Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.4985
Peer-review started: October 30, 2021
First decision: February 14, 2022
Revised: March 4, 2022
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 26, 2022
Processing time: 206 Days and 9.8 Hours
The 2020 European Association of Urology prostate cancer guidelines recommend androgen deprivation therapy (ADT) in combination with apalutamide and enzalutamide, a new generation of androgen receptor antagonists, as first-line therapy. A decrease in prostate-specific antigen (PSA) levels may occur in the early stages of novel hormonal therapy; however, radionuclide bone imaging may suggest disease progression. During follow-up, PSA, radionuclide bone imaging, and prostate-specific membrane antigen (PSMA) positron emission tomography – computed tomography (PET-CT) are needed for systematic evaluation.
We admitted a 56-year-old male patient with metastatic hormone-sensitive prostate cancer. Initial radionuclide bone imaging, magnetic resonance imaging (MRI), and PSMA PET-CT showed prostate cancer with multiple bone metastases. Ultrasound-guided needle biopsy of the prostate revealed a poorly differentiated adenocarcinoma of the prostate with a Gleason score: 5+4 = 9. The final diagnosis was a prostate adenocarcinoma (T4N1M1). ADT with novel hormonal therapy (goseraline sustained-release implant 3.6 mg monthly and apalutamide 240 mg daily) was commenced. Three months later, radionuclide bone imaging and MRI revealed advanced bone metastasis. However, PSMA PET-CT examination showed a significant reduction in PSMA aggregation on the bone, indicating improved bone metastases. Considering that progressive decrease in the presenting lumbar pain, treatment strategies were considered to be effective.
ADT using novel hormonal therapy is effective for treating patients with prostate adenocarcinoma. Careful evaluation must precede treatment plan changes.
Core Tip: In 2018, prostate cancer was ranked as the fifth leading cause of cancer-related deaths in men, worldwide. Some of such cases are metastatic hormone-sensitive prostate cancers (mHSPC) and Apalutamide has been shown to improve survival in such patients. However, a “bone-flare” phenomenon may occur during management with apalutamide. We describe a case of mHSPC with this phenomenon after apalutamide and androgen deprivation therapy, and thus demonstrate the importance of multiple bone imaging modalities, radionuclide bone imaging, magnetic resonance imaging, prostate-specific antigen, prostate-specific membrane antigen positron emission tomography – computed tomography, in determining the treatment course in such patients.
- Citation: Li KH, Du YC, Yang DY, Yu XY, Zhang XP, Li YX, Qiao L. Bone flare after initiation of novel hormonal therapy in patients with metastatic hormone-sensitive prostate cancer: A case report. World J Clin Cases 2022; 10(15): 4985-4990
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/4985.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.4985
Prostate cancer (PCa) is ranked as the second most common cancer and the fifth leading cause of cancer-related deaths in men worldwide in 2018[1]. Unfortunately, some patients develop metastatic castration-resistant prostate cancer (mCRPC), including metastatic hormone-sensitive prostate cancer (mHSPC). In recent years, studies have confirmed that apalutamide sustains a lower level of prostate-specific antigen (PSA) and improves the survival of patients with mHSPC. Several international guidelines recommend apalutamide as the standard treatment for mHSPC. During follow-up, PSA and radionuclide bone imaging are needed for systematic evaluation. Following the application of novel hormonal therapy, PSA was noted to decrease significantly in some patients after treatment. However, radionuclide bone imaging showed progression of bone metastases after 3 mo and with a gradual decrease noted during subsequent follow-up. Ryan et al[2] calls this phenomenon “bone flare”. In this study, we retrospectively analyzed the "bone flare" phenomenon during apalutamide treatment in a patient with mHSPC. General information, clinical symptoms, laboratory and imaging examination, treatment, and follow-up data were collected and analyzed. We also share our experience regarding treatment of a patient who had the "bone flare" phenomenon during apalutamide treatment, and provide a reference for its clinical application.
The patient suffer lumbar pain for 1 mo.
On December 16, 2020, a 56-year-old man visited the outpatient clinic of the Department of Urology, Weifang People's Hospital, with a complaint of lumbar pain for 1 mo.
The patient had no relevant history of past illnesses.
The patient has no relevant personal and family history.
Digital rectal examination revealed a significantly enlarged, hard prostate with palpable non-tender nodules on the left side.
Initial hormonal assay results were as follows: PSA > 100 ng/mL, ALP: 730 U/L. Ultrasound-guided needle biopsy of the prostate. Postoperative pathological results were as follows: poorly differentiated adenocarcinoma of the prostate, Gleason score: 5+4 = 9.
