Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.5064
Peer-review started: December 6, 2021
First decision: January 25, 2022
Revised: February 23, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: May 26, 2022
Processing time: 168 Days and 22.3 Hours
Metaplastic breast carcinoma (MBC) is a rare subtype of invasive breast cancer comprising malignant epithelial and mesenchymal cells. Compared with other invasive breast cancers, MBC is not only histologically distinctly heterogeneous but also has a rapid and aggressive growth pattern, which leads to a significant risk of recurrence and mortality.
In this study, we report the case of a patient with a large left breast mass diagnosed with bilateral invasive ductal carcinoma in both breasts after a preoperative core needle aspiration biopsy of the bilateral breast mass. The patient received neoadjuvant chemotherapy and underwent bilateral breast modified radical mastectomy. Postoperative pathology suggested carcinosarcoma with predominantly chondrosarcoma in the left breast and invasive ductal carcinoma (luminal B) in the right breast. As the patient did not achieve complete pathological remission after six cycles of neoadjuvant chemotherapy, we administered six months of intensive capecitabine treatment. Then the patient was switched to continuous treatment with endocrine therapy using letrozole + goserelin, and the patient is currently in stable condition. However, as MBC of the breast is concurrently diagnosed with chondrosarcoma differentiation, our case is sporadic.
Given the variety of immunohistochemical types of bilateral breast cancer, achieving effective chemotherapy should be a key research focus.
Core Tip: Metaplastic breast carcinoma (MBC) is a rare subtype of invasive breast cancer comprising malignant epithelial and mesenchymal cells, with low incidence. Our patient with a large left breast mass was diagnosed with carcinosarcoma with predominantly chondrosarcoma in the left breast, and postoperative pathology also suggested invasive ductal carcinoma in the right breast. The current knowledge of MBC is limited, particularly in patients with concurrent bilateral breast cancer. Clinicians should fully consider the situation of bilateral mammary glands and formulate a comprehensive treatment plan for such patients.
- Citation: Yang SY, Li Y, Nie JY, Yang ST, Yang XJ, Wang MH, Zhang J. Metaplastic breast cancer with chondrosarcomatous differentiation combined with concurrent bilateral breast cancer: A case report. World J Clin Cases 2022; 10(15): 5064-5071
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/5064.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.5064
The incidence of metaplastic breast carcinoma (MBC) is low, accounting for approximately 1%–2% of all diagnosed breast malignancies[1]. MBC consists of a mixture of ductal carcinoma with areas of clostridial, squamous, cartilaginous, or osteogenic components and both carcinomatous and mesenchymal components. Because of its complex composition, MBC was previously referred to as a “mixed malignant tumor of the breast”[2]. The pathology of MBC is characterized by cancerous tissue consisting of two or more cell subtypes of epithelial and mesenchymal origin[3].
Breast carcinosarcoma, also known as mesenchymal differentiated mammary carcinoma, is a subgroup of carcinosarcomas with a clinical incidence less than 0.2%. It is a rare primary malignant tumor of the breast, although chondrosarcoma is the most rare, with an incidence lower than that of carcinosarcoma[4]. Generally, MBC with concurrent bilateral breast cancer and chondrosarcoma is more aggressive and has a worse prognosis than common breast cancer. There remains a lack of systematic reports regarding the clinical management of MBC, especially with a predominantly chondrosarcomatous component.
