Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6021
Peer-review started: December 7, 2021
First decision: January 25, 2022
Revised: March 23, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 26, 2022
Processing time: 191 Days and 12.8 Hours
Lesions of breast imaging reporting and data system (BI-RADS) 4 at mammo
To evaluate the value of DWI and PWI in diagnosing BI-RADS 4 breast lesions.
This is a retrospective study which included patients who underwent breast MRI between May 2017 and May 2019 in the hospital. The lesions were divided into benign and malignant groups according to the classification of histopathological results. The diagnostic efficacy of DWI and PWI were analyzed respectively and combinedly. The 95 lesions were divided according to histopathological diagnosis, with 46 benign and 49 malignant. The main statistical methods used included the Student t-test, the Mann-Whitney U-test, the chi-square test or Fisher’s exact test.
The mean apparent diffusion coefficient (ADC) values in the parenchyma and lesion area of the normal mammary gland were 1.82 ± 0.22 × 10-3 mm2/s and 1.24 ± 0.16 × 10-3 mm2/s, respectively (P = 0.021). The mean ADC value of the malignant group was 1.09 ± 0.23 × 10-3 mm2/s, which was lower than that of the benign group (1.42 ± 0.68 × 10-3 mm2/s) (P = 0.016). The volume transfer constant (Ktrans) and rate constant (Kep) values were higher in malignant lesions than in benign ones (all P < 0.001), but there were no significant statistical differences regarding volume fraction (Ve) (P = 0.866). The sensitivity and specificity of PWI combined with DWI (91.7% and 89.3%, respectively) were higher than that of PWI or DWI alone. The accuracy of PWI combined with DWI in predicting pathological results was significantly higher than that predicted by PWI or DWI alone.
DWI, combined with PWI, might possibly distinguish between benign and malignant BI-RADS 4 breast lesions at mammography.
Core Tip: Lesions of breast imaging reporting and data system (BI-RADS) at mammography only appeared a wide range of risk of being malignant (2%-96%). The specificity of magnetic resonance imaging in detecting breast is relatively low. This study aimed to evaluate the value of diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI) in diagnosing BI-RADS 4 breast lesions. The diagnostic efficacy of DWI and PWI were analyzed respectively and jointly. The results suggested DWI, combined with PWI, might possibly help distinguish benign breast lesions from malignant ones and provide clear diagnostic results for patients with potentially malignant BI-RADS 4 lesions at mammography.
- Citation: Zhang H, Zhang XY, Wang Y. Value of magnetic resonance diffusion combined with perfusion imaging techniques for diagnosing potentially malignant breast lesions. World J Clin Cases 2022; 10(18): 6021-6031
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6021.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6021
Breast cancer is one of the most common malignant tumors threatening women[1]. In recent years, the incidence of breast cancer has increased year by year, topping all types of cancer in Chinese women[2]. Magnetic resonance imaging (MRI) has become an essential examination method to diagnose breast lesions due to the avoidance of ionizing radiation, high soft-tissue resolution, multi-parameter imaging, multi-sequence imaging, and high sensitivity[3,4]. Nevertheless, the specificity of MRI in detecting breast is relatively low, leading to many false-positive results and high rates of re-examination or biopsy[3,4].
Novel functional MRI techniques, including diffusion-weighted imaging (DWI), perfusion-weighted imaging (PWI), and other non-invasive detection methods, have enabled the detection of pathological conditions of tissues to reach on a molecular-level, as well as the detection of functional status and change in mechanisms of organs, tissues, and cells in vivo[5]. DWI is considered the most effective modality for malignant tumor screening and therapeutic effect assessment for breast cancer[6-9]. PWI can be applied in detecting blood perfusion in tissues, where the perfusion imaging pattern is closely related to the density of newly-generated microvessels in tumors[10,11]. Nevertheless, these techniques have not been widely validated in clinical practice since they mainly act as auxiliary roles for assessing suspicious lesions[8,12,13]. DWI can also monitor the treatment response to neoadjuvant chemotherapy[14] and help to determine the subtypes of breast cancer[15].
According to the MRI breast imaging reporting and data system (BI-RADS) (5th edition), potentially malignant breast lesions are classified as the 4th category (BI-RADS 4) with a wide variation with regard to the risk of malignancy, from 2% to 95%[16]. BI-RADS 4 lesions could be anything from benign to malignant, resulting in the difficulties for clinicians to distinguish between them[17-20].
Therefore, the present study aimed to investigate the diagnostic efficiency of the apparent diffusion coefficient (ADC) combined with PWI in determining the nature of lesions categorized as BI-RADS 4. The results help to decide the exact nature of breast lesions before the patients undergo biopsy.
This retrospective study included patients who underwent breast MRI examination, including symptomatic patients presenting to clinic and patients detected abnormalities in regular screening, between May 2017 and May 2019 at the Department of Radiology of Hospital. The diagnostic criteria were the 5th edition of MRI BI-RADS[16].
The inclusion criteria were: (1) Age over 20 years old; (2) BI-RADS 4 based on mammography only; (3) Lesion > 5.0 mm; (4) The MRI examination of all lesions was completed before needle biopsy; and (5) The MRI examination included DWI and dynamic contrast enhancement imaging (DCE-MRI) in addition to the conventional plain scans. The exclusion criteria were: (1) Received neoadjuvant chemotherapy which might affect the MRI readings[14]; or (2) Clinical or pathological T4 lesion.
