Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6168
Peer-review started: November 4, 2021
First decision: March 7, 2022
Revised: March 15, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 26, 2022
Processing time: 224 Days and 12.7 Hours
Cerebrotendinous xanthomatosis (CTX) is a rare autosomal recessive metabolic disease caused by mutations in CYP27A1. It has a low incidence rate, insidious onset, and diverse clinical manifestations. It can be easily misdiagnosed and can go unrecognized by clinicians, leading to delayed treatment and worsened patient outcomes.
A 38-year-old male was admitted to our hospital with a history of unabating unstable posture and difficulty in walking for more than 30 years. Subsequently based on the patient's medical history, clinical symptoms, magnetic resonance imaging and gene sequencing results, he was finally diagnosed with CTX. Due to the low incidence rate of the disease, clinicians have insufficient knowledge of it, which makes the diagnosis process more tortuous and prolongs the diagnosis time.
Prompt diagnosis and treatment of CTX improve patient outcomes.
Core Tip: Cerebrotendinous xanthomatosis (CTX) is a rare disease for which prompt diagnosis and treatment improve patient outcomes. In addition, unreported new mutation and previously reported mutation were found in this patient. Thus, it provides new data for the further study of the pathogenesis of CTX and enriches the pathogenic mutation spectrum of CYP27A1.
- Citation: Li ZR, Zhou YL, Jin Q, Xie YY, Meng HM. CYP27A1 mutation in a case of cerebrotendinous xanthomatosis: A case report. World J Clin Cases 2022; 10(18): 6168-6174
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6168.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6168
Cerebrotendinous xanthomatosis (CTX) is a rare autosomal recessive lipid deposition disorder characterized by systemic signs and neurological dysfunction[1]. CTX is a treatable genetic metabolic disease, and early diagnosis and treatment can delay the progression of the disease to a considerable extent[2]. We report a case of CTX caused by mutations at two sites in CYP27A1. This case report will help clinicians to better understand CTX and its presentation, leading to early diagnosis and treatment, thereby improving the quality of life of patients.
A 38-year-old male was admitted to our hospital with a history of unabating postural instability and difficulty in walking for more than 30 years.
The patient was first brought for treatment at the age of 5 years. Clinical documentation at that time reported that the patient had exhibited unabating postural instability and difficulty in walking that did not improve with rest. He also exhibited cognitive impairment and irritability.
Since childhood, the patient experienced frequent episodes of chronic diarrhea lasting multiple weeks. At the age of 11 years, the patient underwent bilateral cataract surgery. At the age of 36 years, the patient presented with bilateral masses on the Achilles tendons coupled with thickening of the Achilles tendons.
The patient had no specific personal and family medical history.
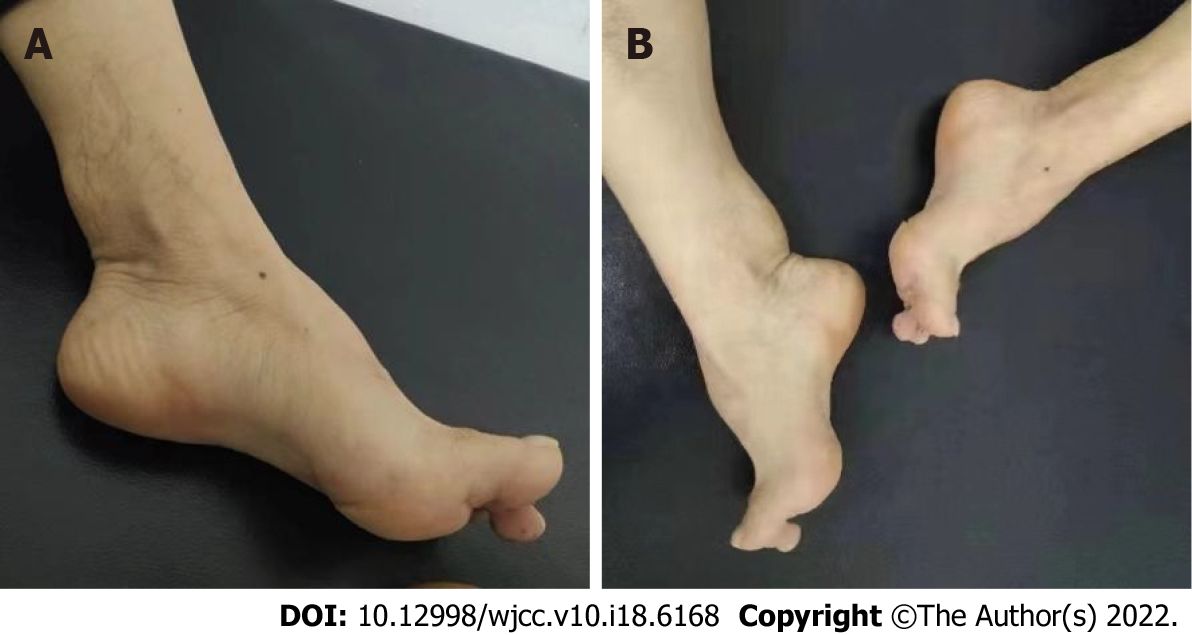
Neurological examination revealed gait ataxia, increased muscle tension in both lower limbs, bilateral hyperreflexia of the Achilles and knee tendons, a bilateral positive Babinski sign, and bilateral positive ankle clonus, indicating that the pyramidal tracts were damaged bilaterally. In addition, the patient also presented with arched feet and egg-sized, hard, painless lumps in both achilles tendons (Figure 1).
Blood lipid level examination revealed a total cholesterol concentration of 4.03 mmol/L (reference range: 2.60–5.20 mmol/L).
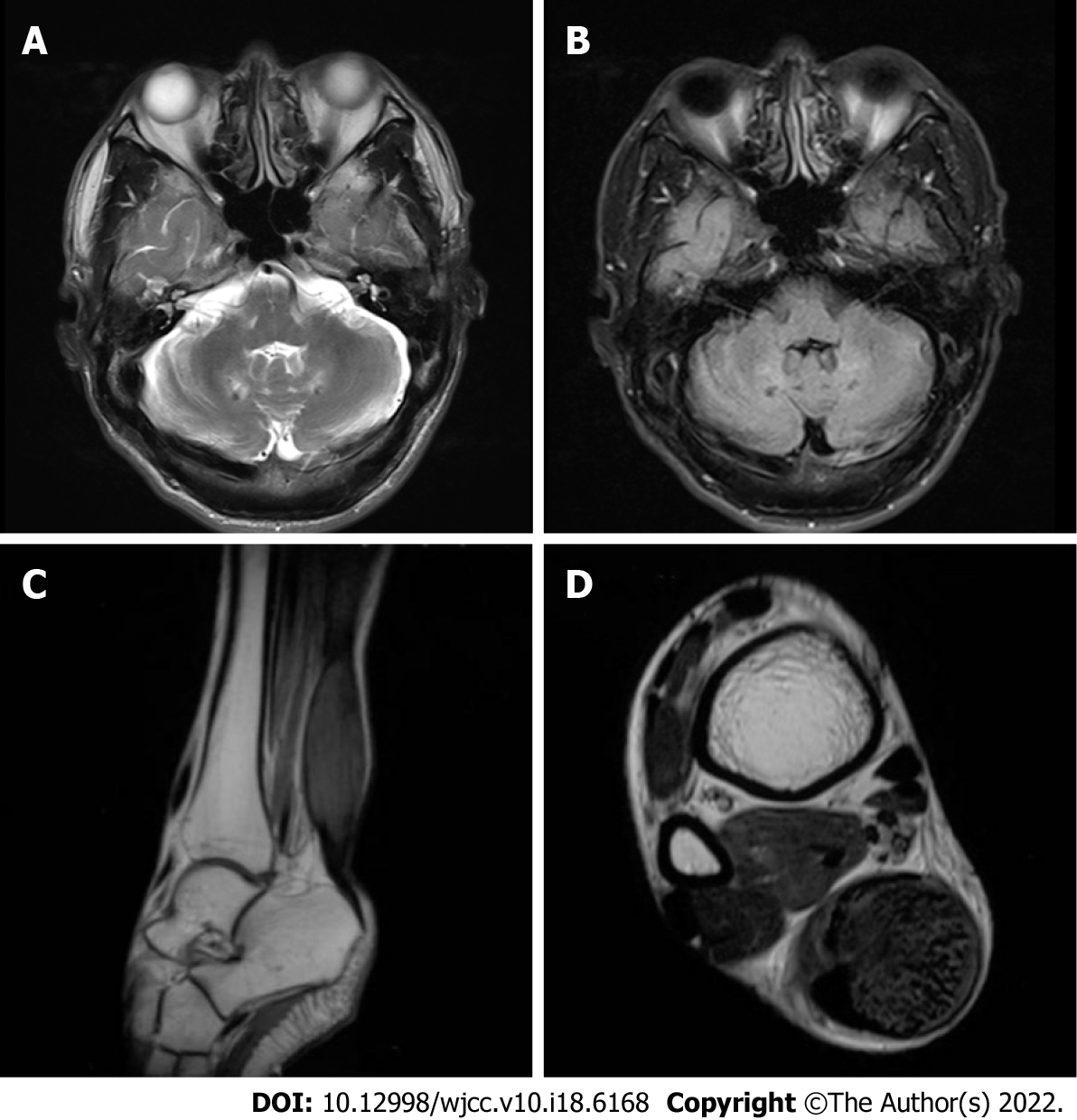
Magnetic resonance imaging (MRI) (Figure 2A and B) of the brain showed T2-weighted and FLAIR imaging hyperintensity in the bilateral cerebellar dentate nuclei. Electroencephalography showed abnormal slow-wave activity, composed of θ and δ waves, bilaterally in the posterior regions. MRI (Figure 2C and D) of the right ankle indicated fusiform swelling and abnormal signals in the Achilles tendons.
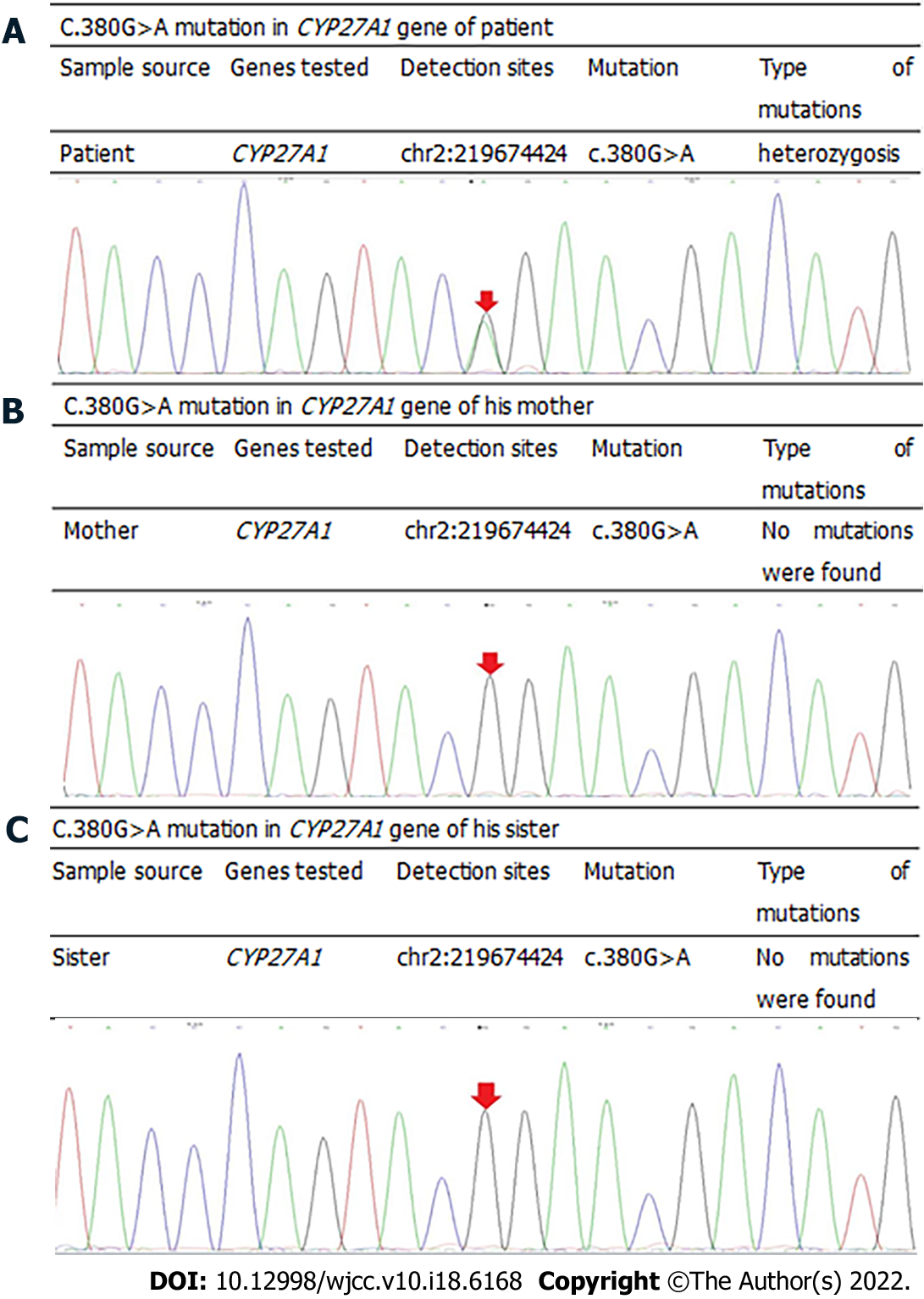
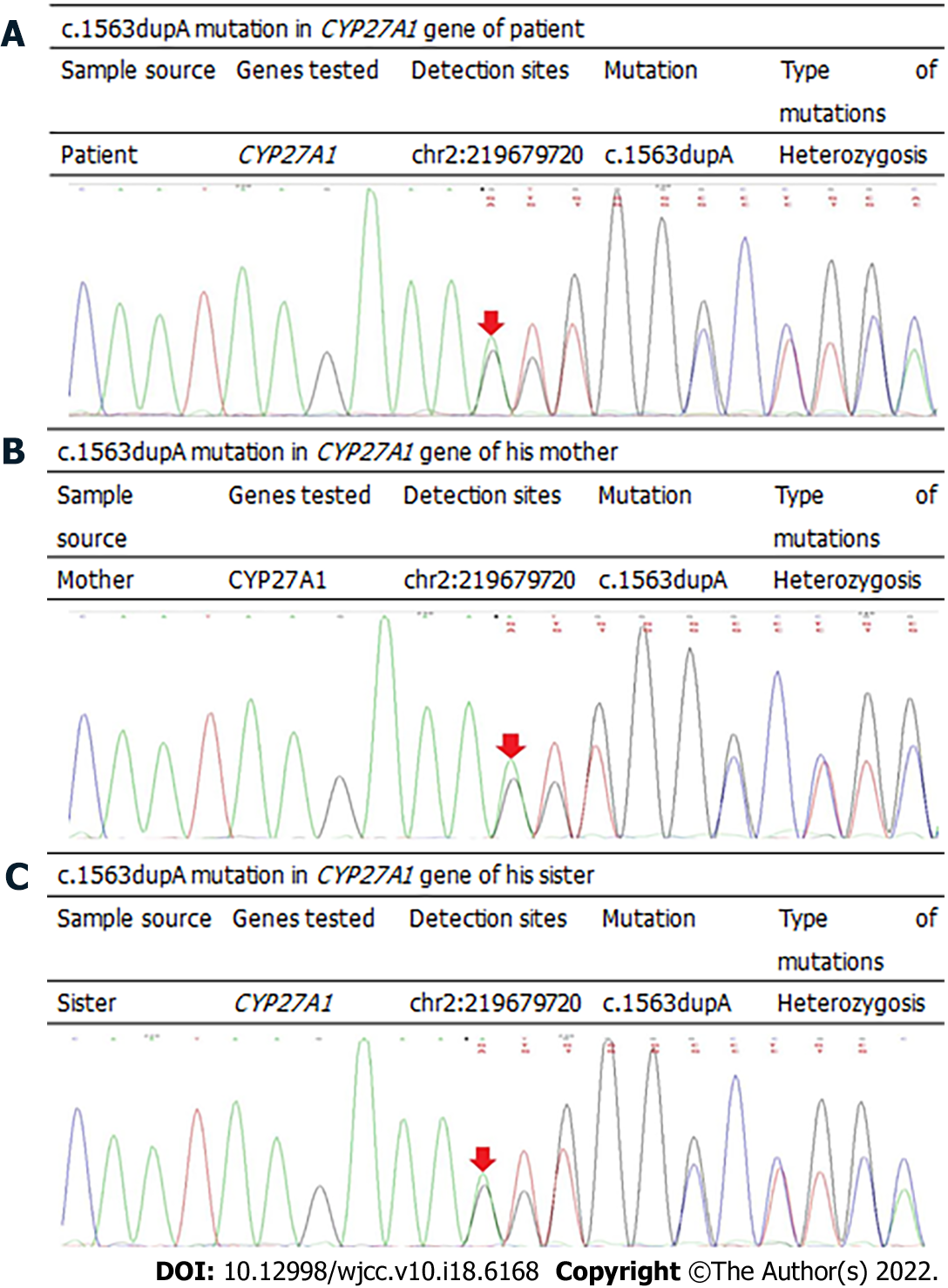
Based on the patient's medical history, clinical manifestations, and imaging analyses, it was unclear if CTX was involved, and gene sequencing was required to confirm the diagnosis. After informing the patient, the patient was eager to identify the underlying cause and had hopes for treatment; therefore he agreed to undergo gene sequencing analyses. Genomic DNA was extracted from the peripheral blood cells of the patient, and first-generation sequencing of the exon coding region of CYP27A1 revealed that the gene had a compound heterozygous mutation of c.380G>A (Figure 3) and c.1563dupA (Figure 4). Further examination demonstrated that the mother and sister of the patient were carriers of the c.1563dupA mutation.
Based on the patient's medical history, clinical manifestations, auxiliary examinations and gene sequencing results, the diagnosis of CTX was confirmed.
After the diagnosis of CTX, the patient was prescribed chenodeoxycholic acid (CDCA) at 250 mg three times per day and instructed to adhere to a low-cholesterol diet.
After 1 year of treatment, the patient felt that the symptoms of weakness in both lower limbs had improved slightly, but he did not report any additional changes. The patient reported no adverse reactions to CDCA.
The main cause of CTX is sterol 27-hydroxylase deficiency caused by the mutation of CYP27A1[3]. CYP27A1 encodes sterol 27-hydroxylase and is the only gene known to be associated with CTX[4]. Sterol 27-hydroxylase is involved in the biosynthesis of primary bile acids, including cholic acid and CDCA[5]. Sterol 27-hydroxylase deficiency obstructs the synthesis of primary bile acids, which causes the accumulation of bile acid synthesis pathway intermediates and derivative metabolites such as cholesterol and cholestanol. These substances are easily deposited in various lipophilic tissues, and therefore, they are more common in the brain, lens, and tendons[6]. They can negatively influence the function of cellular calcium channels, destroy the stability of cell membranes, and initiate the apoptosis pathway[7].
Currently, CTX is considered a rare disease, as are only a few hundred reported cases worldwide. However, we believe that this value is likely underestimated owing to the diversity of symptoms and the frequent delay in diagnosis. Consequently, the number of CTX cases is likely to be considerably far higher than that reported. The primary manifestations of CTX are infant-onset chronic refractory diarrhea, juvenile-onset bilateral cataracts, tendinous xanthomas, and progressive neurological dysfunction[8]. Neurophenotypes include ataxia, pyramidal tract signs, cognitive impairment, and peripheral neuropathy[8]. When the above symptoms are unexplained by common diseases at any age, the possibility of CTX should be considered, and further examinations should be performed. In the case of our patient, almost all the aforementioned symptoms occurred, although they varied in age at presentation. The constellation of symptoms observed in our patient caused us to consider CTX before other possible diseases.
There are no recognized diagnostic criteria for CTX; thus, clinicians must make their diagnoses based on medical history, family history, and clinical characteristics, following which the diagnosis must be confirmed by performing blood biochemistry tests, plasma cholesterol assessment, MRI, and gene sequencing. Our patient’s gene sequencing results showed two mutation sites in CYP27A1: c.380G>A located on exon 2 and c.1563dupA located on exon 9. Among these, the mutation site c.380G>A has been reported previously[9], but the c.1563dupA mutation is novel and yet to be reported. As our patient possesses a known pathogenic mutation in CYP27A1, we are currently unable to determine whether the new gene mutation is pathogenic; further research is needed to confirm the same. In addition, the biochemical diagnosis of CTX is based on the increase in serum cholestanol and urine bile alcohols levels[4]. The typical imaging findings indicating the prevalence of CTX include T2-weighted and FLAIR imaging hyperintensity in the dentate nucleus[10]. The current case supports the inclusion of high signal intensity of the two dentate nuclei on MRI as a typical feature of CTX[11]. The typical imaging manifestations of CTX are high signal in T2 weighted imaging and FLAIR imaging of dentate nucleus.
In CTX treatment, there is currently no clear treatment plan, and the condition can be treated symptomatically based on the different clinical manifestations. Bile acid supplements, such as CDCA, provide a source of primary bile acids, which can inhibit the synthesis of bile acids through a negative feedback mechanism, thereby prevent the accumulation of cholesterol and cholestanol[12]. Consequently, early oral bile acid supplement treatment is recommended[13]. In addition, cataract extraction is common performed in these patients, and xanthoma can be surgically removed.
Our patient was prescribed CDCA replacement therapy. At a follow-up visit 6 months post-treatment, the symptoms of the patient had improved slightly. Based on previous studies, we understand that the clinical process of CTX is progressive[14]. Saussy et al[14] compared three cases of CTX and concluded that early and uninterrupted treatment can delay progression of the disease, avert nervous system involvement, and improve the quality of life of patients. Once the neurological symptoms are completely determined, the therapeutic effect will be considerably reduced.
Herein, we reported a case where first-generation sequencing of CYP27A1 was performed in a patient with CTX, leading to the detection of an unreported new mutation as well as a previously reported mutation. Consequently, this case provides new data for further examination of the pathogenesis of CTX and enriches the pathogenic mutational spectrum of CYP27A1. In addition, the diagnosis of our patient helped him to receive genetic counseling and guidance regarding fertility. We hope that our case report enables other clinicians to more deeply understand the diagnosis and treatment of CTX, leading to early diagnoses and treatment and improved patient prognoses.
We would like to thank the patient and the patient’s family.
| 1. | Dell'Aversano Orabona G, Dato C, Oliva M, Ugga L, Dotti MT, Fratta M, Gisonni P. Multi-imaging study in a patient with cerebrotendinous xanthomatosis: radiology, clinic and pathology correlation of a rare condition. BJR Case Rep. 2020;6:20190047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Degrassi I, Amoruso C, Giordano G, Del Puppo M, Mignarri A, Dotti MT, Naturale M, Nebbia G. Case Report: Early Treatment With Chenodeoxycholic Acid in Cerebrotendinous Xanthomatosis Presenting as Neonatal Cholestasis. Front Pediatr. 2020;8:382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Salen G, Steiner RD. Epidemiology, diagnosis, and treatment of cerebrotendinous xanthomatosis (CTX). J Inherit Metab Dis. 2017;40:771-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Koyama S, Sekijima Y, Ogura M, Hori M, Matsuki K, Miida T, Harada-Shiba M. Cerebrotendinous Xanthomatosis: Molecular Pathogenesis, Clinical Spectrum, Diagnosis, and Disease-Modifying Treatments. J Atheroscler Thromb. 2021;28:905-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Hong X, Daiker J, Sadilek M, DeBarber AE, Chiang J, Duan J, Bootsma AH, Huidekoper HH, Vaz FM, Gelb MH. Toward newborn screening of cerebrotendinous xanthomatosis: results of a biomarker research study using 32,000 newborn dried blood spots. Genet Med. 2020;22:1606-1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Parry AH, Wani AH, Bashir M, Gojwari TA. Cerebrotendinous xanthomatosis - A case report. Indian J Radiol Imaging. 2019;29:332-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Baghbanian SM, Mahdavi Amiri MR, Majidi H. Cerebrotendinous xanthomatosis revisited. Pract Neurol. 2021;21:243-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Verrips A, Dotti MT, Mignarri A, Stelten BML, Verma S, Federico A. The safety and effectiveness of chenodeoxycholic acid treatment in patients with cerebrotendinous xanthomatosis: two retrospective cohort studies. Neurol Sci. 2020;41:943-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Watts GF, Mitchell WD, Bending JJ, Reshef A, Leitersdorf E. Cerebrotendinous xanthomatosis: a family study of sterol 27-hydroxylase mutations and pharmacotherapy. QJM. 1996;89:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Mignarri A, Dotti MT, Federico A, De Stefano N, Battaglini M, Grazzini I, Galluzzi P, Monti L. The spectrum of magnetic resonance findings in cerebrotendinous xanthomatosis: redefinition and evidence of new markers of disease progression. J Neurol. 2017;264:862-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Cao LX, Yang M, Liu Y, Long WY, Zhao GH. Chinese patient with cerebrotendinous xanthomatosis confirmed by genetic testing: A case report and literature review. World J Clin Cases. 2020;8:5446-5456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Gerrish AC, Gaba S. Case 239: Cerebrotendinous Xanthomatosis. Radiology. 2017;282:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Pierre G, Setchell K, Blyth J, Preece MA, Chakrapani A, McKiernan P. Prospective treatment of cerebrotendinous xanthomatosis with cholic acid therapy. J Inherit Metab Dis. 2008;31 Suppl 2:S241-S245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Saussy K, Jain N, Murina A. Cerebrotendinous xanthomatosis: A report of 3 cases. JAAD Case Rep. 2020;6:1205-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Conflicts-of-interest statement: The authors have no conflicts of interest to declare that are relevant to the content of this article.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Parry AH, India; Wiratnaya IGE, Indonesia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