A case report . World J Clin Cases 2022; 10(18): 6307-6313
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6307.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6307
Renal cell carcinoma (RCC) is a malignant tumour that originates from the renal parenchymal urothelial system and accounts for approximately 80%-85% of all malignant renal tumours. It is one of the most common tumours in the urinary system, second only to prostate cancer and bladder cancer[1]. Thyroid follicular RCC (TFRCC) is a rare new subtype of primary RCC that is not a true carcinoma originating from the thyroid gland. Although it is histologically similar to thyroid follicular carcinoma, TFRCC lacks typical thyroid markers. The rarity of these tumours limits our understanding of them, leading to misdiagnosis and inappropriate treatment. Here, we report the case of a 60-year-old man who presented with lower back and abdominal pain. Imaging examination revealed lesions in the right kidney and bilateral thyroid gland. Based on postoperative pathological examination findings, renal metastasis of the thyroid carcinoma was excluded.
A 60-year-old man presented with a > 1-mo history of right lower back and abdominal pain.
The 60-year-old man presented with a > 1-mo history of right lower back and abdominal pain. Computed tomography (CT) revealed small solid nodules under the capsule in the middle and lower part of the right kidney, leading to the suspicion of small RCC.
The patient had a 10-year history of diabetes. He did not take his medicine regularly and had poor blood glucose control.
The patient had no relevant personal or family history.
The patient’s vital signs were normal.
Laboratory examinations on admission revealed a carbohydrate antigen 19-9 level of 99.98 μmol/mL and creatinine level of 73.5 μmol/L (postoperative creatinine level: 79.1 μmol/L). The glomerular filtration rate in the left and right kidneys was 34.88 and 34.89 mL/min, respectively.
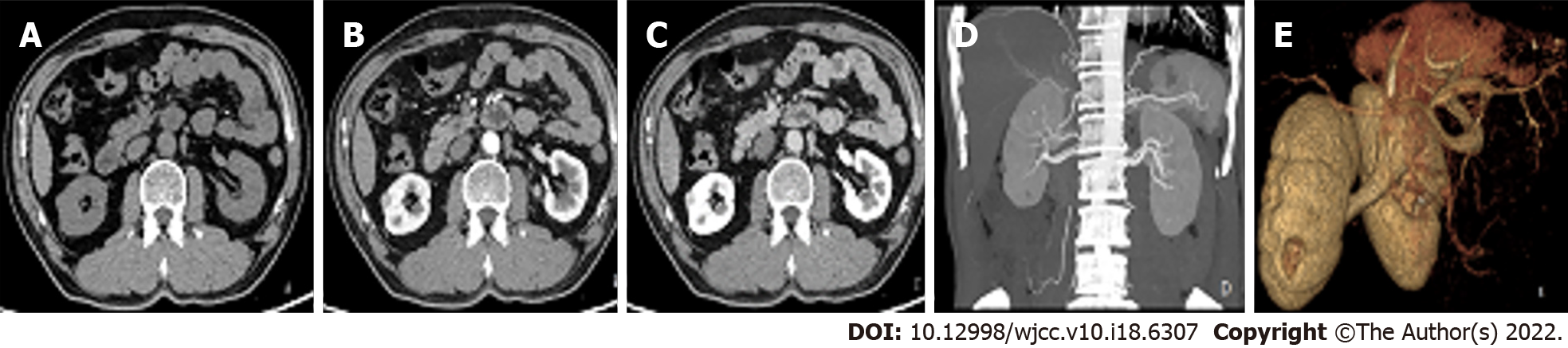
Ultrasound showed a round hypoechoic mass measuring approximately 0.9 × 0.8 cm in the middle and lower parenchyma of the right kidney; however, there was no obvious blood flow signal in the mass. Computed tomography angiography revealed a small nodular iso-density shadow (approximately 1 cm in diameter) in the middle parenchyma of the right kidney that protruded to the edge of the kidney. An enhanced scan showed continuous and obvious enhancement during the arterial phase. However, the enhancement was not uniform, and there were no abnormal tumour-supplying blood vessels (Figure 1). Ultrasound of the thyroid gland performed in our hospital on 5 January 2021 showed bilateral thyroid nodules (TI-RADS3 class).