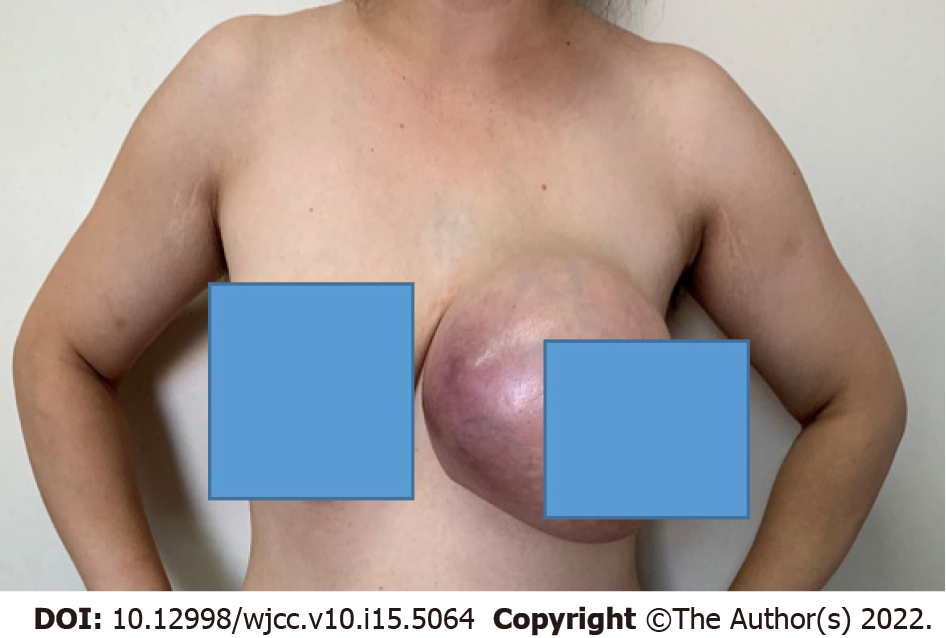
A 35-year-old Chinese woman was admitted to a tertiary care hospital in December 2020 with a painless mass in her left breast for 6 mo (Figure 1).
The patient found a left breast mass 6 mo prior, which slowly grew in size. However, the patient did not have any mass-related clinical symptoms such as fever and pain.
There was no history of past illness.
There was no personal and family history.
The size of the left breast was twice that of the right one. In addition, the skin of the left breast was redder in color and a higher temperature than the left one, especially the outer quadrants. According to physical examination, the left breast palpable mass in the inner quadrants was non-tender, had a clear boundary and poor activity, and was approximately 20 cm × 18 cm in size. No palbable lesions were found in the right breast.
No abnormalities were found in the patient’s laboratory examinations.
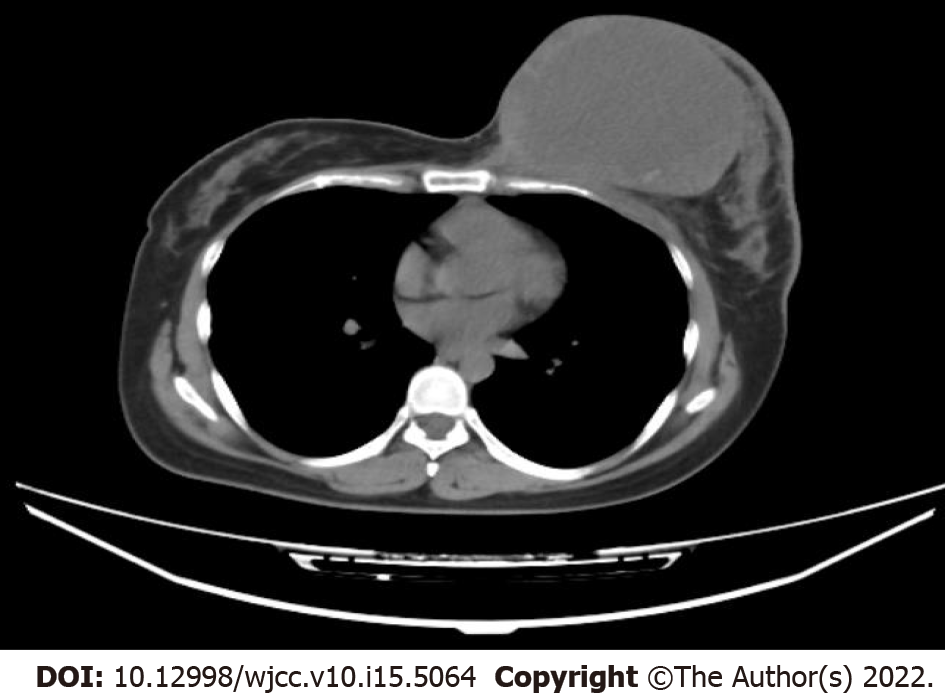
Magnetic resonance imaging (MRI) suggested morphological derangement of the left breast, with multiple occupying lesions within it (Figure 2). The mass was categorized as Breast Imaging Reporting and Data System (BI-RADS) category 5 and the left axillary lymph node metastasis was considered. In addition, an irregular mass in the outer quadrant of the right breast was found using BI-RADS category 5. Therefore, both masses were highly suspicious of malignancy.