The study was reviewed and approved by the First Hospital of Hebei Medical University. The need for individual informed consent was waived by the committee.
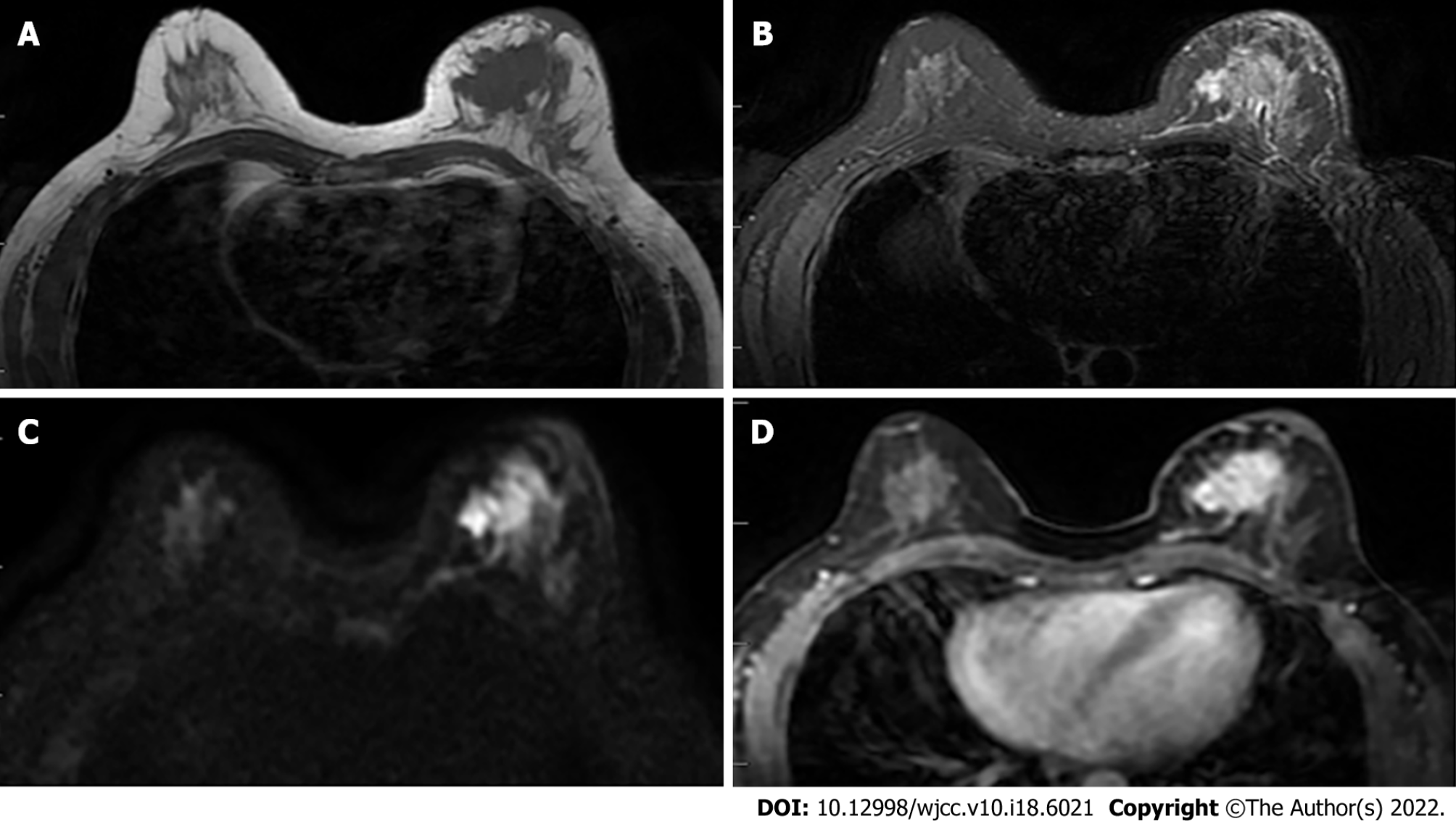
All examinations were performed with the patients in the prone position. MRI was performed by a 1.5-T MRI scanner (Signa Excite HDxT; GE Healthcare, Waukesha, WI, United States) and a 3.0-T MRI scanner (GE Silent Discovery 750W; GE Healthcare, Waukesha, WI, United States) with an 8-channel phased-array bilateral breast coil. The scanning sequences and corresponding parameters were: (1) T2WI fat-suppressed fast spin-echo (FSE): Repetition time (TR) 6079 ms, echo time (TE) 85 ms, flip angle (FA) 111°, field-of-view (FOV) 36 × 36 mm2, matrix size 320 × 256, number of excitation (NEX) 1.0, slice thickness (ST) 5.0 mm, scan time 2.44 s; (2) T1WI FSE: TR 697 ms, TE min full, FA 111°, FOV 36 × 36 mm2, matrix size 320 × 256, NEX 1.0, ST 5.0 mm, scan time 1.05 s; (3) DWI: TR 2881.4 ms, TE minimum, FOV 36 × 36 mm2, matrix size 128 × 128, ST 5.0 mm, b values 0-800 s/mm2, scan time 2.01 s; and (4) T1WI dynamic perfusion: TR 5.5 ms, TE min full, FA 12°, FOV 34 × 34 mm2, matrix size 160 × 150, ST 5.0 mm, 40 phase scanning, scan time 7.12 s (Figure 1). The contrast agent was gadopentetate dimeglumine (Jiangsu Hengrui Pharmaceutical Co., Ltd., China) and was administered at a dose of 0.2 mmol/kg with infusion rate of 3.0 mL/s. The intravenous injection of contrast agent began 30 s after the start of the scanning.
