Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.4964
Peer-review started: November 1, 2021
First decision: December 2, 2021
Revised: December 15, 2021
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: May 26, 2022
Processing time: 204 Days and 2.5 Hours
Metaplastic breast cancer (MBC) is a rare subtype of breast cancer. They constitute less than 1% of breast cancer cases and are much rarer in males. There are few reports of MBC because of its rarity. MBC, an aggressive type of cancer, is refractory to common treatment modalities of breast cancer and has a poor prognosis.
We report a case of MBC in a 78-year-old man. He visited our clinic with a palpable mass on the left breast with no masses in the axillary areas. He had previously undergone robot-assisted laparoscopic radical prostatectomy for prostate cancer, but there was no family history of malignancy. The breast mass was visible on ultrasonography, mammography, and magnetic resonance imaging, and chest computed tomography revealed a lung mass in the posterior basal segment of the right lower lobe. The patient was diagnosed with metaplastic carcinoma on core needle biopsy with lung metastasis. Total mastectomy with sentinel lymph node biopsy and video-assisted segmentectomy of the right lung was performed. However, multiple metastases appeared 3 mo after surgery in the brain, chest, and abdomen, and the patient died 5 mo after the initial diagnosis.
MBC is an aggressive and extremely rare breast cancer type. Further case reports are needed to determine the optimal treatment.
Core Tip: Metaplastic breast cancer is a rare and aggressive form of breast cancer and is even rarer in males. Here, we present a case of a 78-year-old man with metaplastic breast cancer and lung metastasis. While he was treated with mastectomy and video-assisted segmentectomy, multiple metastases throughout the body appeared months later. More cases need to be accumulated to determine the most appropriate method of treatment.
- Citation: Kim HY, Lee S, Kim DI, Jung CS, Kim JY, Nam KJ, Choo KS, Jung YJ. Male metaplastic breast cancer with poor prognosis: A case report . World J Clin Cases 2022; 10(15): 4964-4970
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/4964.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.4964
Metaplastic breast cancer (MBC) is a special subtype of breast cancer. MBC represents less than 1% of breast cancer cases, and male MBC is extremely rare. MBC shows aggressive clinical features. It often presents with a larger mass and rarely with axillary nodal metastasis, and it tends to recur locoregionally and distantly. MBC is not sensitive to common therapies for breast cancer, and there are limited options for the treatment of MBC, especially due to its triple-negative status. There are only a few reports of male MBC. We report a case of MBC in a male patient with pulmonary metastasis at the time of diagnosis.
A 78-year-old man presented to our breast center with a palpable mass on his left breast diagnosed as malignant by core needle biopsy.
This patient recognized a palpable mass on his left breast 10 d earlier when he visited the local clinic. He had no complaints other than the lump.
He previously had a robot-assisted laparoscopic radical prostatectomy for prostate cancer in May 2016. He received routine follow-up in the urology department. He had also been followed-up for coronary artery obstructive disease in the cardiology department. He was taking dual antiplatelet therapy.
The patient did not abuse alcohol and was a non-smoker. There was no specific family history of malignancy.
There was a fixed mass on his left breast, with no visible signs like redness, skin ulcer, or edema. There were no palpable masses in the axillary areas.
Carcinoembryonic antigen, cancer antigen 15-3, and prostate-specific antigen were checked according to his history and were within the normal range (3.9 ng/mL, 7.73 U/mL, and 0.01 ng/mL, respectively). Except for mild anemia, his blood test results were normal. Genetic testing for BRCAness or next generation sequencing was not performed.