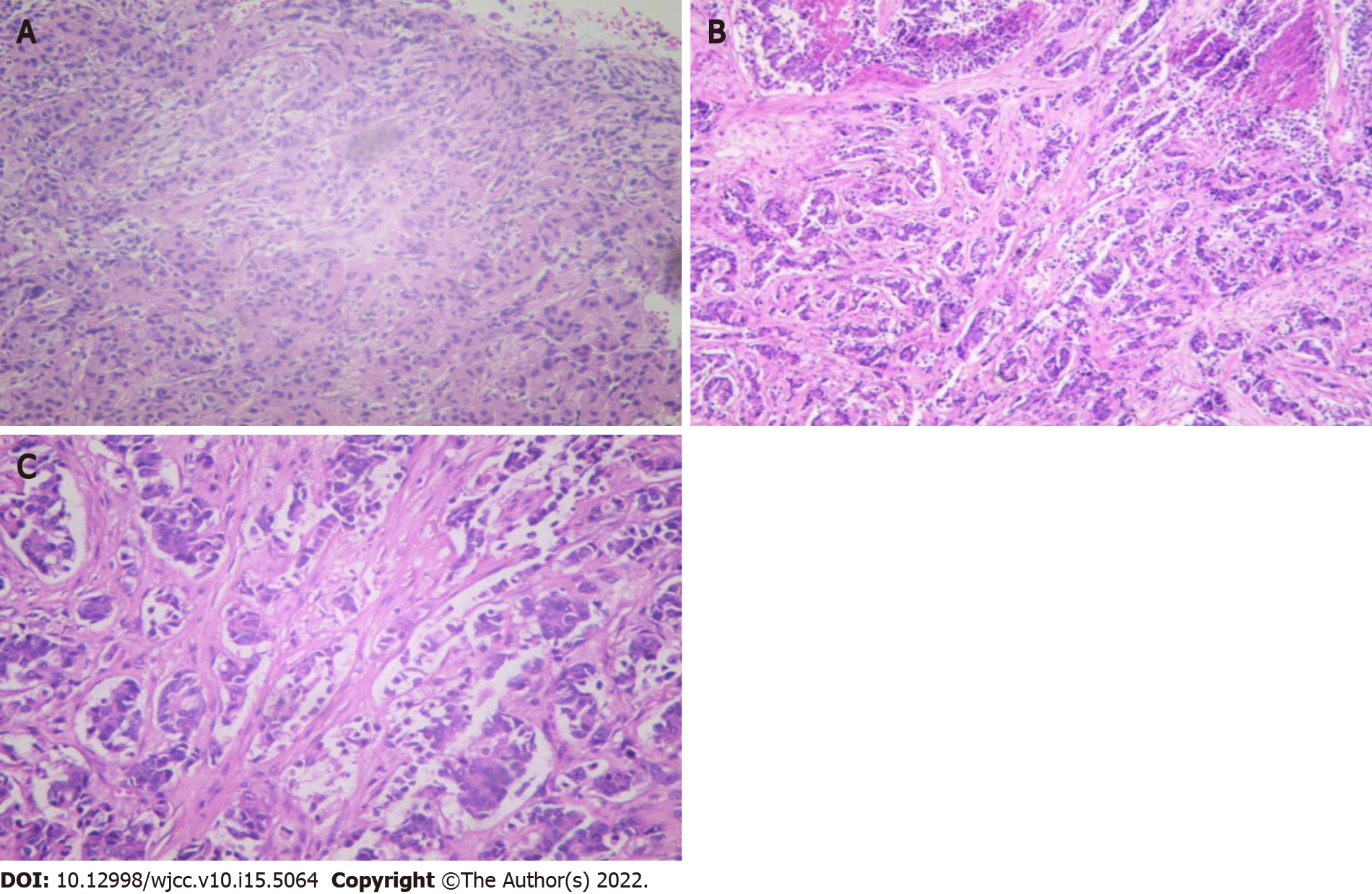
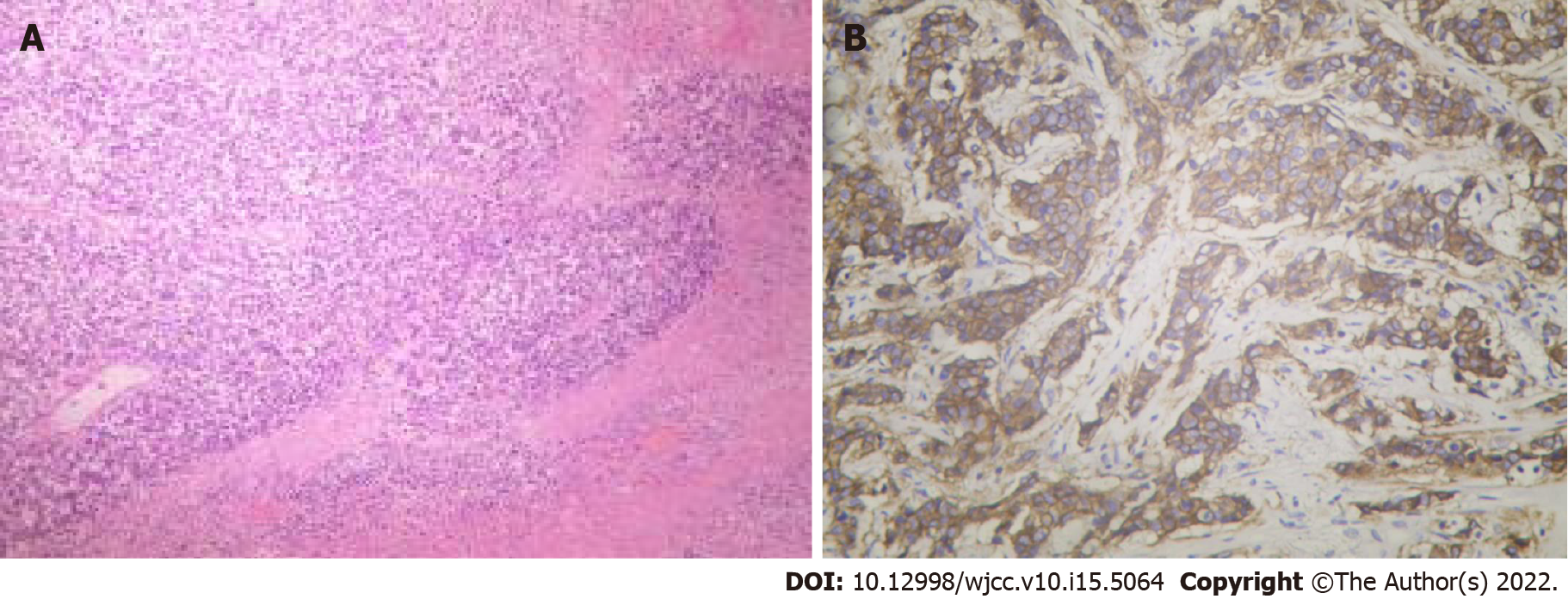
The patient underwent a core needle biopsy (CNB) of both breast masses. Approximately 100 mL fluid was aspirated from the left breast mass, and malignant tumor cells could be seen on cytology (Figure 3A). Postoperative pathology confirmed invasive ductal carcinoma in the left breast (Figure 3B), with immunohistochemical findings of estrogen receptor (ER) (–), progesterone receptor (PR) (+, 5%), Ki-67 (Ki-67) (+, 40%), and human epidermal growth factor receptor 2 (HER-2) (2+). In addition, breast invasive ductal carcinoma was confirmed on the right side, with immunohistochemical results of ER (+, 90%), PR (+, 90%), Ki-67 (+, 30%), and HER-2 (2+) (Figure 3C). Furthermore, a right axillary lymph node aspiration biopsy was performed, and postoperative pathology confirmed involvement of the lateral axillary lymph node.
Based on CNB pathology and the patient’s medical history, the diagnosis before surgery was bilateral breast invasive ductal carcinoma. However, based on the postoperative pathology, the final diagnosis was bilateral breast invasive ductal carcinoma with left breast MBC (carcinosarcoma with predominantly chondrosarcoma).