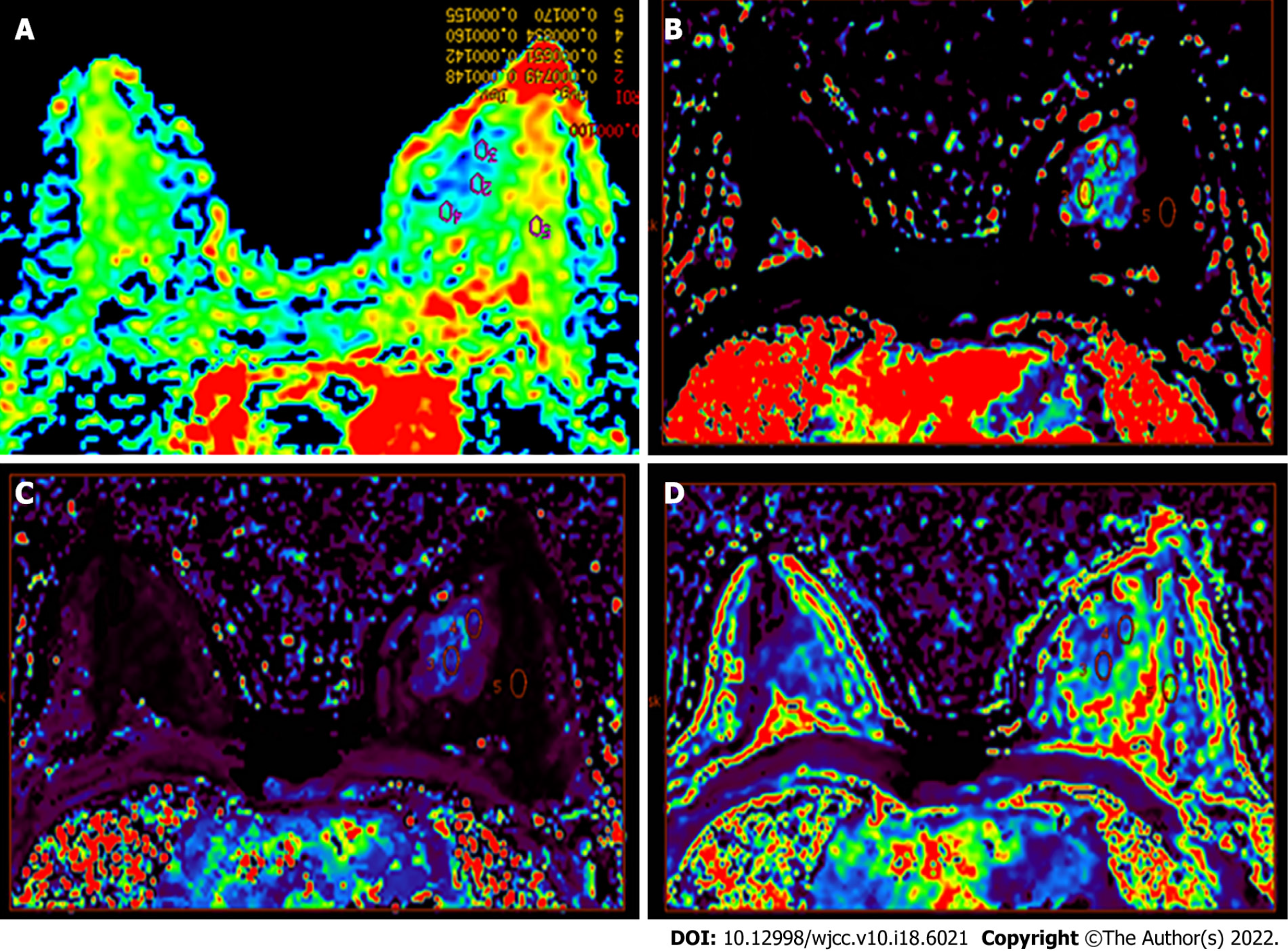
The collected data were transmitted to the General Electric Assistant Diagnostic Workstation 4.6, and the image data were analyzed. Then two radiologists with more than 5 years of experience in breast lesion diagnosis provided an independent analysis and assessment without knowing the clinical and pathological results. A consensus was reached through discussion in case of any inconsistencies between them. Regions with enhancements in DCE-MRI sequence and high signal intensity in the DWI sequence were considered to be lesions. After obtaining the ADC map by post-processing, the ADC values were measured by manually placing the elliptic in the lesion area, and covering at least four minimum pixels, with the average value based on three measurements. Meanwhile, the volume transfer constant (Ktrans), rate constant (Kep), and extravascular extracellular volume fraction (Ve) in this region were measured (Figure 2).