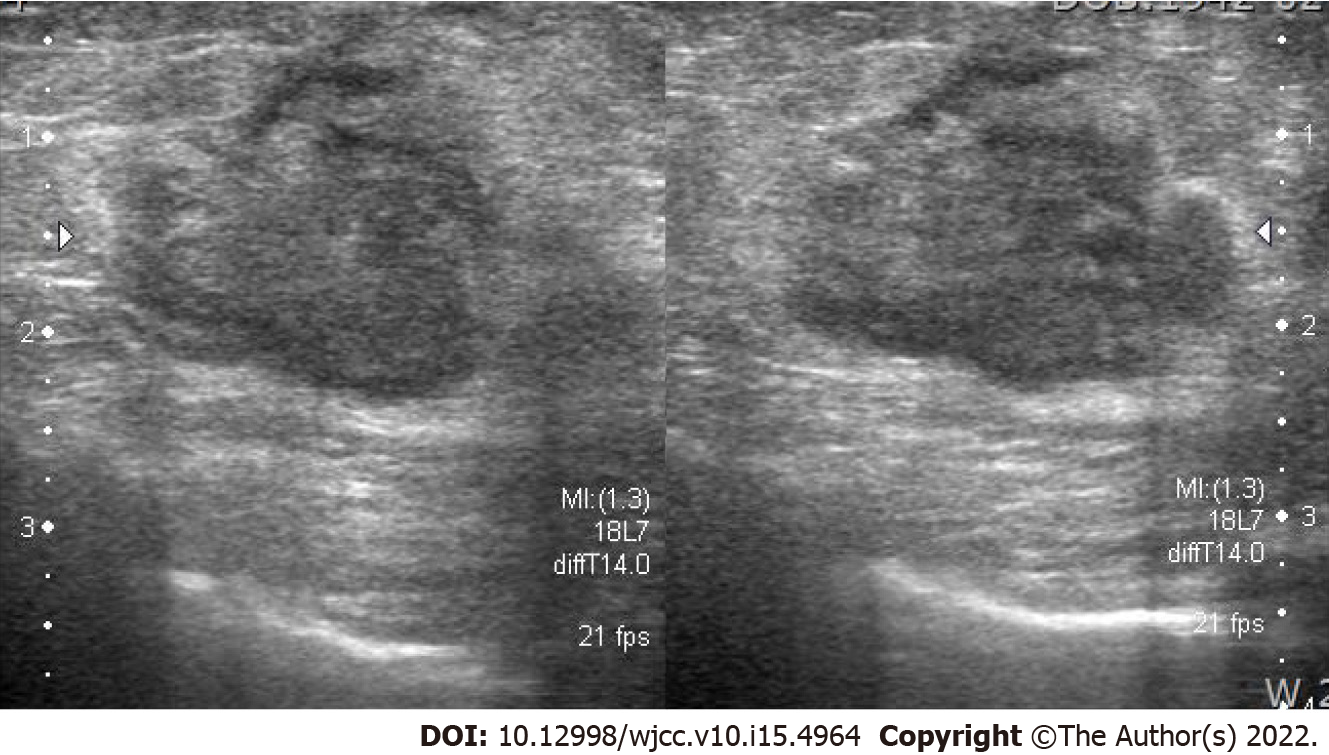
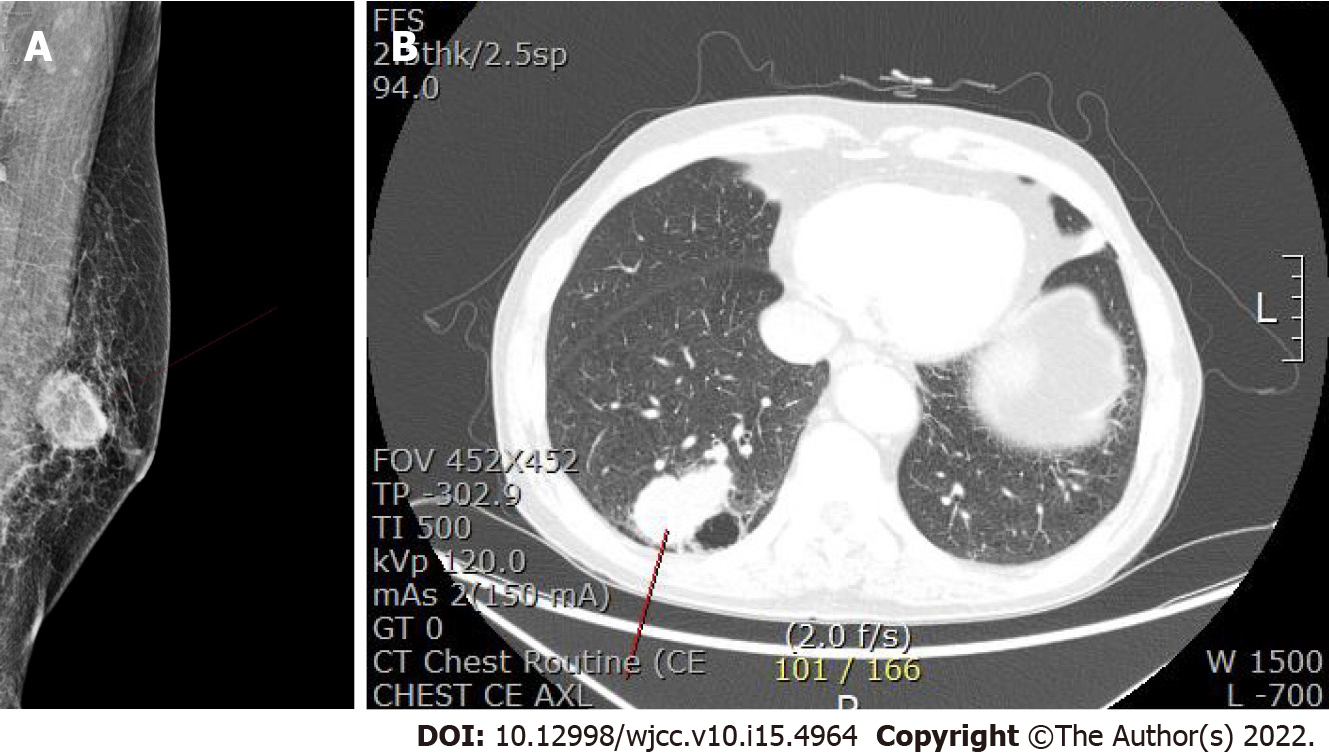
Ultrasonography showed an ill-defined, lobulated, heterogeneous hypoechoic mass 2 cm in size on the left breast (Figure 1). Mammography identified a 3.2-cm sized oval, indistinct, hyperdense mass on his left mid inner breast (Figure 2A). On magnetic resonance imaging (MRI), there was a 2.9 cm × 2.9 cm × 3 cm sized irregular, rim-enhancing mass with an adjacent satellite-enhancing focus on the left breast. The mass showed diffusion restriction. There were two round enhancing lymph nodes on level I, which were suspicious for metastasis. For the metastasis workup, a bone scan and Computed tomography (CT) of the chest, abdomen, and pelvis were performed. There were no metastatic findings in the bones or abdomen. Examination of chest CT revealed a 5.1 cm × 3.0 cm sized lobulated lung mass in the posterior basal segment of the right lower lobe with multiple enlarged sub-centimeter-sized lymph nodes in the subaortic area (Figure 2B).