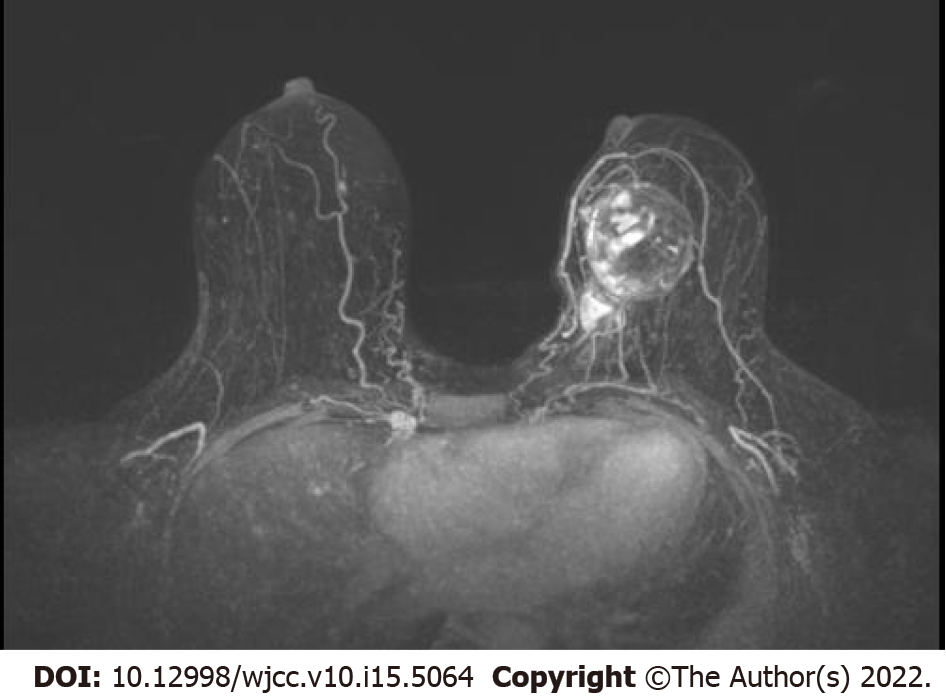
The patients underwent six cycles of neoadjuvant chemotherapy, including albumin paclitaxel and epoetin. After neoadjuvant chemotherapy, the patient’s bilateral breast cancer outcome was assessed as PR, and MRI showed significant regression of the left breast mass (Figure 4). In May 2021, the patient underwent modified radical surgery for bilateral breast cancer. Postoperative pathology confirmed residual left invasive ductal carcinoma with a chondrosarcoma component, with left axillary lymph node involvement (0/18) and immunohistochemical findings of ER (–), PR (+, 5%), Ki-67 (+, 70%) for invasive ductal carcinoma, and vimentin (VIM) (+), cytokeratin 5/6 (CK5/6) (–), CK7 (–), CK8 (–), and synuclein (–) for the chondrosarcoma component (Figure 5), which was diagnosed as MBC (carcinosarcoma with predominantly chondrosarcoma ) in the left breast. Postoperative pathology of the right breast detected only a small amount of residual ductal carcinoma in situ and achieved complete pathological remission.
Postoperatively, the patient received local radiotherapy and continued oral capecitabine during radiotherapy. In August 2021, the patient finished radiotherapy and continued capecitabine. The patient took capecitabine for 6 mo, after which she was treated with letrozole + goserelin for endocrine therapy. The patient is currently in overall stable condition with no recurrence or metastasis.
The age of MBC occurrence (> 50 years old) is similar to that of invasive ductal carcinoma[5,6]. However, the biological behavior of MBC can vary significantly due to the type and proportion of the mesenchymal component, with some tumors showing rapid growth and a mixture of cystic and solid components, which are often prominent at the time of initial presentation. In the present case, the rapid growth of the left breast mass in 6 mo indicated a clue of this characteristic.