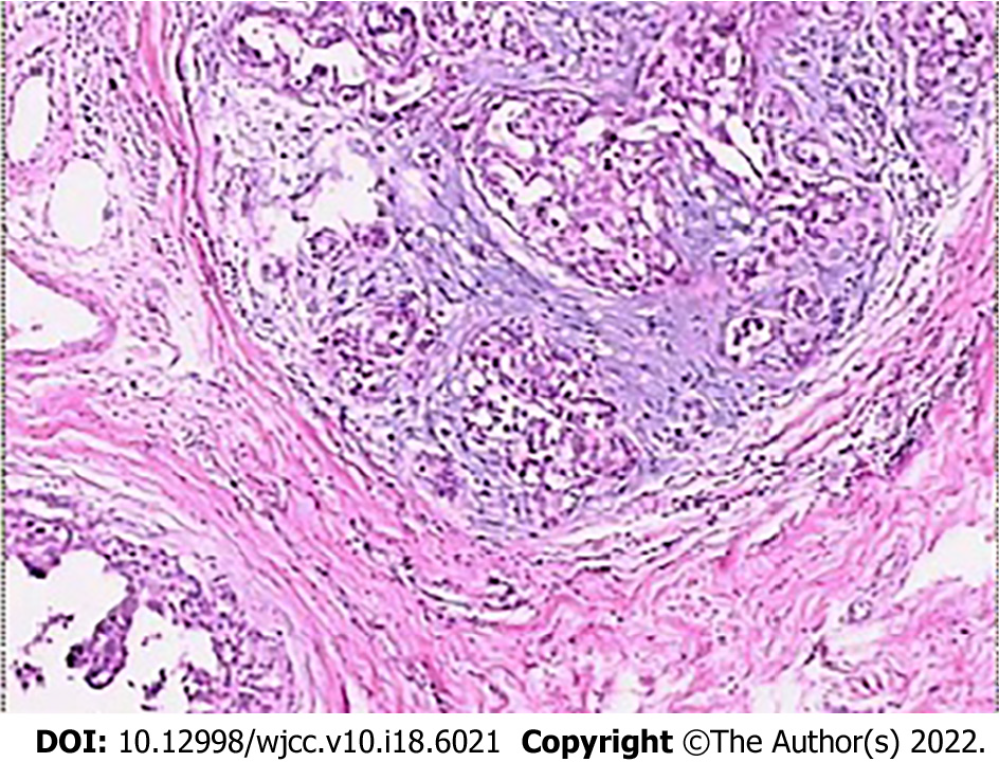
The lesion samples gained from needle biopsy were sent to the Department of Pathology at the Hospital to obtain the pathological results. The lesions were divided into benign and malignant groups according to the classification of histopathological results. The benign group included non-hyperplasic, hyperplasic, and atypical hyperplasic lesions. The malignant group included ductal carcinoma in situ and any type of invasive carcinoma[21]. For samples with mixed pathological characteristics, the more severe lesion prevailed (Figure 3). Surgical resection was performed for all patients whose pre-surgical needle biopsy results demonstrated either malignant lesions or atypical lesions.
All data were analyzed using SPSS Version 18.0 (IBM, Armonk, NY, United States). Continuous data were presented as means ± SD or medians (range), according to the results of the Kolmogorov-Smirnov test for normal distribution, and analyzed using the Student t-test or the Mann-Whitney U-test, as appropriate. Categorical data were presented as numbers (percentages) and analyzed using the chi-square test or Fisher’s exact test. Differences with P < 0.05 were considered statistically significant.
This study included 95 breast lesions in 83 female patients, of which 36 patients were in the benign group, and 47 patients were in the malignant group. All women were Chinese Han. There were no statistically significant differences between the benign and malignant groups in terms of age, family history, history of benign breast disease, history of marriage, history of delivery, long-term use of exogenous estrogen, alcohol abuse, and age of menarche, but there was a statistically significant difference between the groups in terms of the age of menopause (P = 0.021) (Table 1). As for the 95 lesions detected, 46 (48.4%) lesions were in the benign group, and 49 (51.6%) lesions were in the malignant group. The average size of the lesions was 2.2 cm (0.6-5.8 cm); the lesions were larger in the malignant group than in the benign group (median, 2.4 cm vs 1.5 cm, P = 0.007) (Table 2).
| Variables | Benign (n = 36) | Malignant (n = 47) | P value |
| Age (yr) | 44 (28-54) | 57 (27-79) | 0.238 |
| Family history, n (%) | 3 (9.7) | 2 (3.8) | 0.316 |
| History of benign breast diseases, n (%) | 4 (14.8) | 5 (9.6) | 0.673 |
| History of marriage, n (%) | 31 (86.1) | 45 (95.7) | 0.583 |
| History of delivery, n (%) | 29 (80.6) | 42 (89.4) | 0.402 |
| Long term use of exogenous estrogen, n (%) | 0 | 6 (11.8) | 0.242 |
| Alcohol abuse, n (%) | 0 | 1 (1.9) | 0.709 |
| Age of menarche, n (%) | 0.477 | ||
| < 12 years old | 2 (6.5) | 5 (9.6) | |
| ≥ 12 years old | 29 (93.5) | 47 (90.4) | |
| Age of menopause, n (%)1 | 0.021 | ||
| > 55 years old | 3 (9.7) | 7 (13.5) | |
| ≤ 55 years old | 21 (67.7) | 38 (73.1) |
| Parameters | Benign (n = 46) | Malignant (n = 49) | P value | |
| Lesion size (cm) | 1.5 (0.6-2.7) | 2.4 (1.6-5.7) | 0.007 | |
| Perfusion imaging parameters | Ktrans | 0.076 ± 0.001 | 0.681 ± 0.013 | < 0.001 |
| Kep | 0.140 ± 0.004 | 1.892 ± 0.021 | < 0.001 | |
| Ve | 0.577 ± 0.012 | 0.316 ± 0.010 | 0.866 |
Table 2 shows that the Ktrans and Kep values were both larger in the malignant group compared with the benign group (both P < 0.001), but there were no significant statistical differences regarding Ve (P = 0.866).