We reviewed the breast biopsy specimen. A percutaneous needle biopsy was also performed on the lung mass to differentiate between metastasis and primary lung cancer. The percutaneous needle biopsy revealed poorly differentiated carcinoma with spindle cell differentiation, suggesting metastatic metaplastic carcinoma. GATA3, pancytokeratin, and p53 were positive. Alpha-Methylacyl-CoA Racemase (AMACR) and TTF-1 were negative. These findings helped us rule out primary lung cancer.
The final diagnosis of the presented case was metaplastic carcinoma of the breast with metastatic carcinoma of the lung.
The patient did not wish to undergo chemotherapy. Subsequently, a multidisciplinary discussion was conducted, following which the surgical treatment option was chosen.
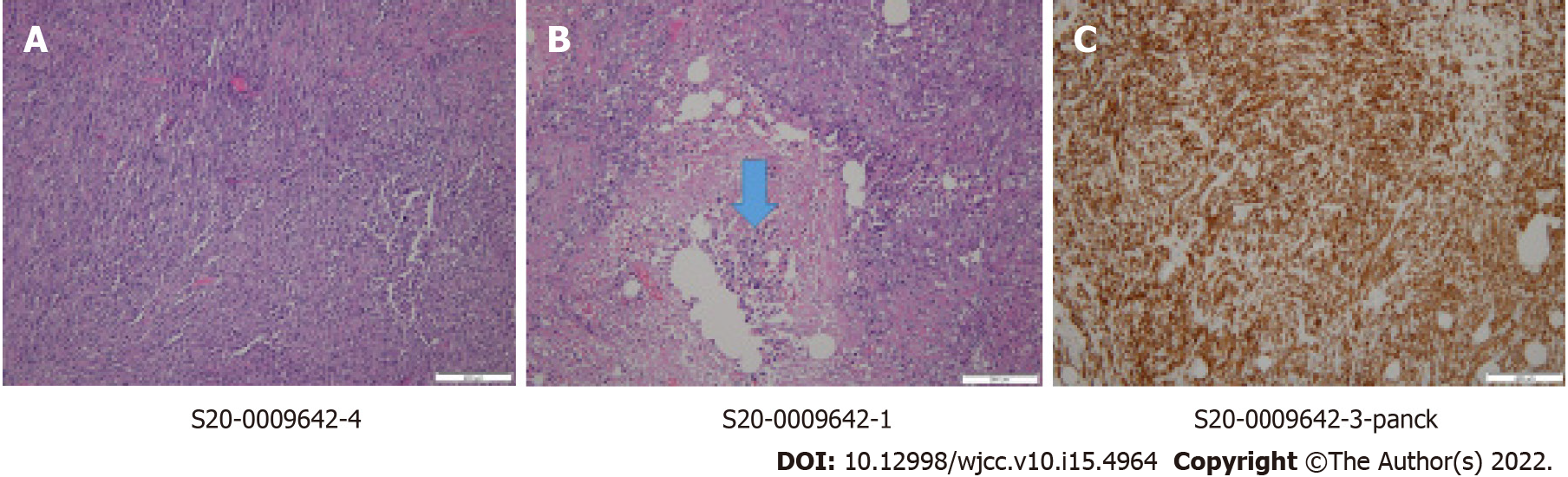
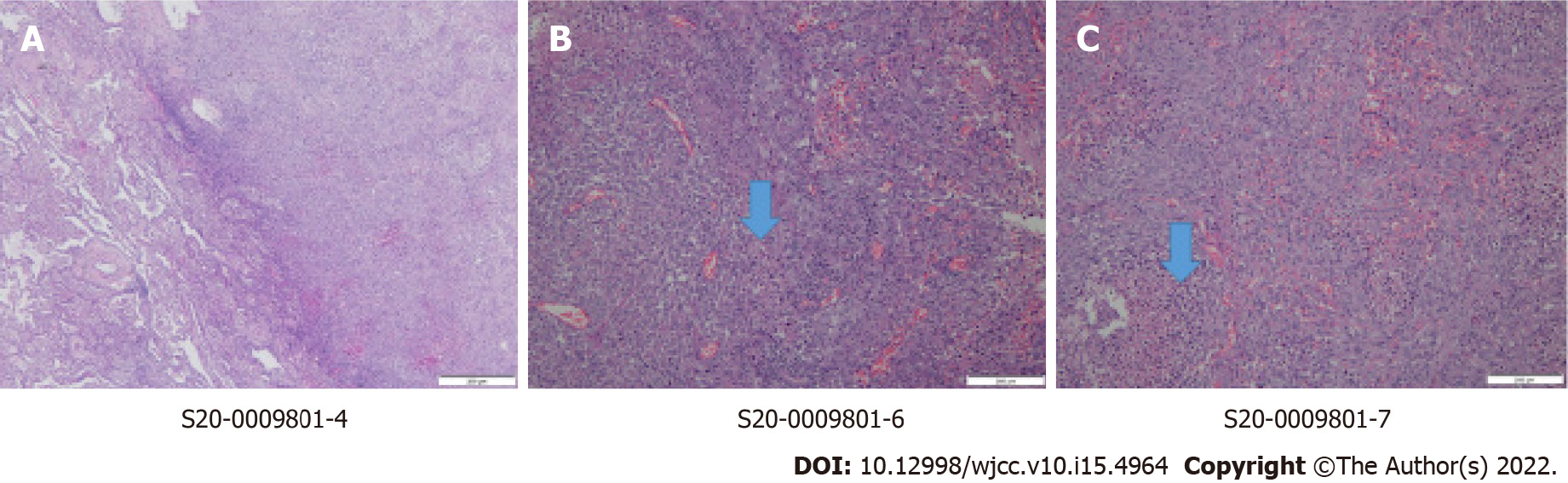
Total mastectomy, sentinel lymph node biopsy, and video-assisted segmentectomy of the right lung were performed. There were no tumor cells in the sentinel lymph nodes. The final pathologic report of the breast confirmed poorly differentiated metaplastic carcinoma (Figure 3). It revealed triple-negative breast cancer with a Ki67 index of up to 90%. p53 and pancytokeratin were positive. Pathology of the lung also revealed metastatic carcinoma from the metaplastic carcinoma of the breast (Figure 4). Finally, the patient was staged as stage IV, pT2pN0pM1. Adjuvant chemotherapy was also omitted considering his age and medical condition.
After 3 mo, the patient visited the emergency department due to headache, degradation of cognitive function, and general weakness. CT of the brain revealed multiple isodense masses with peritumoral edema at the bilateral cerebral hemisphere, which were suspicious of metastasis. Further MRI evaluation showed multiple rim-enhancing masses or enhancing foci at both cerebral hemispheres. The largest mass of 1.5 cm in the right temporal lobe was reported as a metastasis. The patient was administered palliative radiotherapy to the whole brain (30 Gy in 10 fractions) to relieve the severe headache.