MBC may appear as relatively regular mass contours[7], whereas the tumor seen on ultrasound may be irregularly shaped (hypoechoic lobulated), with a cystic component visible within it, with posterior echogenic enhancement and calcification. Typically, ultrasound suggestive of complex lesions with cystic tissue portions and posterior echogenic enhancement represent necrosis or hemorrhage[8,9]. This was similar to our patient. Because of the lack of specificity in imaging, mammography, ultrasonography, and MRI are not satisfactory for diagnosing MBC[10].
In practice, preoperative diagnosis by cytology (i.e., CNB) can effectively identify malignant cells. However, because breast cancer can exhibit various metaplastic changes, including those relating to spindle cells, squamous cells, and heterogeneous mesenchymal components, as well as an insufficient amount of biopsy tissue, the heterogeneous component may not be identified by CNB[11]. In addition, although it is possible to achieve a definitive diagnosis in cases where the cancerous or sarcomatous component is predominant, most cases are still overlooked[12] because of the low interstitial component. In the current case, because the left breast mass was large and the right breast mass was highly suspicious of malignancy, we preferred to prioritize neoadjuvant treatment once the breast cancer diagnosis was confirmed. Therefore, the patient was biopsied using CNB to confirm the malignancy. Our experience makes it possible to obtain more pathological tissue through multiple punctures at different sites for inoperable tumors using CNB. However, multiple punctures can significantly increase the cost and patient pain. Furthermore, it may even lead to life-threatening severe events such as major bleeding, so we did not perform multiple punctures in this case.
Frozen section diagnosis of breast masses is highly accurate during the operation, with sensitivities and specificities exceeding 90% and 99%, respectively[13]. However, frozen section diagnosis cannot supply the information of immunohistochemistry results. Immunohistochemistry is currently the most common method for diagnosis and differential diagnosis. Most MBC is triple-negative, but a minority of patients may be ER-, PR-, and/or HER2-positive. Studies have shown that patients with sarcomatous breast cancer have an ER- and/or PR-positive expression rate of 12% and a HER2-positive expression rate of 15%, whereas only approximately 2% of patients have positive expression of ER, PR, and HER2[14]. Additionally, because carcinosarcomas contain malignant epithelial components and malignant mesenchymal components, the epithelial components stain positive for CK, whereas the mesenchymal components stain negative for CK but positive for VIM. Moreover, carcinomas that produce stroma contain cancerous tissue that is transformed into cartilaginous or bone-like components[15,16]. Xu and Hou[17] reported that CK and VIM in chondrocytic sarcoma tissues have positive expression rates of 19.74% and 82.89%, respectively. However, in most cases, the metaplastic component tends to be insignificant, or the foci are confined or difficult to observe by conventional hematoxylin and eosin staining or histopathological sections. In our patient, the metaplastic component was not detected at the time of CNB because the patient had a huge mass that showed mixed cystic-solid changes. At this stage, there is increasing evidence that the diversity of breast cancer phenotypes is associated with gene expression diversity[18]. The majority of pathological immunohistochemical typing of MBC is consistent with the basal-like type[19]. The development of new gene sequencing technologies will provide us with more clinical diagnostic tools in the future. Because of the low incidence and inconsistent classification of metaplastic breast cancer, and the even greater rarity of metaplastic chondrosarcoma-like metastases, expertise and evidence-based information on optimal treatment is limited. The vast majority of clinical evidence comes from case reports and series with small clinical sample sizes, which do not provide a definitive basis for treatment. In most case series, the most common surgical approach is mastectomy, with the need for combined axillary lymph node dissection determined on the basis of axillary lymph node status. This is mainly due to the aggressive growth pattern of MBC, which results in large tumors (most > 5 cm) and lymph node metastasis[20]. However, although the clinical presentation in the current patient was highly suspicious of saprophytic carcinoma or even breast sarcoma, since only invasive ductal carcinoma was found in the pathological findings before surgery, our treatment strategy was more oriented toward targeting breast cancer, i.e., neoadjuvant chemotherapy was used first to achieve tumor reduction and stage reduction for larger masses and more advanced stages.
Tseng and Martinez[21] demonstrated by multivariate analysis that adjuvant radiotherapy improved overall survival in patients with MBC, with a 36% reduction in overall mortality and a 26% reduction in breast cancer-related mortality in patients who received adjuvant radiotherapy after surgery, regardless of whether the patient underwent mastectomy alone or breast lumpectomy alone. Our patient also received chest wall radiotherapy after surgery. Based on the prognosis of the present case, we conclude that patients with MBC can benefit from postoperative adjuvant radiotherapy, which has become an important component of the comprehensive treatment of MBC and is recommended for clinical use[21-23].