The ADC values in the parenchyma and lesion area of the normal mammary gland were 1.82 ± 0.22 × 10-3 mm2/s and 1.24 ± 0.16 × 10-3 mm2/s, respectively (P = 0.021). The mean ADC value of the malignant group was 1.09 ± 0.23 × 10-3 mm2/s, which was lower than that of the benign group (1.42 ± 0.68 × 10-3 mm2/s) (P = 0.016). Based on the literature[22], an ADC value of 1.20 × 10-3 mm2/s was used as the threshold for malignant lesions. Lesions with an ADC value lower than the threshold were considered malignant lesions, and those with an ADC value higher than the threshold were considered benign lesions (Table 3).
| Histopathological results | Number of lesions | < 1.20 × 10-3 mm2/s, n (%) | ≥ 1.20 × 10-3 mm2/s, n (%) | |
| Benign | 46 | 1.42 ± 0.24 | 9 (19.6) | 37 (80.4) |
| Fibrocystic hyperplasia | 19 | 1.44 ± 0.32 | 4 (21.1) | 15 (78.9) |
| Fibroadenoma | 7 | 1.49 ± 0.27 | 2 (28.6) | 5 (71.4) |
| Adenopathy | 13 | 1.38 ± 0.26 | 2 (15.4) | 11 (84.6) |
| Catheter dilatation | 2 | 1.69 ± 0.36 | 1 (50.0) | 1 (50.0) |
| Inflammation | 5 | 1.16 ± 0.08 | 3 (60.0) | 2 (40.0) |
| Malignant | 49 | 1.08 ± 0.30 | 35 (71.4) | 14 (28.6) |
| Ductal carcinoma in situ | 16 | 1.24 ± 0.25 | 9 (56.3) | 7 (43.7) |
| Invasive ductal carcinoma | 24 | 0.92 ± 0.23 | 21 (87.5) | 3 (12.5) |
| Invasive lobular carcinoma | 5 | 1.16 ± 0.19 | 3 (60.0) | 2 (40.0) |
| Neuroendocrine carcinoma | 3 | 0.95 ± 0.67 | 2 (66.7) | 1 (33.3) |
| Invasive mucinous carcinoma | 1 | 1.84 ± 0.56 | 0 (0) | 1 (100) |
We evaluated the diagnostic efficiency of PWI and DWI and combined examination techniques compared to the pathological results. The sensitivity and specificity of combined PWI and DWI were higher than those of PWI or DWI alone. The accuracy of combining the two test methods in predicting pathological results was also significantly higher than that predicted by PWI or DWI alone (Table 4).
| Positive | False-positive | Negative | False-negative | Total | Sensitivity (%) | Specificity (%) | Accuracy (%) | |
| PWI | 41 | 8 | 39 | 7 | 95 | 85.4 | 83.0 | 84.2 |
| DWI | 35 | 14 | 37 | 9 | 95 | 79.5 | 72.5 | 75.8 |
| PWI combined with DWI | 44 | 5 | 42 | 4 | 95 | 91.7 | 89.3 | 90.5 |
BI-RADS 4 breast lesions at mammography only appeared a wide range of risk of being malignant (2%-96%)[17-20]. DWI and PWI could help discriminate benign from malignant lesions[6,10,11], but those techniques are mainly considered as accessory to standard imaging modalities[12]. Therefore, this study aimed to evaluate the efficiency of DWI and PWI in diagnosing breast lesions categorized as BI-RADS 4 at mammography. The results suggested that DWI, combined with PWI, might possibly distinguish between benign and malignant BI-RADS 4 breast lesions at mammography.
DWI has the advantages of short acquisition time, unnecessary for a paramagnetic contrast agent, and high sensitivity[23]. Therefore, DWI is widely used in the differential diagnosis of breast diseases, with the scanning parameters and diagnostic specificity being constantly optimized[24]. Among the new MRI techniques, DWI is considered a useful diagnostic method in differentiating benign from malignant lesions and assessing the therapeutic effect[2]. Studies showed that the ADC values of typical malignant tumors are lower than those of benign hyperplasic tissues and normal tissues[25,26]. This finding is partly attributable to the small extracellular space resulting from the high cell density of malignant tumors, which leads to the restricted diffusion of water molecules[25]. This complicated microscopic phenomenon can be partially converted into quantifiable parameters by measuring ADC values, which can thus be applied in distinguishing between different tissue sources[25,26]. In the present study, the mean ADC value of the observed lesion areas was lower than that of the normal breast tissues, and the mean ADC value of the malignant group was the lowest (except for necrotic areas). This finding is consistent with the results reported in previous studies[27,28]. Tsushima et al[29] found that DWI is extremely helpful for diagnosing breast cancer, which its sensitivity and specificity achieved 89% and 77%, respectively.