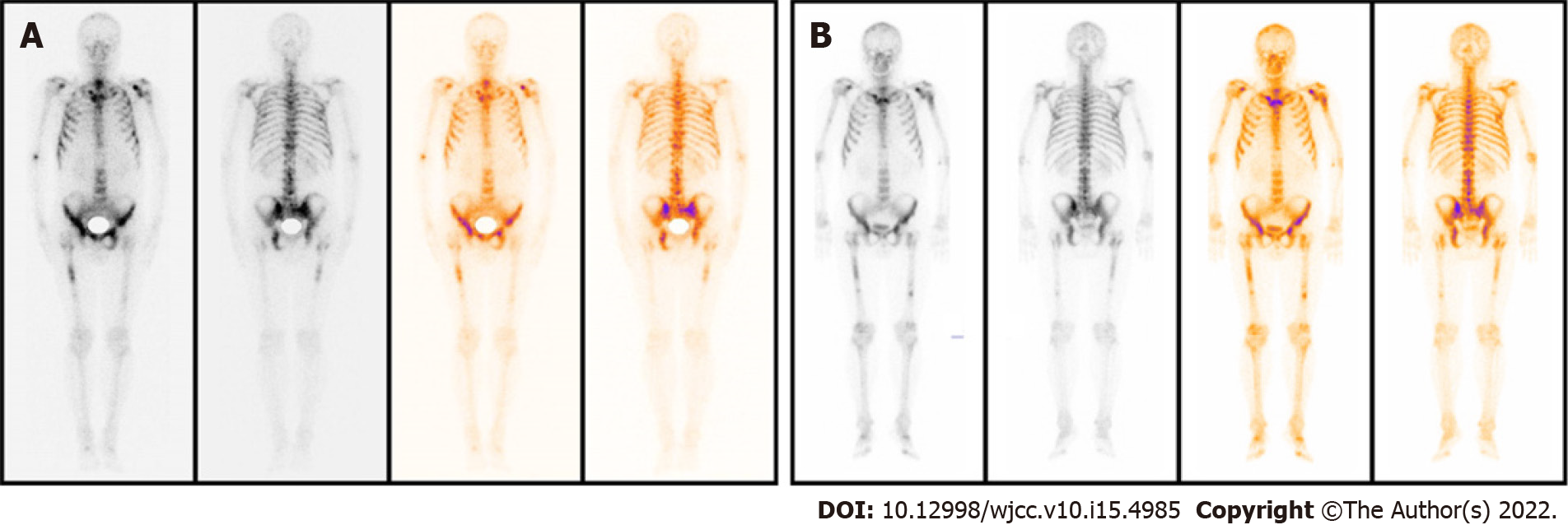
Magnetic resonance imaging (MRI) revealed a malignant mass in the peripheral zone of the prostate with multiple bone metastases. Radionuclide bone imaging revealed multiple bone metastases. Prostate-specific membrane antigen (PSMA) positron emission tomography – computed tomography (PET-CT) examination revealed PSMA aggregation in multiple bone sites.
Based on the results obtained from imaging and histopathology, the final diagnosis was prostate adenocarcinoma (T4N1M1).
Androgen deprivation therapy (ADT) using novel hormonal therapy (goseraline sustained-release implant 3.6 mg monthly and apalutamide 240 mg/d was initiated on December 24, 2020.
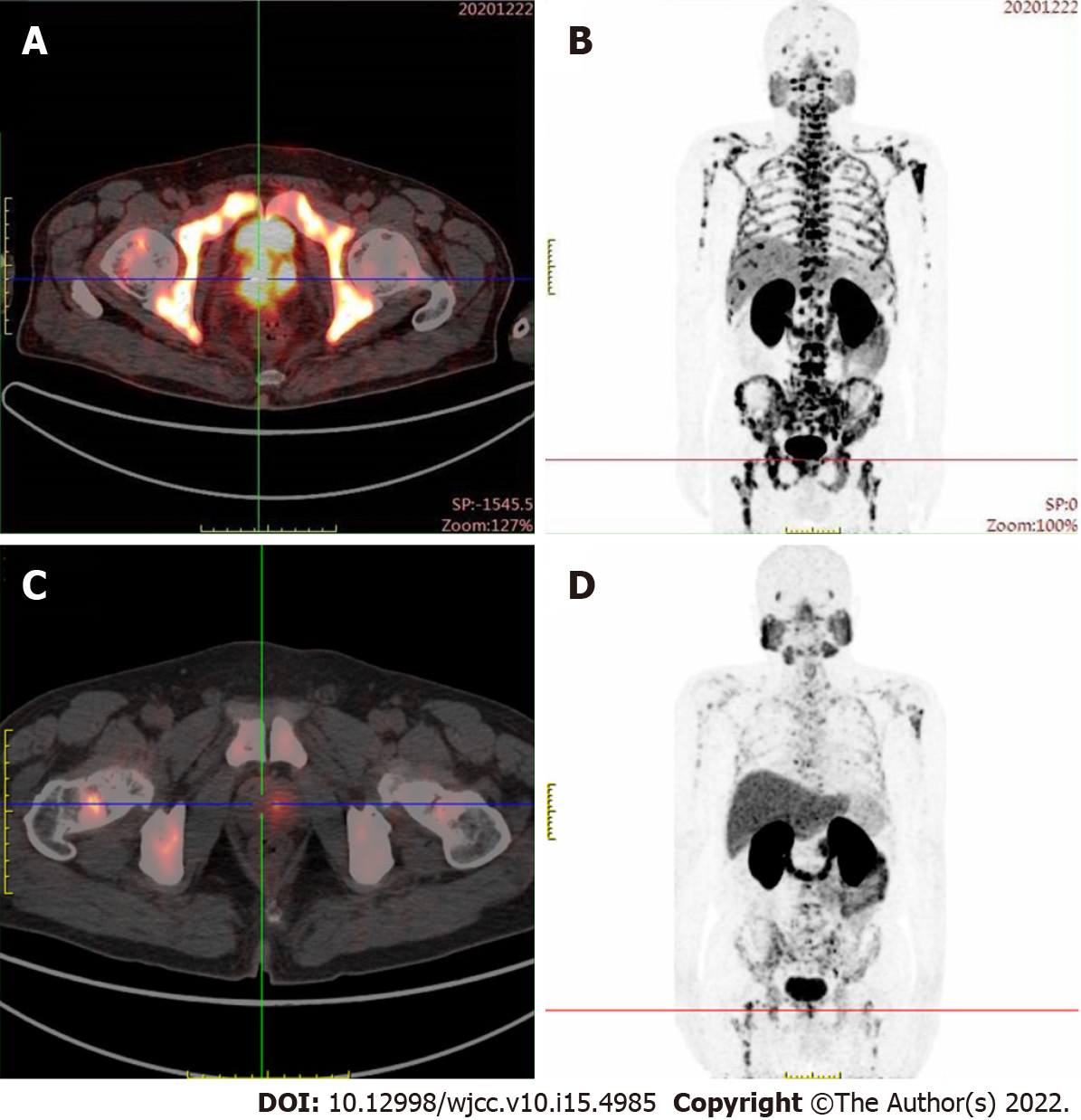
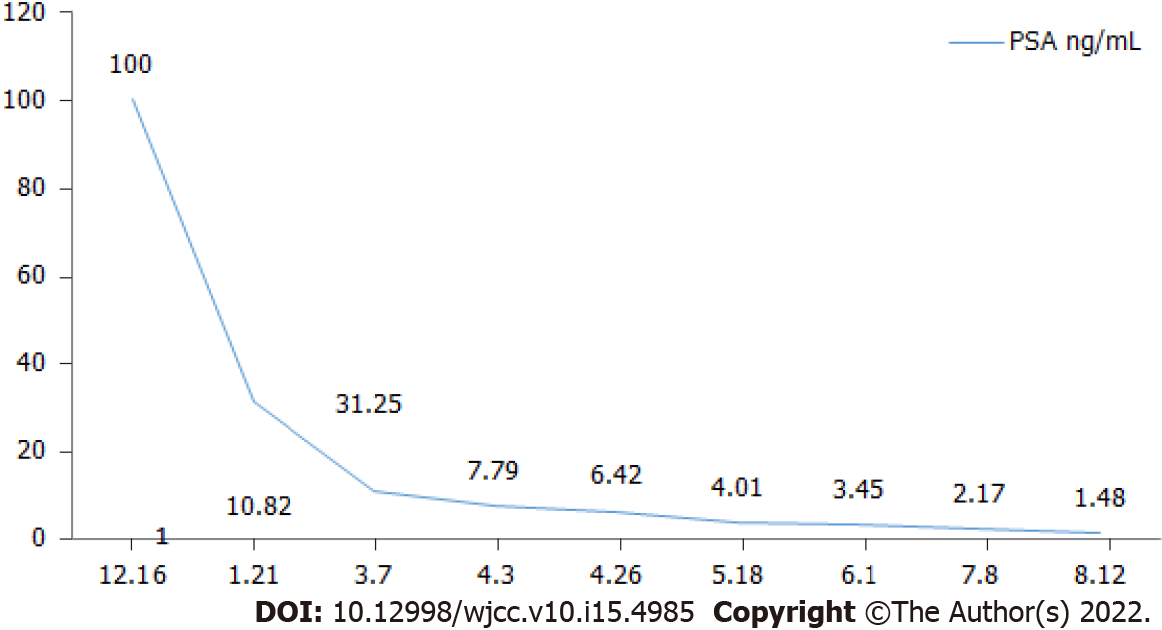
PSA levels and alkaline phosphatase levels were reviewed monthly, and imaging examinations were reviewed every three months. The PSA level decreased significantly during the treatment; however, three months later, radionuclide bone imaging and MRI indicated progression of the bone metastasis (Figure 1). Contrarily, PSMA PET-CT examination showed significantly decreased PSMA aggregation on the bone, indicating improved bone metastases (Figure 2). Considering that the lumbar pain, which he initially presented with, continued to decrease, the treatment strategies were considered to be effective. The original treatment strategies were continued for over 4 mo. The patient's PSA decreased to normal levels (Figure 3), and his clinical symptoms reduced significantly; therefore, treatment was continued.
In most patients diagnosed with prostate cancer with distant metastases at the clinic, bone metastasis is the most common. Studies have shown that androgen receptors (AR) play an important role in the development of prostate cancer[3]. Therefore, ARs are an important target for the treatment of prostate cancer. However, with prolonged conventional AR-targeted drug therapy, patients with prostate cancer experience a reduction in therapeutic effect[4]. Presently, research and development of new AR-targeting drugs has become a hotspot in the field of prostate cancer treatment.
Apalutamide, an oral non-steroidal antiandrogen agent that binds directly to the ligand-binding domain of the androgen receptor and prevents androgen receptor translocation, DNA binding, and androgen receptor–mediated transcription[5], has shown good efficacy and safety in clinical practice. The 2020 EAU prostate cancer guidelines recommend ADT in combination with apalutamide and enzalutamide, a new generation of androgen receptor antagonists, as first-line therapy for metastatic prostate cancer. Ryan et al[2] reported that after abiraterone treatment for 3 mo, the PSA level decreased by more than 50%, but bone metastasis lesions increase. Furthermore, after 6 mo of continued treatment, bone scan revealed reduced or stable lesions, and this was termed the “bone flare” phenomenon. During treatment, the laboratory results of patients with the bone flare phenomenon showed no significant change in the alkaline phosphatase level, however, PSA level continued to decrease. Our case revealed that bone flare phenomenon also occurs with apalutamide therapy. Some studies have also shown that bone flare occurs in some breast cancer patients with bone metastasis after chemotherapy and other treatments, and is considered to be a sign of effective treatment of bone metastases. Cook et al[6] reported that of 22 patients with unequivocal skeletal metastases on baseline scan, flares occurred in 9 (41%). Out of 36 high-risk localized prostate cancer patients with normal pre-treatment bone scintigraphy, the scan became positive for metastases at 6 wk in 4 (11%). Furthermore, out of 41 patients, pre-treatment scintigraphic abnormalities of uncertain etiology and flares occurred in 8 patients (20%).
In the present study, the patient was diagnosed with mHSPC and bone metastasis. The initial treatment strategy was ADT and apalutamide. However, bone flares occurred 3 mo after treatment, and a bone scan showed metastatic progression. However, PSMA PET-CT examination showed that abnormal PSMA aggregation was significantly reduced, and bone metastasis was significantly improved compared to the baseline. Therefore, the temporary phenomenon of increased lesions in the bone scan was considered a bone flare. During treatment, the PSA level decreased significantly, and the alkaline phosphatase level did not change significantly. Vincenza et al[7] pointed out that therapy for metastatic disease may lead to healing and new bone formation, which causes an initial increase in tracer uptake (akin to callus formation). This deterioration, followed by subsequent improvement in the bone scan appearance after successful therapy, is defined as a flare response. Due to the shortcomings of the current assessment based on PSA and imaging examinations, more research results show that the changes in the number of circulating tumor cells in peripheral blood can be used as a new means of evaluating efficacy. If the treatment is effective, the number of circulating tumor cells will decline rapidly. The increase or decrease in the number of circulating tumor cells after treatment is also a predictor of patient survival. The prognostic value is significantly better than PSA and imaging examination[8,9]. In the latest PCWG3 study, assessment of circulating tumor cell number was used as an important marker for disease evaluation and to assess drug efficacy[10].