The effectiveness of neoadjuvant chemotherapy is low and the rate of pathological remission is suboptimal[24,25] considering the late stage of the disease in most patients presenting with MBC[26]. In the present case, although the metaplastic carcinoma component was not detected before neoadjuvant therapy, a 3-wk chemotherapy regimen of albumin paclitaxel combined with epirubicin was chosen for neoadjuvant chemotherapy, considering such a possibility. Encouragingly, the bilateral breast masses continued to decrease throughout the treatment course and eventually achieved partial clinical remission before surgery.
Endocrine therapy is otherwise known as hormone therapy. Most MBCs are hormone receptor negative, so endocrine therapy is not fully effective and lacks clinical application. The lack of effective targeted therapy options due to the patient’s triple-negative invasive ductal carcinoma component of the left breast, coupled with the aggressive biological behavior, is associated with a high risk of early recurrence, especially organ metastasis. As an oral antimetabolite chemotherapeutic agent, capecitabine can convert to fluorouracil in vivo to exert antitumor effects. Indeed, several clinical studies have demonstrated that adding capecitabine to standard chemotherapy regimens can help patients with triple-negative breast cancer achieve better long-term outcomes, demonstrating the success of a capecitabine regimen for the postoperative clearance of residual lesions[27,28]. Thus, at the end of radiation therapy, our patients continue to receive capecitabine therapy for up to 6 mo. Due to the patient’s bilateral concurrent breast malignancy and based on the pathological findings in the right breast, we plan to use goserelin and letrozole endocrine therapy following completion of capecitabine therapy.
The incidence of MBC is extremely low, and most previous studies have involved small samples, in which the treatment options and long-term prognosis remain unclear. MBC is mainly treated with a combination of surgery, chemotherapy, and radiotherapy. Early diagnosis and selection of the most appropriate treatment is critical for MBC, which requires the comprehensive participation of multiple disciplines. The current knowledge of MBC is still limited, particularly in cases with concurrent bilateral breast cancer. There is a lack of systematic studies and more detailed analysis in multicenter or registry studies is necessary. For such cases, it is important to consider them in a comprehensive manner in the context of the actual situation.
| 1. | Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. 2007;14:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Johnson MC. Anatomy and physiology of the breast. In: Management of breast diseases. edn. Edited by Ismail J, Manfred K. Heidelberg, Berlin, Germany: Springer Berlin Heidelberg; 2010: 1–36. |
| 3. | Llombart-Bosch A, Peydro A. Malignant mixed osteogenic tumours of the breast. An ultrastructural study of two cases. Virchows Arch A Pathol Anat Histol. 1975;366:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Esses KM, Hagmaier RM, Blanchard SA, Lazarchick JJ, Riker AI. Carcinosarcoma of the breast: two case reports and review of the literature. Cases J. 2009;2:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Bhosale SJ, Kshirsagar AY, Sulhyan SR, Jagtap SV, Nikam YP. Metaplastic carcinoma with predominant chondrosarcoma of the right breast. Case Rep Oncol. 2010;3:277-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Chao TC, Wang CS, Chen SC, Chen MF. Metaplastic carcinomas of the breast. J Surg Oncol. 1999;71:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Kato T, Tohnosu N, Suwa T, Takahashi H, Tokuizumi M, Uehara T, Kobayashi TK. Metaplastic breast carcinoma with chondrosarcomatous differentiation: fine-needle aspiration cytology findings. A case report. Diagn Cytopathol. 2006;34:772-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Schwartz TL, Mogal H, Papageorgiou C, Veerapong J, Hsueh EC. Metaplastic breast cancer: histologic characteristics, prognostic factors and systemic treatment strategies. Exp Hematol Oncol. 2013;2:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Lai YC, Hsu CY, Chou YH, Tiu CM, Tseng LM, Wang HK, Chiou HJ. Sonographic presentations of metaplastic breast cancers. J Chin Med Assoc. 2012;75:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Tokudome N, Sakamoto G, Sakai T, Sarumaru S, Okuyama N, Hori F, Horii R, Akiyama F, Tanabe M, Saito K, Takahashi K, Kasumi F. A case of carcinosarcoma of the breast. Breast Cancer. 2005;12:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Sternberg SS, Mills SE, Carter D: Sternberg's diagnostic surgical pathology, 4th edn. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2004. |