Many studies have been conducted on the threshold point of the ADC value used to distinguish benign lesions from malignant ones, and their conclusions differ[28,30,31]. By referring to the methods used in the literature[22] and diagnostic tests, the present study used 1.20 × 10-3 mm2/s as the critical ADC value to distinguish benign lesions from malignant ones in the DWI examination of breast lesions. The accuracy obtained by comparison with the pathological results was approximately 73%. Using a relatively high ADC value as the critical point can effectively avoid over-diagnosis of BI-RADS 4 Lesions at mammography. Except for 5.5% of the cases in this study, which were mucinous carcinomas (with an ADC value of 2.20 × 10-3 mm2/s), all lesions with ADC values > 1.74 × 10-3 mm2/s were nonmalignant, as indicated by the final pathological results. This critical point is close to the average of the critical values ranging from 1.60-1.81 × 10-3 mm2/s, as reported by previous studies[22,28,30]. Ren et al[31] showed that the ADC values could be used to evaluate breast lesions’ malignancy. Spick et al[32] also stated that DWI might partially eliminate the need for MRI-guided biopsies. They revealed that when the ADC value of 1.58 × 10-3 mm2/s was used as the threshold, no false-negative occurred.
PWI is an imaging method that can clearly display microvessel density[33] and reflect the neovascularization of tumors, which is a necessary condition for tumor growth, progression, and metastasis[7,8,12,34]. The hemodynamic information that it provides has enabled quantitative analysis. The parameters include Ktrans (which refers to the rate of the contrast agent diffusing from the inside to the outside of the blood vessel), Kep (which refers to the rate of the contrast agent in the extravascular tissue space returning to the blood vessel after diffusing for a period of time), and Ve (which is the volumetric ratio of the extravascular extracellular space to the total voxel). These parameters are able to quantitatively evaluate blood perfusion in the diseased tissue[7,35], thereby enabling quantitative and differential diagnoses of lesions. In the present study, there was a significant increase in the number of blood vessels throughout the breast in sequence of PWI. The reason may be that tumor blood vessels face smaller growth resistance, and the high metabolism level of tumors gradually stimulates the regeneration of blood vessels throughout the breast. In addition, studies have shown that breast cancer with multifocal lesions, large masses, and axillary lymph node metastases have also exhibited pronounced neovascularization throughout the breast, suggesting a poor prognosis[36]. It can thus be inferred that the significant increase in the number of new blood vessels in the breast with cancerous lesions will suggest the progressive growth of malignant lesions and intramammary metastasis.
Conventional MRI scanning techniques combined with DWI and PWI can provide information on the internal structure of the breast[27], reflect the pathological characteristics of the tissue more accurately, and improve the diagnostic accuracy for breast lesions by measuring ADC values and quantitative parameters of PWI[7]. This method achieves the transition from qualitative diagnosis to quantitative diagnosis, thereby associating BI-RADS 4 lesions with corresponding histopathological grades. By using a DWI sequence combined with the quantitative parameters of PWI, a pathological classification of potentially malignant lesions, which were diagnosed as BI-RADS 4 lesions by mammography, was performed in this study to improve the accuracy of imaging diagnosis and provide different clinical recommendations. The present study proved that the sensitivity and specificity of DWI combined with PWI were higher than that of DWI and PWI alone. Nevertheless, additional studies are necessary to further confirm those results. Follow-up observations with imaging methods should be performed for those diagnosed with benign lesions, and needle biopsies should be performed, if necessary, for those diagnosed as malignant lesions.
This study has limitations. Firstly, the purpose of this study was to group BI-RADS 4 lesions at mammography based on their histological features by dividing them into two groups. Including atypical hyperplasic lesions in the benign group may result in bias, and different histological subtypes are included in the same group. As a result, the ADC values of the group may vary greatly. Secondly, two radiologists independently evaluated the slides, but the concordance was not examined. Thirdly, ultrasound data were not available for many patients because the physicians decided not to perform it, the patient refused, or it was done at another hospital, and thus could not be analyzed. Fourthly, the sample size was small, and receiver operating characteristics and multivariable analyses could not be performed. Studies with larger sample size are necessary to determine the real diagnostic value of DWI combined for PWI and determine the adequate cutoff values for the different quantitative parameters.
In conclusion, DWI, combined with PWI, might possibly help distinguish benign breast lesions from malignant ones and provide clear diagnostic results for patients with potentially malignant BI-RADS 4 lesions at mammography.
In recent years, the incidence of breast cancer has increased year by year, topping all types of cancer in Chinese women. Novel functional magnetic resonance imaging (MRI) techniques, including diffusion-weighted imaging (DWI), perfusion-weighted imaging (PWI), and other non-invasive detection methods, have enabled the detection of pathological conditions of tissues to reach on a molecular-level, as well as the detection of functional status and change in mechanisms of organs, tissues, and cells in vivo. According to the MRI breast imaging reporting and data system (BI-RADS) (5th edition), potentially malignant breast lesions are classified as the 4th category (BI-RADS 4) with a wide variation with regard to the risk of malignancy, from 2% to 95%. The results of this study help to decide the exact nature of breast lesions before the patients undergo biopsy.