Therefore, during the novel hormonal therapy in mHSPC patients with bone metastasis, it is important to identify whether the treatment is ineffective or whether the bone flare phenomenon is observed when bone scan indicates bone disease progression. This will determine the subsequent treatment strategy for patients. If PSA levels are not reduced after treatment and clinical symptoms worsen, the possibility of disease progression should be considered, and a new treatment strategy should be selected. Otherwise, bone flare should be considered if the PSA level decreases significantly after treatment, and the patient's clinical symptoms improve significantly. In this case, the original treatment was maintained with close follow-up. Bone scan showed increased metastases, but the PSA level decreased significantly. Following PSAM, PET-CT results showed significant improvement in bone metastasis, and the bone flare phenomenon was confirmed.
When apalutamide therapy is used in the treatment of prostate cancer, an increase in metastatic bone lesions on radionuclide bone imaging and MRI; warrants the use of other examinations including PSA, PSMA PET-CT, to determine the efficacy of ongoing treatment, so as to determine the course of treatment for such patients.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56698] [Article Influence: 7087.3] [Reference Citation Analysis (135)] |
| 2. | Ryan CJ, Shah S, Efstathiou E, Smith MR, Taplin ME, Bubley GJ, Logothetis CJ, Kheoh T, Kilian C, Haqq CM, Molina A, Small EJ. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854-4861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Dai C, Heemers H, Sharifi N. Androgen Signaling in Prostate Cancer. Cold Spring Harb Perspect Med. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 316] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 4. | Paschalis A, Sharp A, Welti JC, Neeb A, Raj GV, Luo J, Plymate SR, de Bono JS. Alternative splicing in prostate cancer. Nat Rev Clin Oncol. 2018;15:663-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 5. | Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, Chen Y, Grillot K, Bischoff ED, Cai L, Aparicio A, Dorow S, Arora V, Shao G, Qian J, Zhao H, Yang G, Cao C, Sensintaffar J, Wasielewska T, Herbert MR, Bonnefous C, Darimont B, Scher HI, Smith-Jones P, Klang M, Smith ND, De Stanchina E, Wu N, Ouerfelli O, Rix PJ, Heyman RA, Jung ME, Sawyers CL, Hager JH. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 532] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 6. | Cook GJ, Venkitaraman R, Sohaib AS, Lewington VJ, Chua SC, Huddart RA, Parker CC, Dearnaley DD, Horwich A. The diagnostic utility of the flare phenomenon on bone scintigraphy in staging prostate cancer. Eur J Nucl Med Mol Imaging. 2011;38:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Conteduca V, Poti G, Caroli P, Russi S, Brighi N, Lolli C, Schepisi G, Romeo A, Matteucci F, Paganelli G, Marchetti P, De Giorgi U. Flare phenomenon in prostate cancer: recent evidence on new drugs and next generation imaging. Ther Adv Med Oncol. 2021;13:1758835920987654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Thalgott M, Heck MM, Eiber M, Souvatzoglou M, Hatzichristodoulou G, Kehl V, Krause BJ, Rack B, Retz M, Gschwend JE, Andergassen U, Nawroth R. Circulating tumor cells versus objective response assessment predicting survival in metastatic castration-resistant prostate cancer patients treated with docetaxel chemotherapy. J Cancer Res Clin Oncol. 2015;141:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Vogelzang NJ, Fizazi K, Burke JM, De Wit R, Bellmunt J, Hutson TE, Crane E, Berry WR, Doner K, Hainsworth JD, Wiechno PJ, Liu K, Waldman MF, Gandhi A, Barton D, Jungnelius U, Fandi A, Sternberg CN, Petrylak DP. Circulating Tumor Cells in a Phase 3 Study of Docetaxel and Prednisone with or without Lenalidomide in Metastatic Castration-resistant Prostate Cancer. Eur Urol. 2017;71:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, Antonarakis ES, Beer TM, Carducci MA, Chi KN, Corn PG, de Bono JS, Dreicer R, George DJ, Heath EI, Hussain M, Kelly WK, Liu G, Logothetis C, Nanus D, Stein MN, Rathkopf DE, Slovin SF, Ryan CJ, Sartor O, Small EJ, Smith MR, Sternberg CN, Taplin ME, Wilding G, Nelson PS, Schwartz LH, Halabi S, Kantoff PW, Armstrong AJ; Prostate Cancer Clinical Trials Working Group 3. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 1307] [Article Influence: 130.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Anazawa U, Japan; Di Zazzo E, Italy; Jeyaraman M, India; Salvadori M, Italy S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