| 12. | Stanley MW, Tani EM, Skoog L. Metaplastic carcinoma of the breast: fine-needle aspiration cytology of seven cases. Diagn Cytopathol. 1989;5:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Sultana N, Kayani N. Validity of frozen section in the diagnosis of breast lumps: 5 years experience at the Aga Khan University Hospital. J Pak Med Assoc. 2005;55:533-536. [PubMed] |
| 14. | Lee H, Jung SY, Ro JY, Kwon Y, Sohn JH, Park IH, Lee KS, Lee S, Kim SW, Kang HS, Ko KL, Ro J. Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol. 2012;65:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Abd El Hafez A, Shawky Ael-A. Analysis of metaplastic breast carcinoma: FNAC; histopathology and immunohistochemistry are complementary for diagnosis. Breast Dis. 2013;34:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Jia Y, He C, Liu L, Sun L, Chen Y, Luo Y, Yu T. A Retrospective Study of the Imaging and Pathological Features of Metaplastic Breast Carcinoma and Review of the Literature. Med Sci Monit. 2019;25:248-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Xu D, Hou L. Clinicopathologic characteristics of mixed epithelial/mesenchymal metaplastic breast carcinoma (carcinosarcoma): a meta-analysis of Chinese patients. Pol J Pathol. 2019;70:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Altaf FJ, Mokhtar GA, Emam E, Bokhary RY, Mahfouz NB, Al Amoudi S, Al-Gaithy ZK. Metaplastic carcinoma of the breast: an immunohistochemical study. Diagn Pathol. 2014;9:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Wang H, Guan B, Shi Q, Ma H, Zhou H, Wang X, Zhou X. May metaplastic breast carcinomas be actually basal-like carcinoma? Med Oncol. 2011;28:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Shah DR, Tseng WH, Martinez SR. Treatment options for metaplastic breast cancer. ISRN Oncol. 2012;2012:706162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Tseng WH, Martinez SR. Metaplastic breast cancer: to radiate or not to radiate? Ann Surg Oncol. 2011;18:94-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Rakha EA, Tan PH, Varga Z, Tse GM, Shaaban AM, Climent F, van Deurzen CH, Purnell D, Dodwell D, Chan T, Ellis IO. Prognostic factors in metaplastic carcinoma of the breast: a multi-institutional study. Br J Cancer. 2015;112:283-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Cooper R, Rajak R, Valentine K, Bhargava V. Metaplastic carcinoma of the breast. Diagn Histop. 2018;24:83-85. [DOI] [Full Text] |
| 24. | Takuwa H, Ueno T, Ishiguro H, Mikami Y, Kanao S, Takada M, Sugie T, Toi M. A case of metaplastic breast cancer that showed a good response to platinum-based preoperative chemotherapy. Breast Cancer. 2014;21:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Esbah O, Turkoz FP, Turker I, Durnali A, Ekinci AS, Bal O, Sonmez OU, Budakoglu B, Arslan UY, Oksuzoglu B. Metaplastic breast carcinoma: case series and review of the literature. Asian Pac J Cancer Prev. 2012;13:4645-4649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Park HS, Park S, Kim JH, Lee JH, Choi SY, Park BW, Lee KS. Clinicopathologic features and outcomes of metaplastic breast carcinoma: comparison with invasive ductal carcinoma of the breast. Yonsei Med J. 2010;51:864-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Wu SY, Wang H, Shao ZM, Jiang YZ. Triple-negative breast cancer: new treatment strategies in the era of precision medicine. Sci China Life Sci. 2021;64:372-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Huo X, Li J, Zhao F, Ren D, Ahmad R, Yuan X, Du F, Zhao J. The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gursel B, Turkey; Rossi T, Italy S-Editor: Fan JR L-Editor: A P-Editor: Fan JR