Follow-up CT of the chest and abdomen revealed multiple metastatic findings, including an enlarged left supradiaphragmatic lymph node and multiple metastases of the liver, adrenal gland, omentum, muscles of the thigh, and the subcutaneous fat layer of the abdominal wall and buttock. The patient died 5 mo after the initial diagnosis.
Although breast cancer is one of the most frequently diagnosed cancers, MBC is a rare clinical entity among breast cancer cases. MBC accounts for less than 1% of breast cancer diagnoses and is extremely rare in males. We found only 5 cases of male MBC reported in the literature[1-5].
MBC was first described by Huvos et al[6] in 1973. MBC is a heterogeneous group of neoplasms. Wargotz et al[7] classified MBC into 5 types: matrix-producing carcinoma, squamous cell carcinoma, spindle cell carcinoma, carcinosarcoma, and metaplastic carcinoma with osteoclastic giant cells. However, in 2012, the World Health Organization[8] suggested 7 types of metaplastic carcinomas, including low-grade adenosquamous carcinoma, fibromatosis-like metaplastic carcinoma, squamous cell carcinoma, spindle cell carcinoma, metaplastic carcinoma with mesenchymal differentiation, mixed metaplastic carcinoma, and myoepithelial carcinoma. These types are also subdivided into low- or high-grade tumors according to their cellular features.
MBCs are usually seen in women in their 5th and 6th decades of life. The clinical presentation of MBC is similar to common breast cancers; however, MBCs often manifest as a rapidly growing mass; therefore, they tend to be larger at the time of diagnosis. According to the previously reported cases, most male patients presented with palpable masses accompanied by common signs of cancer, such as edema, erythema, and nipple retraction, but those signs were absent in our case. MBC shows lower rates of axillary nodal involvement but higher rates of both local recurrence and metastasis. Luini et al[9] reported high rates of pulmonary metastasis, suggesting that MBCs were more associated with hematogenous spread than with lymphatic spread. Compared to invasive ductal carcinoma (IDC), more MBCs present with stage IV disease at diagnosis, and more than 50% of MBCs develop local or distant metastases within 5 years. MBC is associated with a poor prognosis.
Radiologic findings of MBCs are similar to other types of breast cancers. They are also similar to benign lesions, as is common for triple-negative breast cancers. Therefore, careful examination and active histologic confirmation must be performed. On MRI, MBC presents as isointense or hypointense, similar to other histologic types of invasive breast carcinoma. High signal intensity on T2-weighted images is a frequent finding. The commonly reported kinetic pattern is an early enhancement and a delayed washout corresponding to the enhancing peripheral portion and non-enhancing internal components[10].
Pathologic confirmation is the gold standard for diagnosis. The most common type of MBC is squamous cell carcinoma, followed by spindle cell carcinoma. MBC must be differentiated from phyllodes tumors, primary sarcomas, and fibromatoses. Although male breast cancers are usually hormone-receptor positive, most MBCs are negative for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2. However, MBC shows even more aggressive behavior than the more common type of triple-negative invasive ductal carcinoma[11]. Lien et al[12] recently proved that the epithelial-mesenchymal transition (EMT) related genes were notably upregulated in MBC. EMT is known to be related to cancer invasion, progression, and resistance to chemotherapy. These findings validate the poor prognosis of MBC.
Surgery is the treatment of choice for MBCs. Due to their larger sizes and high propensity for local recurrence than IDCs, mastectomy is preferred as the surgical treatment. Axillary lymph node dissection is not recommended initially because nodal involvement is not common. Sentinel lymph node biopsy is preferable. It is known that MBCs are more resistant to chemotherapy.
There is no optimal chemotherapeutic regimen for MBC. Katz et al[2] reported on the youngest man to be treated with surgery and chemotherapy for MBC. The patient received adjuvant chemotherapy with four cycles of dose-dense adriamycin and cyclophosphamide followed by 12 wk of paclitaxel, one of the standard regimens.
Chen et al[13] reported on systemic chemotherapy for MBC. They insisted that MBC behaves more like a low-grade sarcoma rather than IDC. Although the data is limited, it is reported that taxane-based regimens show better results than other regimens. Hormone therapy is usually unnecessary because of the high prevalence of triple-negative status. The role of radiotherapy is also uncertain; however, it can be performed as part of palliative care. The lack of data makes it difficult for physicians to manage patients with MBC. In our case, chemotherapy was not administered considering the patient’s the advanced age and comorbidities.