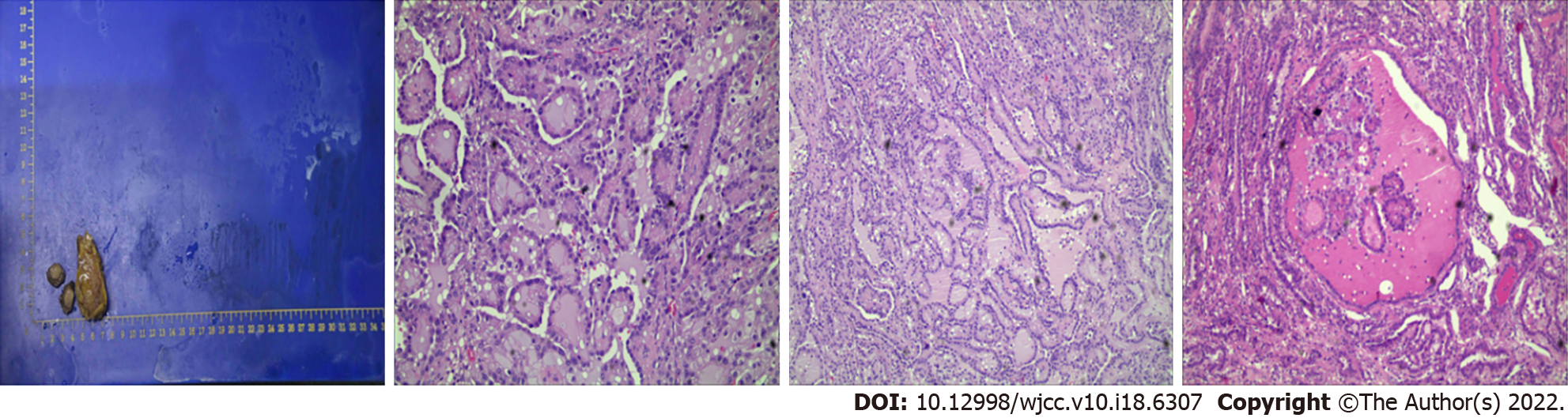
Postoperative pathology showed: Part of the renal tissue, 2.1 cm × 1.5 cm × 1.5 cm in size, had been cut in half in the clinic. Analysis of the tissue section revealed a nodular body measuring 1.2 cm × 1.0 cm × 0.6 cm; it was greyish-yellow or greyish white, slightly tough, and had slightly unclear boundaries. Moreover, it contained free adipose tissue (5.5 cm × 3.3 cm × 1.2 cm) and was not in contact with lymph nodes. Microscopy revealed glandular, cystic follicular, or papillary tumour cells of uniform size. The cytoplasm was medium stained, lightly stained, or empty bright, and the nucleus was round or slightly irregular with small nucleoli and red-stained lumen. Immunohistochemistry showed CK (3+); EMA) (+); Vimentin (+); PAX-8 (+); CK7 (+); Ki67 (2%+); P504S (+); E-cd (+); CD117 (-); CD10 (+); RCC (-); Calponin (-); TTF-1 (-); TG (-); TFE3 (weak+); S-100 (-); WT-1 (-); CA9 (-). Therefore, TFRCC of the right kidney was considered (Figure 2).
Laparoscopic partial nephrectomy was performed in December 2020. The patient recovered well after surgery and was discharged after 3 d. Fine-needle biopsy of the thyroid nodules was performed in 2021, and revealed no obvious pathological abnormalities .
The patient underwent re-examination on 30 March 2021, and the CT scan showed that the right kidney had changed after partial nephrectomy, but there were no other abnormalities. At the last follow-up, the patient was alive and healthy.
Previously, TFRCC was known as thyroid-like follicular carcinoma of the kidney or thyroid follicular carcinoma-like renal tumour, a special type of RCC with thyroid follicular carcinoma-like histomorphology. This rare, new type of RCC was described in detail at the 2012 International Society of Urological Pathology. In 2016, the World Health Organization Classification of Urological Oncology reclassified renal tumour subtypes, and TFRCC was listed as a ‘tentative renal cell carcinoma’ due to its extremely low incidence rate and small number of cases available for review[2]. This type of tumour was first described by Angell[3] in 1996. Immunohistochemical staining of the reported cases showed positive expression of TG and TTF-1 in tumour cells, although no space-occupying lesions were detected by thyroid examination. However, considering that papillary thyroid carcinoma can develop lymph node or distant metastasis with very small primary foci, we believe that the first case of TFRCC was reported in an abstract published by Amin[4] in 2004 and in a case report published by Jung[5] in 2006. Thus far, approximately 41 cases have been reported[6].