This study aimed to evaluate the value of DWI and PWI in diagnosing BI-RADS 4 breast lesions and analyze the diagnostic efficacy of DWI and PWI respectively and jointly, which could help to decide the exact nature of breast lesions.
The main objective of this study is to improve the specificity of MRI in detecting breast. MRI has become an essential examination method to diagnose breast lesions due to the avoidance of ionizing radiation, high soft-tissue resolution, multi-parameter imaging, multi-sequence imaging, and high sensitivity. When realizing the objective, the diagnostic efficiency of the apparent diffusion coefficient (ADC) combined with PWI in determining the nature of lesions categorized as BI-RADS 4 will be improved.
This retrospective study included patients who underwent breast MRI between May 2017 and May 2019. The lesions were divided into benign and malignant groups according to the classification of histopathological results. The diagnostic efficacy of DWI and PWI were analyzed respectively and combinely.
The mean ADC value of the malignant group was lower than that of the benign group (P = 0.016). The volume transfer constant (Ktrans) and rate constant (Kep) values were higher in malignant lesions than in benign ones (all P < 0.001). The sensitivity and specificity of PWI combined with DWI (91.7% and 89.3%, respectively) were higher than that of PWI or DWI alone. Studies with larger sample size are necessary to determine the real diagnostic value of DWI combined for PWI and determine the adequate cutoff values for the different quantitative parameters.
The sensitivity and specificity of combined PWI and DWI were higher than those of PWI or DWI alone. DWI, combined with PWI, might possibly help distinguish benign breast lesions from malignant ones and provide clear diagnostic results for patients with potentially malignant BI-RADS 4 lesions at mammography.
To improve the accuracy of combining PWI and DWI in predicting pathological results.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56683] [Article Influence: 7085.4] [Reference Citation Analysis (135)] |
| 2. | Wong IO, Schooling CM, Cowling BJ, Leung GM. Breast cancer incidence and mortality in a transitioning Chinese population: current and future trends. Br J Cancer. 2015;112:167-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Radhakrishna S, Agarwal S, Parikh PM, Kaur K, Panwar S, Sharma S, Dey A, Saxena KK, Chandra M, Sud S. Role of magnetic resonance imaging in breast cancer management. South Asian J Cancer. 2018;7:69-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Mann RM, Cho N, Moy L. Breast MRI: State of the Art. Radiology. 2019;292:520-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 5. | Soares JM, Magalhães R, Moreira PS, Sousa A, Ganz E, Sampaio A, Alves V, Marques P, Sousa N. A Hitchhiker's Guide to Functional Magnetic Resonance Imaging. Front Neurosci. 2016;10:515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | Ei Khouli RH, Jacobs MA, Mezban SD, Huang P, Kamel IR, Macura KJ, Bluemke DA. Diffusion-weighted imaging improves the diagnostic accuracy of conventional 3.0-T breast MR imaging. Radiology. 2010;256:64-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Baltzer P, Mann RM, Iima M, Sigmund EE, Clauser P, Gilbert FJ, Martincich L, Partridge SC, Patterson A, Pinker K, Thibault F, Camps-Herrero J, Le Bihan D; EUSOBI international Breast Diffusion-Weighted Imaging working group. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur Radiol. 2020;30:1436-1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 314] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 8. | Iima M, Honda M, Sigmund EE, Ohno Kishimoto A, Kataoka M, Togashi K. Diffusion MRI of the breast: Current status and future directions. J Magn Reson Imaging. 2020;52:70-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Partridge SC, Amornsiripanitch N. DWI in the Assessment of Breast Lesions. Top Magn Reson Imaging. 2017;26:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Jiang L, Lu X, Hua B, Gao J, Zheng D, Zhou Y. Intravoxel Incoherent Motion Diffusion-Weighted Imaging Versus Dynamic Contrast-Enhanced Magnetic Resonance Imaging: Comparison of the Diagnostic Performance of Perfusion-Related Parameters in Breast. J Comput Assist Tomogr. 2018;42:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Jia ZZ, Shi W, Shi JL, Shen DD, Gu HM, Zhou XJ. Comparison between perfusion computed tomography and dynamic contrast-enhanced magnetic resonance imaging in assessing glioblastoma microvasculature. Eur J Radiol. 2017;87:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Zervoudis S, Iatrakis G, Tomara E, Bothou A, Papadopoulos G, Tsakiris G. Main controversies in breast cancer. World J Clin Oncol. 2014;5:359-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Avendano D, Marino MA, Leithner D, Thakur S, Bernard-Davila B, Martinez DF, Helbich TH, Morris EA, Jochelson MS, Baltzer PAT, Clauser P, Kapetas P, Pinker K. Limited role of DWI with apparent diffusion coefficient mapping in breast lesions presenting as non-mass enhancement on dynamic contrast-enhanced MRI. Breast Cancer Res. 2019;21:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Liu Z, Li Z, Qu J, Zhang R, Zhou X, Li L, Sun K, Tang Z, Jiang H, Li H, Xiong Q, Ding Y, Zhao X, Wang K, Liu Z, Tian J. Radiomics of Multiparametric MRI for Pretreatment Prediction of Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A Multicenter Study. Clin Cancer Res. 2019;25:3538-3547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 339] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 15. | Maric J, Boban J, Ivkovic-Kapicl T, Djilas D, Vucaj-Cirilovic V, Bogdanovic-Stojanovic D. Differentiation of Breast Lesions and Distinguishing Their Histological Subtypes Using Diffusion-Weighted Imaging and ADC Values. Front Oncol. 2020;10:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | American College of Radiology. ACR BI-RADS®, Breast Imaging Reporting and Data System. Reston: American College of Radiology (2013).. |