Recently, some studies have attempted to find new therapeutic approaches. Basho et al[14] reported a high frequency of alterations in the PI3K/AKT/mTOR pathway in MBC. According to this report, MBC treated with an mTOR-based systemic therapy regimen showed a better outcome than non-metaplastic triple-negative breast cancer treated with the same regimen. Adams[15] reported remarkable responses to immune checkpoint inhibitors, like pembrolizumab and durvalumab, in advanced MBC with PD-L1 expression.
MBC is a distinct disease entity that shows a dismal prognosis compared to other types of breast cancer. There is no standard mode of treatment, but surgery is the treatment of choice. Conventional therapeutic modalities like chemotherapy, radiotherapy, hormone therapy, and molecular-targeted therapy can be applied, but their effects are not certain. Future studies of effective treatments and agents, like immunotherapy and molecular-targeted treatment, are needed to improve the course of the disease.
| 1. | Barr JG, Jane Clayton ES, Sotheran W. A case of metaplastic breast cancer in a man. J Surg Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Katz H, Jafri H, Dougherty T, Lebowicz Y. Rare case of metaplastic breast cancer in a man. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Kuo SH, Chen CL, Huang CS, Cheng AL. Metaplastic carcinoma of the breast: analysis of eight Asian patients with special emphasis on two unusual cases presenting with inflammatory-type breast cancer. Anticancer Res. 2000;20:2219-2222. [PubMed] |
| 4. | Rehman A. Triple-negative phenotype of poorly-differentiated metaplastic breast carcinoma in a male: an oncological rarity. J Coll Physicians Surg Pak. 2013;23:370-372. [PubMed] |
| 5. | Tampakis A, Tampaki EC, Trafalis D, Nonni A, Kontzoglou K, Patsouris E, Kontos M, Kouraklis G. Nestin and CD146 expression in metaplastic breast cancer: stem-cell therapy in need? Eur Rev Med Pharmacol Sci. 2017;21:4137-4140. [PubMed] |
| 6. | Huvos AG, Lucas JC Jr, Foote FW Jr. Metaplastic breast carcinoma. Rare form of mammary cancer. N Y State J Med. 1973;73:1078-1082. [PubMed] |
| 7. | Wargotz ES, Deos PH, Norris HJ. Metaplastic carcinomas of the breast. II. Spindle cell carcinoma. Hum Pathol. 1989;20:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 223] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 8. | Sinn HP, Kreipe H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care (Basel). 2013;8:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Luini A, Aguilar M, Gatti G, Fasani R, Botteri E, Brito JA, Maisonneuve P, Vento AR, Viale G. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. 2007;101:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Kim HJ, Kim SY, Huh S. Multimodality Imaging Findings of Metaplastic Breast Carcinomas: A Report of Five Cases. Ultrasound Q. 2018;34:88-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Jung SY, Kim HY, Nam BH, Min SY, Lee SJ, Park C, Kwon Y, Kim EA, Ko KL, Shin KH, Lee KS, Park IH, Lee S, Kim SW, Kang HS, Ro J. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat. 2010;120:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Lien HC, Hsiao YH, Lin YS, Yao YT, Juan HF, Kuo WH, Hung MC, Chang KJ, Hsieh FJ. Molecular signatures of metaplastic carcinoma of the breast by large-scale transcriptional profiling: identification of genes potentially related to epithelial-mesenchymal transition. Oncogene. 2007;26:7859-7871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Chen IC, Lin CH, Huang CS, Lien HC, Hsu C, Kuo WH, Lu YS, Cheng AL. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat. 2011;130:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Basho RK, Yam C, Gilcrease M, Murthy RK, Helgason T, Karp DD, Meric-Bernstam F, Hess KR, Valero V, Albarracin C, Litton JK, Chavez-MacGregor M, Hong D, Kurzrock R, Hortobagyi GN, Janku F, Moulder SL. Comparative Effectiveness of an mTOR-Based Systemic Therapy Regimen in Advanced, Metaplastic and Nonmetaplastic Triple-Negative Breast Cancer. Oncologist. 2018;23:1300-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Adams S. Dramatic response of metaplastic breast cancer to chemo-immunotherapy. NPJ Breast Cancer. 2017;3:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Deng DT, China; Seeman MV, Canada S-Editor: Xing YX L-Editor: A P-Editor: Xing YX