Due to its rarity, the pathogenic factors of TFRCC are unclear. Recent studies have found that a previous history of malignant tumour and chemotherapy, especially the use of a platinum-based chemotherapy regimen, significantly increased the risk of TFRCC, but the relationship between the mutual development needs to be determined in future studies[7]. Moreover, relevant genetic data on TFRCC are limited. In only a few groups of genetic tests, there were obvious genetic changes in TFRCC, but the chromosomal changes were significantly different to each other, and the genetic changes were not consistent with those of other known types of RCC[8]. More cases and studies are needed to find causative factors and genes.
The existing case data suggest that the disease mainly occurs in women. Currently, the youngest reported case pertains to a 10-year-old child[9] in whom the right side was more affected than the left side. The clinical symptoms are not obvious and most lack specificity; they are found accidently during physical examination. In most symptomatic patients, symptoms manifest as gross haematuria and abdominal pain, while some patients show hypertension[10,11,12], repeated urinary stimulation symptoms[12,13], weight loss[13], and other symptoms(Table 1). For the preoperative diagnosis of common RCC, CT is the first choice. However, for TFRCC, preoperative ultrasound seems to be more accurate than CT in visualizing the mass. On plain CT, cystic-solid changes are usually seen with high-density shadow, clear boundary, haemorrhage, and necrosis. Most of them show weak enhancement on enhanced scan, which is different to the obvious enhancement of other types. Moreover, eggshell-like calcification has been observed around the tumour, while the calcification in other types of RCC generally appears in the centre of the tumour. However, in our case, plain CT showed a moderate density shadow, while an enhanced scan showed continuous obvious uneven enhancement, inhomogeneous enhancement, and no obvious calcification, which was rarely seen in previous case reports. Magnetic resonance imaging generally shows a high signal on T1-weighted imaging and a low signal on T2-weighted imaging compared to the signal in the renal parenchyma. These characteristic imaging findings may have a certain suggestive value for preoperative consideration of TFRCC; however, pathological and immunological examination need to be performed for diagnosis.
| Features | Non-metastatic (n = 34) | Metastatic (n = 7) |
| Age (yr), mean ± SD (range) | 41.7 ± 17 (10-83) | 42.6 ± 14 (27-68) |
| Female sex | 18/33 (55%; 1 unknown) | 4/7 (57%) |
| Clinical presentation | ||
| Incidental | 24 (71%) | 4 (57%) |
| Flank pain/haematuria | 10 (29%) | 3 (43%) |
| History of malignancy (other sites) | 8 (24%) | 1 (14%) |
| Tumour characteristics | ||
| Size (cm), mean ± SD (range) | 4.2 ± 2.3 (1.1-11.8) | 5.3 ± 2.3 (3.5-10) |
| Right/left sided | 17/16 (1 unknown) | 5 2 |
| Necrosis | 9 (24%) | 3 (43%) |
| Ki-67 proliferation index | 1% to 30% (n = 6) | 6% (n = 1) |
| pT stage | ||
| 1a | 20 | 3 |
| 1b | 10 | 3 |
| 2a | 1 | 0 |
| 2b | 2 | 0 |
| Immunohistochemical features | ||
| CK7 | 24/25 (96%) | 5/6 (83%) |
| CK20 | 3/14 (21%) | 2/4 (50%) |
| CD10 | 4/23 (17%) | 1/5 (20%) |
| Vimentin | 20/25 (80%) | 3/4 (75%) |
| PAX8 | 10/11 (91%) | 3/4 (75%) |
| Site of metastasis | - | Bone (2), lung (3), lymph node (2) |
| Follow-up period (mo) | 22.1 ± 19.8 | 30.6 ± 28.8 |
| Disease/death at follow-up | 0 | 2 (28%) |
Macroscopically, most of the tumours are clear with a false capsule and both cystic and solid, with occasional bleeding, necrosis, or cystic degeneration. The section has a medium texture and is greyish white to greyish yellow, which differs from the multi-coloured appearance of clear cell RCC. Microscopically, the most prominent feature of the tumour is the formation of the thyroid follicular carcinoma-like structure, and the follicular cavity is filled with a red dye colloid-like substance, which is similar to thyroid colloid-like substance. The cytoplasm of the tumour cells is bichromatic or eosinophilic and empty and bright, the nucleus is round and oval, and the heteromorphism is not obvious. Occasionally, the nuclear groove can be seen, the mitotic image is rare, and the Fuhrman grade is mainly grade 2. Most cases show positive expression of CK, CK7, vimentin, and EMA; negative expression of the thyroid markers TTF-1 and TG; and low Ki-67 proliferation index[14]. In our case, the tumour was solid, its boundary was unclear, the other histological characteristics were similar to those previously reported, and the Ki-67 proliferation index was 2%. The degree of malignancy of the tumour was low, and the postoperative recovery was good.
The pathological diagnosis should be differentiated from thyroid carcinoma with renal metastasis and ovarian monodermal teratoma with renal metastasis, and the history can not completely exclude the primary TFRCC. The immunohistochemical markers TTF-1 and TG have important value in differential diagnosis. However, in poorly differentiated or sarcomatoid-differentiated thyroid carcinoma, TG and TTF-1 are absent, which can be distinguished according to whether there is a primary tumour. In addition, attention should be paid to the differentiation with renal thyroidisation[11], other nephrogenic tumours[12], and atrophic kidney[15]. It is not difficult to distinguish them in combination with clinical and histological characteristics. Our patient had bilateral thyroid mass; therefore, we needed to rule out the possibility that the kidney lesion was metastasized by thyroid carcinoma. Thyroid cancer often metastasises to the bone, lung, and liver and rarely to the kidney. There are <30 reported cases of renal metastasis of thyroid cancer origin, and the expression of TTF-1 and TG is both positive and strongly positive[16,17]. The specimens from our patient were repeatedly examined by the Pathology Department in our hospital, and both TTF-1 and TG continued to be absent. Therefore, the possibility of thyroid origin was ruled out, and TFRCC was presumed to be the primary tumour in the kidney. The diagnosis was then confirmed by thyroid tumour biopsy, similar to the method used in the case reported by Cai[16] and Tretiakova[13].
TFRCC has a certain degree of invasiveness, up to T3, and can have retroperitoneal lymph node metastasis and distant metastasis such as skull and meninges[6]. These tumours may metastasise through the blood-derived pathway, but the degree of malignancy is generally low. Surgical resection is the main treatment, which is supplemented by postoperative follow-up. Surgical methods include radical nephrectomy, partial nephrectomy, and resection of the metastatic lesion, which can be performed even if distant metastasis occurs. Except for a few patients with dedifferentiation of sarcomatoid areas[18] or with highly malignant cells[8], there is currently no clear clinicopathological feature that can predict the occurrence of metastasis and its poor outcome. There are limited reports on adjuvant therapy after surgery. At present, surgery is selected according to the general guidelines for RCC, and its unique treatment scheme needs to be actively explored in clinical practice to avoid unnecessary over-treatment and ensure the quality of life of patients. Following effective treatment, patients do not easily relapse, have a good prognosis, and can achieve long-term survival.
In summary, TFRCC is a rare subtype of low-grade malignant renal cell carcinoma with certain invasiveness, which usually occurs in young and middle-aged women. Its clinical and imaging manifestations have certain suggestive value, with unique morphological and immunohistochemical characteristics. The diagnosis depends on pathology and immunohistochemistry, and surgical resection is the preferred treatment. The overall prognosis is good, but there is a certain malignant potential, which needs long-term and close follow-up. If the disease progresses, the treatment plan for metastatic RCC should be considered. Due to the low incidence of TFRCC, there are few studies on this tumour; therefore, further studies are needed to enhance understanding and provide valuable information for diagnosis and treatment. No recurrence or metastasis was found in our case; however, further observation is needed in terms of survival time.
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 12203] [Article Influence: 2440.6] [Reference Citation Analysis (7)] |
| 2. | Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 2163] [Article Influence: 216.3] [Reference Citation Analysis (2)] |
| 3. | Angell SK, Pruthi R, Freiha FS. Primary thyroidlike carcinoma of the kidney. Urology. 1996;48:632-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Amin MB, Gupta R, Ondrej H, McKenney JK, Michal M, Young AN, Paner GP, Junker K, Epstein JI. Primary thyroid-like follicular carcinoma of the kidney: report of 6 cases of a histologically distinctive adult renal epithelial neoplasm. Am J Surg Pathol. 2009;33:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Jung SJ, Chung JI, Park SH, Ayala AG, Ro JY. Thyroid follicular carcinoma-like tumor of kidney: a case report with morphologic, immunohistochemical, and genetic analysis. Am J Surg Pathol. 2006;30:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Agrawal V, Neyaz Z, Kapoor R. Thyroid-Like Follicular Carcinoma of the Kidney With Oncocytic Cells: A Case Report and Review of Metastatic and Non-metastatic Tumors. Int J Surg Pathol. 2020;28:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Dong L, Huang J, Huang L, Shi O, Liu Q, Chen H. Thyroid-Like Follicular Carcinoma of the Kidney in a Patient with Skull and Meningeal Metastasis: A Unique Case Report and Review of the Literature. Medicine (Baltimore). 2016;95:e3314. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Ko JJ, Grewal JK, Ng T, Lavoie JM, Thibodeau ML, Shen Y, Mungall AJ, Taylor G, Schrader KA, Jones SJM, Kollmannsberger C, Laskin J, Marra MA. Whole-genome and transcriptome profiling of a metastatic thyroid-like follicular renal cell carcinoma. Cold Spring Harb Mol Case Stud. 2018;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | de Jesus LE, Fulgêncio C, Leve T, Dekermacher S. Thyroid-like follicular carcinoma of the kidney presenting on a 10 year-old prepubertal girl. Int Braz J Urol. 2019;45:834-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Wang H, Yu J, Xu Z, Li G. Clinicopathological study on thyroid follicular carcinoma-like renal tumor related to serious hypertension: Case report and review of the literature. Medicine (Baltimore). 2017;96:e6419. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Wu Y, Huang F, Zhou X, Yu S, Tang Q, Li S, Wang J, Chen L. Hypoxic Preconditioning Enhances Dental Pulp Stem Cell Therapy for Infection-Caused Bone Destruction. Tissue Eng Part A. 2016;22:1191-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Yang J, Zhang M, Meng Z, Song W, Yang L, Li L, Wang D, Shi T. Thyroid follicular carcinoma-like renal tumor: A case report and literature review. Medicine (Baltimore). 2018;97:e10815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Tretiakova MS, Kehr EL, Gore JL, Tykodi SS. Thyroid-Like Follicular Renal Cell Carcinoma Arising Within Benign Mixed Epithelial and Stromal Tumor. Int J Surg Pathol. 2020;28:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Eble JN, Delahunt B. Emerging entities in renal cell neoplasia: thyroid-like follicular renal cell carcinoma and multifocal oncocytoma-like tumours associated with oncocytosis. Pathology. 2018;50:24-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Herlitz L, Hes O, Michal M, Tretiakova M, Reyes-Múgica M, Nguyen JK, Troxell ML, Przybycin CG, Magi-Galluzzi C, McKenney JK. "Atrophic Kidney"-like Lesion: Clinicopathologic Series of 8 Cases Supporting a Benign Entity Distinct From Thyroid-like Follicular Carcinoma. Am J Surg Pathol. 2018;42:1585-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Cai DM, Wang HY, Jiang Y, Parajuly SS, Tian YE, Ma BY, et al. Primary follicular thyroid carcinoma metastasis to the kidney and widespread dissemination: A case report. Oncol Lett. 2016;11:3293-3297. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Cavalcante A, Kuwano AY, Costa-Matos A, Spanholi EF, Souza T, Mascarenhas FM. Thyroid-like follicular carcinoma of the kidney - Case report. Urol Case Rep. 2017;15:36-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Jenkins TM, Rosenbaum J, Zhang PJ, Schwartz LE, Nayak A, Cooper K, Tickoo SK, Lal P. Thyroid-Like Follicular Carcinoma of the Kidney With Extensive Sarcomatoid Differentiation: A Case Report and Review of the Literature. Int J Surg Pathol. 2019;27:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bari SU, India; Moez R, Tunisia S-Editor: Wang LL L-Editor: Webster JR P-Editor: Wang LL