| 17. | Elverici E, Barça AN, Aktaş H, Özsoy A, Zengin B, Çavuşoğlu M, Araz L. Nonpalpable BI-RADS 4 breast lesions: sonographic findings and pathology correlation. Diagn Interv Radiol. 2015;21:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Liu G, Zhang MK, He Y, Liu Y, Li XR, Wang ZL. BI-RADS 4 breast lesions: could multi-mode ultrasound be helpful for their diagnosis? Gland Surg. 2019;8:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Bennani-Baiti B, Dietzel M, Baltzer PA. MRI for the assessment of malignancy in BI-RADS 4 mammographic microcalcifications. PLoS One. 2017;12:e0188679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Strobel K, Schrading S, Hansen NL, Barabasch A, Kuhl CK. Assessment of BI-RADS category 4 Lesions detected with screening mammography and screening US: utility of MR imaging. Radiology. 2015;274:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, Suman VJ, Johnson J, Blake C, Tlsty T, Vachon CM, Melton LJ 3rd, Visscher DW. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 621] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 22. | Maltez de Almeida JR, Gomes AB, Barros TP, Fahel PE, de Seixas Rocha M. Subcategorization of Suspicious Breast Lesions (BI-RADS Category 4) According to MRI Criteria: Role of Dynamic Contrast-Enhanced and Diffusion-Weighted Imaging. AJR Am J Roentgenol. 2015;205:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Baliyan V, Das CJ, Sharma R, Gupta AK. Diffusion weighted imaging: Technique and applications. World J Radiol. 2016;8:785-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 166] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (8)] |
| 24. | Thomassin-Naggara I, De Bazelaire C, Chopier J, Bazot M, Marsault C, Trop I. Diffusion-weighted MR imaging of the breast: advantages and pitfalls. Eur J Radiol. 2013;82:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Pereira FP, Martins G, Carvalhaes de Oliveira Rde V. Diffusion magnetic resonance imaging of the breast. Magn Reson Imaging Clin N Am. 2011;19:95-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Partridge SC, Nissan N, Rahbar H, Kitsch AE, Sigmund EE. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J Magn Reson Imaging. 2017;45:337-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 27. | Pereira FP, Martins G, Figueiredo E, Domingues MN, Domingues RC, da Fonseca LM, Gasparetto EL. Assessment of breast lesions with diffusion-weighted MRI: comparing the use of different b values. AJR Am J Roentgenol. 2009;193:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Brandão AC, Lehman CD, Partridge SC. Breast magnetic resonance imaging: diffusion-weighted imaging. Magn Reson Imaging Clin N Am. 2013;21:321-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Tsushima Y, Takahashi-Taketomi A, Endo K. Magnetic resonance (MR) differential diagnosis of breast tumors using apparent diffusion coefficient (ADC) on 1.5-T. J Magn Reson Imaging. 2009;30:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Parsian S, Rahbar H, Allison KH, Demartini WB, Olson ML, Lehman CD, Partridge SC. Nonmalignant breast lesions: ADCs of benign and high-risk subtypes assessed as false-positive at dynamic enhanced MR imaging. Radiology. 2012;265:696-706. [RCA] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Ren C, Zou Y, Zhang X, Li K. Diagnostic value of diffusion-weighted imaging-derived apparent diffusion coefficient and its association with histological prognostic factors in breast cancer. Oncol Lett. 2019;18:3295-3303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Spick C, Pinker-Domenig K, Rudas M, Helbich TH, Baltzer PA. MRI-only lesions: application of diffusion-weighted imaging obviates unnecessary MR-guided breast biopsies. Eur Radiol. 2014;24:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Shi R, Jiang T, Si L, Li M. Correlations of magnetic resonance, perfusion-weighed imaging parameters and microvessel density in meningioma. J BUON. 2016;21:709-713. [PubMed] |
| 34. | Katayama Y, Uchino J, Chihara Y, Tamiya N, Kaneko Y, Yamada T, Takayama K. Tumor Neovascularization and Developments in Therapeutics. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 35. | Ryu JK, Rhee SJ, Song JY, Cho SH, Jahng GH. Characteristics of quantitative perfusion parameters on dynamic contrast-enhanced MRI in mammographically occult breast cancer. J Appl Clin Med Phys. 2016;17:377-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Han M, Kim TH, Kang DK, Kim KS, Yim H. Prognostic role of MRI enhancement features in patients with breast cancer: value of adjacent vessel sign and increased ipsilateral whole-breast vascularity. AJR Am J Roentgenol. 2012;199:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karavaş E, Turkey; Yu RQ, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR