Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3698
Peer-review started: November 24, 2021
First decision: December 9, 2021
Revised: December 26, 2021
Accepted: March 5, 2022
Article in press: March 5, 2022
Published online: April 26, 2022
Processing time: 148 Days and 1.4 Hours
Breast cancer mainly occurs in young and premenopausal women; its incidence is increasing annually. Patients with triple-negative breast cancer (TNBC) have relatively high recurrence and transfer rates during the operation and 3 years after postoperative adjuvant chemotherapy. Currently, the treatment for patients with TNBC is mainly based on a comprehensive combination of surgery and chemotherapy. Therefore, identifying additional effective treatments to improve patient prognosis is important.
To explore and discuss the effects and prognostic factors of neoadjuvant chemotherapy in TNBC.
In total, 118 patients diagnosed with TNBC from January 2016 to January 2020 in our hospital were selected and divided into the observation (n = 60) and control
The karyopherin A2 (KPNA2)-positive and SRY-related HMG box-2 (SOX2)-positive expression rates of patients with TNBC with intravascular tumor thrombus and tumor-node-metastasis (TNM) stage IV were 92.00% and 91.67% and 96.00% and 95.83%, respectively, which were significantly higher than those of patients with no intravascular tumor thrombus and TNM stage III (P < 0.05). KPNA2 was positively associated with SOX2 expression (rs = 0.514, P < 0.50). The short-term curative effect of the observation group was better than that of the control group (P < 0.05), and the total effective rate was 58.33%. After treatment, carcinoembryonic antigen, cancer antigen (CA) 19-9, and CA125 Levels in the observation group were 11.40 ± 2.32 mg/L, 19.92 ± 3.42 kU/L, and 54.30 ± 12.28 kU/L, respectively, which were significantly lower than those in the control group (P < 0.05). The median survival time of the observation group was 33 mo (95%CI: 31.21-34.79), which was significantly longer than that of the control group (P < 0.05). TNM stage, degree of differentiation, lymph node metastasis, KPNA2 and SOX2 expressions, and treatment plan were prognostic factors of TNBC (relative risk = 1.575, 1.380, 1.366, 1.433, 1.411, and 0.581, respectively, P < 0.05).
Neoadjuvant chemotherapy for TNBC treatment can achieve good curative effects. TNM stage, differentiation degree, lymph node metastasis, KPNA2 and SOX2 expressions, and treatment plan are prognostic factors of TNBC.
Core Tip: Neoadjuvant chemotherapy for triple-negative breast cancer (TNBC) treatment can achieve good curative effects. Moreover, tumor-node-metastasis stage, differentiation degree, lymph node metastasis, karyopherin A2 and SRY-related HMG box-2 expressions, and treatment plan are prognostic factors of patients with TNBC.
- Citation: Ding F, Chen RY, Hou J, Guo J, Dong TY. Efficacy and prognostic factors of neoadjuvant chemotherapy for triple-negative breast cancer. World J Clin Cases 2022; 10(12): 3698-3708
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3698.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3698
Breast cancer has several types, and each subtype has different biological behaviors and clinicopathological and molecular characteristics. The corresponding treatment methods and prognoses of breast cancer are also different[1]. Triple-negative breast cancer (TNBC) is a type of breast cancer with no expression of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2, and its incidence accounts for approximately one-fifth of the incidence of breast cancer[2]. TNBC has high morbidity and shows an upward trend annually. However, due to the lack of effective targeted endocrine therapy, only conventional treatment can be provided in clinical practice. However, the curative effect of conventional treatment is poor, and its local recurrence rate is high, which has become one of the areas of interest in breast cancer studies in recent years[3]. For the past few years, drugs (anthracyclines, taxanes) are often used for TNBC treatment in clinical settings, although the therapeutic regimen for TNBC remains unclear. Some patients are drug-resistant, which can influence the treatment effect[4]. Currently, neoadjuvant chemotherapy is one of the most ideal treatments for locally advanced breast cancer, which can effectively improve the overall efficacy for breast cancer[5]. According to a previous study[6], tumor markers are consistent with the biological characteristics of breast cancer, and cytokines can predict the occurrence and development of tumors and the prognosis of patients. Patients with TNBC in our hospital were selected to explore and discuss the effects and prognostic factors of neoadjuvant chemotherapy in TNBC.
A total of 118 patients with TNBC from January 2016 to January 2020 in our hospital were selected. The inclusion criteria were as follows: (1) Patients who were pathologically diagnosed with TNBC; (2) patients with tumor-node-metastasis (TNM) stages III–IV; (3) patients who were all first-treated; (4) female patients; and (5) patients or their family members who provided an informed consent. The exclusion criteria were as follows: (1) Patients with an estimated survival period of < 3 mo; (2) patients complicated with other systemic malignant tumors; (3) patients complicated with liver, kidney, and other important organ diseases and immune system diseases; (4) patients with mental illness; and (5) patients with incomplete clinical follow-up data. Patients were divided into the observation group (n = 60) and the control group (n = 58) according to therapeutic regimen.
Recent therapeutic efficacy[7]: Complete response (CR) was defined as complete disappearance of the lesion, partial response (PR) was defined as tumor shrinkage ≥ 50% compared with that before treatment, stable disease was defined as tumor shrinkage < 50% or increase < 20% compared with that before treatment, and progressive disease was defined as tumor enlargement ≥ 20%. Total effectiveness was achieved using the following formula: CR + PR.
Patients in the two groups received chemotherapy on the first day after the admission. The control group received routine chemotherapy: on the first day of chemotherapy, intravenous cyclophosphamide (Baxter Oncology GmbH, batch no. 20151211) (500 mg/m2), intravenous fluorouracil (on day 1 and day 8) (Shanghai Xudong Haipu Pharmaceutical Co., Ltd., 20151022) (700 mg/m2), and methotrexate [Pfizer (Perth) Pty Limited, 20151103] (35 mg/m2) were administered. All patients received granulocyte colony-stimulating factor support therapy on the second day after chemotherapy in a 21-d cycle. After four consecutive cycles of chemotherapy, surgery could be performed if the effect of chemotherapy was significant.
The observation group received neoadjuvant chemotherapy for the epirubicin-paclitaxel (ET) regimen. Epirubicin [Pfizer (Wuxi) Co., Ltd., 20150724] (75 mg/m2) and paclitaxel (Hainan Haiyao Co., Ltd., 20151202) (75 mg/m2) were administered intravenously for 21 d. After four consecutive cycles of chemotherapy, surgery could be performed if the effect of chemotherapy was significant.
Cancer antigen (CA) 125, CA19-9, and carcinoembryonic antigen (CEA) levels were detected using a Roche Cobas E601 automatic electrochemiluminescence immunoanalyzer, which was purchased from Roche. The next day before and after treatment, 5 mL of the patient’s fasting venous blood was collected and centrifuged at 3000 r/min for 5 min, and the serum was separated. Electrochemical luminescence automatic immunoanalyzer and corresponding reagents were used for detection. All operations were performed in strict accordance with the instructions to avoid hemolysis and contamination.
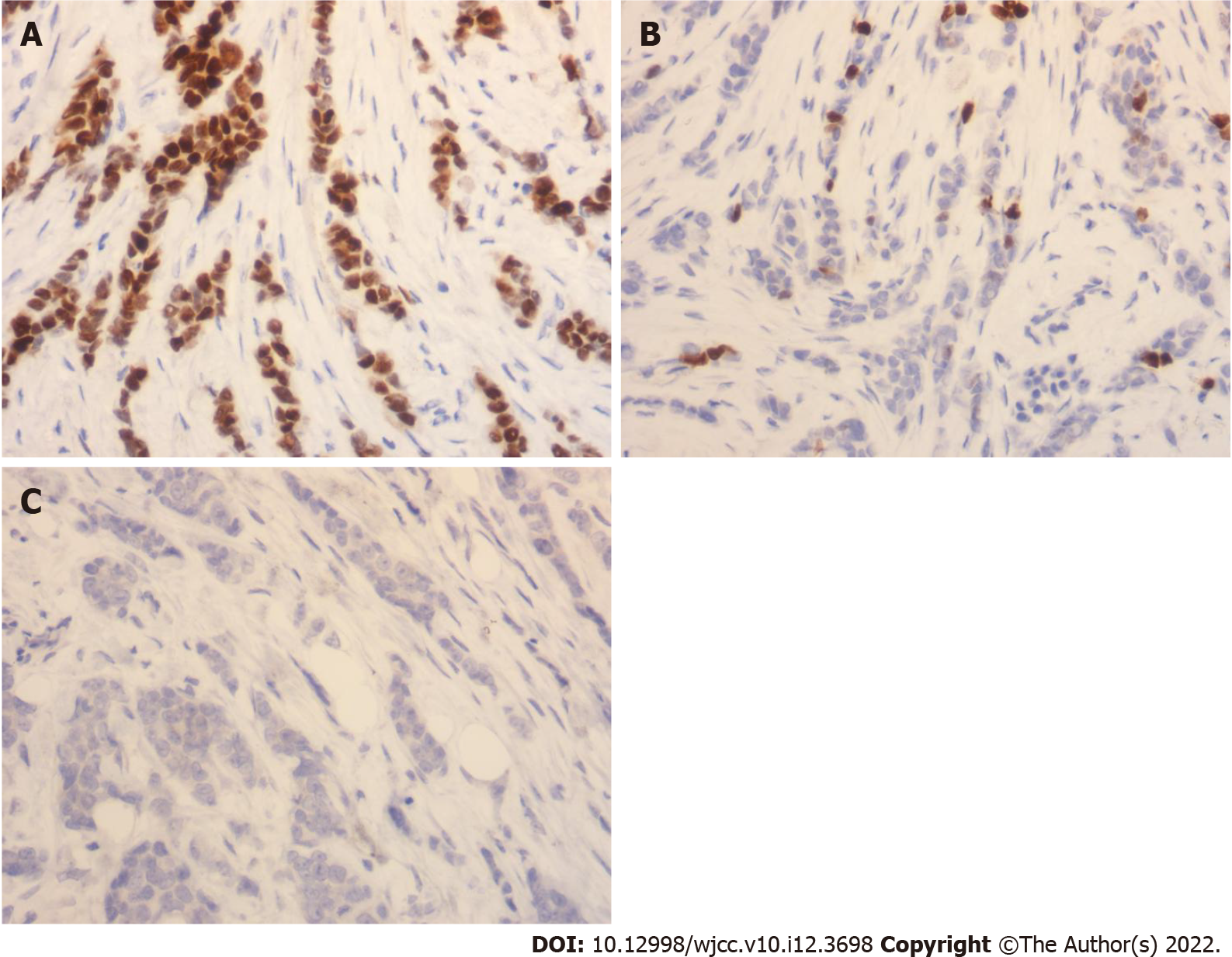
The monoclonal antibodies karyopherin A2 (KPNA2), protein 53, and KI-67 were purchased from Roche, and BenchMark ULTRA was used for immunohistochemical staining. The experimental procedures were performed according to the provided instructions. Known positive tissue was used as the positive control, and phosphate buffered saline was used instead of a primary antibody as the negative control. Organization immunohistochemical staining results were provided by two senior pathologists using the semi-quantitative method, according to the density of dyeing (negative = 0, weakly positive = 1, moderately positive = 2, strongly positive = 3) and the positive cell percentage (0: negative, 1: < 25%, 2: 25%-50%, 3: 51%-75%, 4: > 75%), and five high-power electric field immune response scores (IRSs) were calculated. The final results were as follows: negative (IRS = 0) (−); weakly positive (IRS = 1–4) (+); moderately positive (IRS = 5–8) (++); and strongly positive (IRS = 9–12) (+++).
The Statistical Package for the Social Sciences version 22.0 (IBM Corp., Armonk, NY, USA) was used for data analysis. Normally distributed data are expressed as mean ± SD, and t-tests were used for comparisons between groups. Counting data are expressed as n (%), and the χ2 test was used for comparisons between groups. Survival curves were analyzed using the Kaplan-Meier method. Cox proportional risk regression analysis was used for multiple factors. Spearman rank correlation analysis was used to assess correlation. Inspection level was set at an α level of 0.05.
The KPNA2-positive expression rates of patients with TNBC with intravascular tumor thrombus and TNM stage IV were significantly higher than those of patients with no intravascular tumor thrombus and TNM stage III (P < 0.05) (Table 1). The SRY-related HMG box-2 (SOX2)-positive expression rates of patients with TNBC with intravascular tumor thrombus and TNM stage IV were significantly higher than those of patients with no intravascular tumor thrombus and TNM stage III (P < 0.05) (Table 2).
| Clinical data | Cases | KPNA2 positive expression rate | χ2 | P value |
| Age (yr) | 2.868 | 0.090 | ||
| < 50 | 55 | 33 (60.00) | ||
| ≥ 50 | 63 | 47 (74.60) | ||
| BMI (kg/m2) | 0.271 | 0.602 | ||
| < 22 | 60 | 42 (70.00) | ||
| ≥ 22 | 58 | 38 (65.52) | ||
| Karnofsky score (points) | 0.088 | 0.767 | ||
| < 70 | 52 | 36 (69.23) | ||
| ≥ 70 | 66 | 44 (66.67) | ||
| Differentiation degree | 0.393 | 0.531 | ||
| High differentiation | 51 | 33 (64.71) | ||
| Medium low differentiation | 67 | 47 (70.15) | ||
| Intravascular tumor thrombus | 8.511 | 0.004 | ||
| Yes | 25 | 23 (92.00) | ||
| No | 93 | 57 (61.29) | ||
| Tumor size | 0.136 | 0.712 | ||
| > 5 cm | 53 | 35 (66.04) | ||
| ≤ 5 cm | 65 | 45 (69.23) | ||
| TNM stages | 7.863 | 0.005 | ||
| III | 94 | 58 (61.70) | ||
| IV | 24 | 22 (91.67) | ||
| Pathological type | 0.301 | 0.583 | ||
| Invasive ductal carcinoma | 96 | 64 (66.67) | ||
| Other | 22 | 16 (72.73) | ||
| Ki-67 | 2.764 | 0.096 | ||
| ≤ 14% | 37 | 29 (78.38) | ||
| > 14% | 81 | 51 (62.96) | ||
| P53 | 0.034 | 0.854 | ||
| Positive | 79 | 54 (68.35) | ||
| Negative | 39 | 26 (66.67) |
| Clinical data | Cases | SOX2 positive expression rate | χ2 | P value |
| Age (yr) | 0.000 | 0.994 | ||
| < 50 | 55 | 41 (74.55) | ||
| ≥ 50 | 63 | 47 (74.60) | ||
| BMI (kg/m2) | 0.545 | 0.460 | ||
| < 22 | 60 | 43 (71.67) | ||
| ≥ 22 | 58 | 45 (77.59) | ||
| Karnofsky score (points) | 0.574 | 0.449 | ||
| < 70 | 52 | 37 (71.15) | ||
| ≥ 70 | 66 | 51 (77.27) | ||
| Differentiation degree | 0.000 | 0.988 | ||
| High differentiation | 51 | 38 (74.51) | ||
| Medium low differentiation | 67 | 50 (74.63) | ||
| Intravascular tumor thrombus | 7.689 | 0.006 | ||
| Yes | 25 | 24 (96.00) | ||
| No | 93 | 64 (68.82) | ||
| Tumor size | 0.420 | 0.517 | ||
| > 5 cm | 53 | 38 (71.70) | ||
| ≤ 5 cm | 65 | 50 (76.92) | ||
| TNM stages | 7.180 | 0.007 | ||
| III | 94 | 65 (69.15) | ||
| IV | 24 | 23 (95.83) | ||
| Pathological type | 0.049 | 0.825 | ||
| Invasive ductal carcinoma | 96 | 72 (75.00) | ||
| Other | 22 | 16 (72.73) | ||
| Ki-67 | 0.073 | 0.787 | ||
| ≤ 14% | 37 | 27 (72.97) | ||
| > 14% | 81 | 61 (75.31) | ||
| P53 | 0.741 | 0.389 | ||
| Positive | 79 | 57 (72.15) | ||
| Negative | 39 | 31 (79.49) |
The expression of KPNA2 was positively correlated with the expression of SOX2 (rs = 0.514, P < 0.50) (Table 3).
| KPNA2 expression | SOX2 expression | rs | P value | |
| Positive | Negative | |||
| Positive | 72 | 8 | 0.514 | 0.000 |
| Negative | 16 | 22 | ||
The clinical data of the two groups were compared (Table 4).
| Clinical data | Observation group (n = 60) | Control group (n = 58) | t/χ2 | P value |
| Age (yr) | 54.49 ± 4.29 | 55.70 ± 5.10 | -1.396 | 0.165 |
| BMI (kg/m2) | 22.02 ± 2.05 | 22.10 ± 2.54 | -0.189 | 0.851 |
| Karnofsky score (points) | 74.40 ± 5.12 | 75.52 ± 6.02 | -1.090 | 0.278 |
| Differentiation degree | 1.188 | 0.276 | ||
| High differentiation | 23 (38.33) | 28 (48.28) | ||
| Medium low differentiation | 37 (61.67) | 30 (51.72) | ||
| Intravascular tumor thrombus | 0.337 | 0.562 | ||
| Yes | 14 (23.33) | 11 (18.97) | ||
| No | 46 (78.33) | 47 (81.03) | ||
| Tumor size | 0.576 | 0.448 | ||
| > 5 cm | 29 (48.33) | 24 (41.38) | ||
| ≤ 5 cm | 31 (51.67) | 34 (58.62) | ||
| TNM stages | 0.009 | 0.926 | ||
| III | 48 (80.00) | 46 (79.31) | ||
| IV | 12 (20.00) | 12 (20.69) | ||
| Pathological type | 0.735 | 0.391 | ||
| Invasive ductal carcinoma | 47 (78.33) | 49 (84.48) | ||
| Other | 13 (21.67) | 9 (15.52) | ||
| KPNA2 expression | 0.016 | 0.899 | ||
| Positive | 41 (68.33) | 39 (67.24) | ||
| Negative | 19 (31.67) | 19 (32.76) | ||
| SOX2 expression | 0.012 | 0.914 | ||
| Positive | 45 (75.00) | 43 (74.14) | ||
| Negative | 15 (25.00) | 15 (25.86) | ||
| Ki-67 | 0.518 | 0.472 | ||
| ≤ 14% | 17 (28.33) | 20 (34.48) | ||
| > 14% | 43 (71.67) | 38 (65.52) | ||
| P53 | 0.210 | 0.647 | ||
| Positive | 39 (65.00) | 40 (68.97) | ||
| Negative | 21 (35.00) | 18 (31.03) |
The short-term therapeutic effects of the observation group were better than those of the control group (P < 0.05), and the total effective rate was 58.33% (Table 5).
| Group | Cases | CR | PR | SD | PD | Z | P value |
| Observation group | 60 | 0 (0.00) | 35 (58.33) | 19 (31.67) | 6 (10.00) | -2.183 | 0.029 |
| Control group | 58 | 0 (0.00) | 23 (39.66) | 23 (39.66) | 12 (20.69) |
There were no significant differences in CEA, CA19-9, and CA125 Levels between the observation and control groups before treatment (P > 0.05). CEA, CA19-9, and CA125 Levels in the observation and control groups were lower after treatment than those before treatment (P < 0.05). CEA, CA19-9, and CA125 Levels in the observation group were significantly lower than those in the control group (P < 0.05) (Table 6).
| Group | Cases | CEA (mg/L) | CA19-9 (kU/L) | CA125 (kU/L) | |||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | ||
| Observation group | 60 | 38.49 ± 6.12 | 11.40 ± 2.32a | 50.30 ± 12.21 | 19.92 ± 3.42a | 163.30 ± 34.43 | 54.30 ± 12.28a |
| control group | 58 | 40.02 ± 7.05 | 20.24 ± 2.50a | 52.29 ± 11.73 | 28.38 ± 2.95a | 168.29 ± 37.71 | 79.10 ± 14.42a |
| t | -1.260 | -19.919 | -0.902 | -14.368 | -0.751 | -10.070 | |
| P value | 0.210 | 0.000 | 0.369 | 0.000 | 0.454 | 0.000 | |
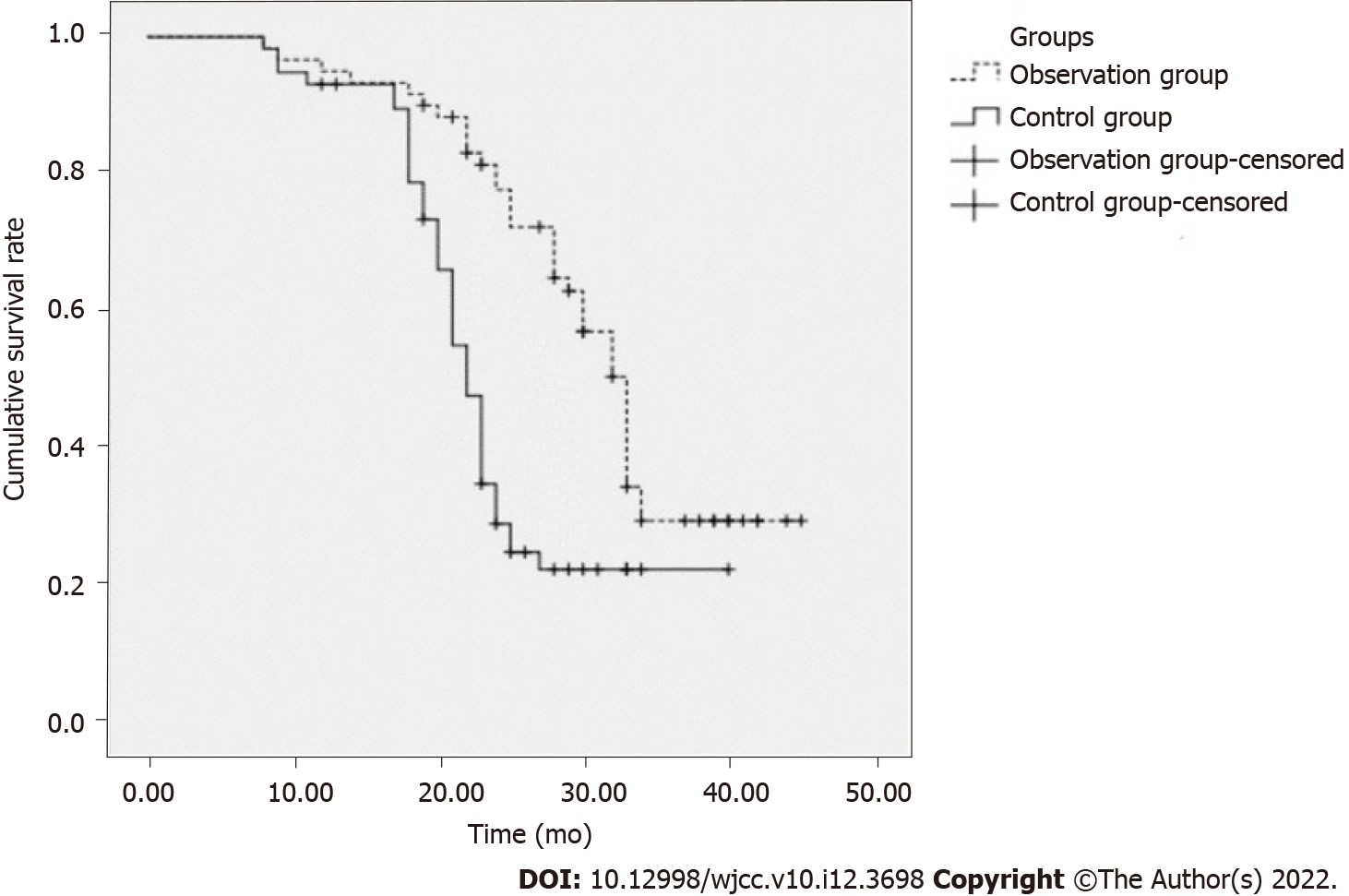
The median survival time of the observation group was 33 mo (95%CI: 31.21–34.79), which was significantly longer than that of the control group (22 mo, 95%CI: 20.69–23.31), and the difference was statistically significant (χ2 = 15.994, P = 0.000 < 0.05) (Figure 1).
Cox proportional risk regression analysis showed that TNM stage, differentiation degree, lymph node metastasis, KPNA2 and SOX2 expressions, and treatment plan were prognostic factors of TNBC (relative risk = 1.575, 1.380, 1.366, 1.433, 1.411, and 0.581, respectively, P < 0.05) (Table 7) (Figure 2).
| Factors | SE | Walds | P value | RR (95%CI) | |
| TNM staging | 0.454 | 0.102 | 19.811 | 0.000 | 1.575 (1.289-1.923) |
| Degree of differentiation | 0.322 | 0.104 | 9.586 | 0.000 | 1.380 (1.125-1.692) |
| Lymph node metastasis | 0.312 | 0.112 | 7.760 | 0.000 | 1.366 (1.097-1.702) |
| KPNA2 | 0.360 | 0.121 | 8.852 | 0.000 | 1.433 (1.131-1.817) |
| SOX2 | 0.344 | 0.132 | 6.792 | 0.000 | 1.411 (1.089-1.827) |
| Chemotherapy regimens | -0.543 | 0.142 | 14.623 | 0.000 | 0.581 (0.440-0.767) |
The ET regimen uses neoadjuvant chemotherapy before surgery, and its main target population is patients with locally advanced breast cancer[8-10]. The paclitaxel used in the regimen was a taxane antitumor drug, which can bind to free tubulin, accelerate the assembly speed of microtubules, inhibit the aggregation of microtubules, and effectively inhibit the growth of tumor cells[11]. The chemical composition of epirubicin, an anthracycline antitumor drug, is similar to that of adriacin, which plays an anticancer role mainly by inhibiting nucleic acid synthesis. The drug is inserted directly into the double strand of DNA, stopping the process of cell division and killing tumor cells. When used in combination, the two drugs have significant antitumor effects with few side effects[12].
According to the study results, the short-term curative effect of the observation group was significantly better than that of the control group, and the median survival time of the observation group was significantly longer than that of the control group (P < 0.05), indicating that the two chemotherapy regimens can all achieve good treatment effects, and the efficacy of the neoadjuvant chemotherapy regimen is relatively significant. The mechanisms of action of the two regimens are different. Cyclophosphamide mainly inhibits the proliferation of breast cancer cells by inhibiting the expression of the key protein pKAT in the PBK pathway. Neoadjuvant chemotherapy can control micrometastases in the body and effectively reduce the clinical stage of breast cancer. Paclitaxel binds specifically to specific parts of tubulin in cancer cells, preventing it from depolymerization, so that the division of cancer cells will always stay in the G2 and M phases, leading to the inability of the cancer cells to replicate and ultimately to the death of the cancer cells. Epirubicin can inhibit DNA replication and RNA synthesis, inhibit the division of cancer cells, and can affect the DNA superhelical DNA replication and transcription process by inhibiting topoisomerase II. It also chelates iron ions, producing free radicals that damage DNA, proteins, and cell membrane structures. KPNA2 is a member of the nuclear transport signal superfamily, transporting mRNA, DNA, and RNA polymerase and transcription factors into and out of the nucleus, thereby promoting cell proliferation and differentiation, and participating in cell development, apoptosis, migration, and DNA damage response.
Studies have shown that[13,14] tumor markers are closely related to the biological behavior of tumors, among which CEA, CA19-9, and CA125 are common. As a common type of hormone in humans, CA125 is a marker for ovarian cancer and can be highly expressed in breast cancer. CEA is elevated in advanced breast cancer, and CA19-9 can indicate the nature of the tumor. The combined detection of three tumor markers can improve the diagnosis rate of tumor, with high prognostic value, and the change level of cytokines after treatment is also of high value in predicting the prognosis of breast cancer[15-17]. In our study, CEA, CA19-9, and CA125 Levels in the observation and control groups after treatment were lower than those before treatment (P < 0.05). CEA, CA19-9, and CA125 Levels in the observation group after treatment were lower than those of the control group (P < 0.05), suggesting that neoadjuvant chemotherapy with the ET regimen can significantly reduce the levels of tumor markers and cytokines in patients with good therapeutic effect. According to the literature[18], patients with TNBC have the worst prognosis and a short survival time and are at risk of developing distant metastasis. Cox proportional risk regression analysis in this study showed that TNM stage, differentiation degree, lymph node metastasis, KPNA2 and SOX2 expressions, and treatment plan were prognostic factors of TNBC, which could be used as important indicators for clinical observation of efficacy and prognosis and may be related to the reduced sensitivity of tumor cells to chemotherapy drugs.
In the comprehensive treatment of breast cancer, neoadjuvant chemotherapy is no longer limited to breast preservation, staging surgery, and other advantages. It can evaluate the sensitivity of chemotherapy, realize personalized treatment, and develop targeted drugs[18-20]. There are many studies on the side effects of chemotherapy drugs, but few studies on the factors that affect the prognosis of patients after chemotherapy have been conducted. This study had certain reference value for clinical treatment. However, due to the short follow-up duration of this study and considering that influencing factors were not assessed in this study, larger multicenter study sample sizes to explore the clinicopathological characteristics and prognosis of elderly patients with TNBC are required in the future to develop a standard treatment plan for better prognosis.
In conclusion, neoadjuvant chemotherapy for TNBC treatment can achieve good curative effects. Moreover, TNM stage, differentiation degree, lymph node metastasis, KPNA2 and SOX2 expressions, and treatment plan are prognostic factors of patients with TNBC.
Patients with triple-negative breast cancer (TNBC) have relatively high recurrence and transfer rates during the operation and 3 years after postoperative adjuvant chemotherapy.
This study identified the additional effective treatments to improve patient prognosis.
This study aimed to explore and discuss the effects and prognostic factors of neoadjuvant chemotherapy in TNBC.
Total 118 patients diagnosed with TNBC from January 2016 to January 2020 in our hospital were selected and divided into the observation (n = 60) and control (n = 58) groups according to therapeutic regimen. The control group received routine chemotherapy, and the observation group received neoadjuvant chemotherapy.
The short-term curative effect of the observation group was significantly better than that of the control group, and the median survival time of the observation group was significantly longer than that of the control group (P < 0.05). The epirubicin-paclitaxel regimen can significantly reduce the levels of tumor markers and cytokines in patients with good therapeutic effect.
Neoadjuvant chemotherapy for TNBC treatment can achieve good curative effects. Moreover, tumor-node-metastasis stage, differentiation degree, lymph node metastasis, karyopherin A2 and SRY-related HMG box-2 expressions, and treatment plan are prognostic factors of patients with TNBC.
Next, we will investigate the mechanism of neoadjuvant chemotherapy for TNBC.
| 1. | Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 1630] [Article Influence: 271.7] [Reference Citation Analysis (0)] |
| 2. | Kim C, Gao R, Sei E, Brandt R, Hartman J, Hatschek T, Crosetto N, Foukakis T, Navin NE. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell. 2018;173:879-893.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 772] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 3. | Medina MA, Oza G, Sharma A, Arriaga LG, Hernández Hernández JM, Rotello VM, Ramirez JT. Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 4. | Wang X, Wang SS, Huang H, Cai L, Zhao L, Peng RJ, Lin Y, Tang J, Zeng J, Zhang LH, Ke YL, Wang XM, Liu XM, Chen QJ, Zhang AQ, Xu F, Bi XW, Huang JJ, Li JB, Pang DM, Xue C, Shi YX, He ZY, Lin HX, An X, Xia W, Cao Y, Guo Y, Su YH, Hua X, Wang XY, Hong RX, Jiang KK, Song CG, Huang ZZ, Shi W, Zhong YY, Yuan ZY; South China Breast Cancer Group (SCBCG). Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial. JAMA. 2021;325:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 5. | Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:1194-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 1462] [Article Influence: 243.7] [Reference Citation Analysis (0)] |
| 6. | DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1518] [Article Influence: 126.5] [Reference Citation Analysis (0)] |
| 7. | Deepak KGK, Vempati R, Nagaraju GP, Dasari VR, S N, Rao DN, Malla RR. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol Res. 2020;153:104683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 434] [Article Influence: 72.3] [Reference Citation Analysis (2)] |
| 8. | Li X, Tan Q, Li H, Yang X. Predictive value of tumor-infiltrating lymphocytes for response to neoadjuvant chemotherapy and breast cancer prognosis. J Surg Oncol. 2021;123:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Poggio F, Bruzzone M, Ceppi M, Pondé NF, La Valle G, Del Mastro L, de Azambuja E, Lambertini M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29:1497-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (1)] |
| 10. | Jung YY, Hyun CL, Jin MS, Park IA, Chung YR, Shim B, Lee KH, Ryu HS. Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. J Breast Cancer. 2016;19:261-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Alqahtani FY, Aleanizy FS, El Tahir E, Alkahtani HM, AlQuadeib BT. Paclitaxel. Profiles Drug Subst Excip Relat Methodol. 2019;44:205-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Bai X, Ni J, Beretov J, Graham P, Li Y. Triple-negative breast cancer therapeutic resistance: Where is the Achilles' heel? Cancer Lett. 2021;497:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 13. | Bareche Y, Buisseret L, Gruosso T, Girard E, Venet D, Dupont F, Desmedt C, Larsimont D, Park M, Rothé F, Stagg J, Sotiriou C. Unraveling Triple-Negative Breast Cancer Tumor Microenvironment Heterogeneity: Towards an Optimized Treatment Approach. J Natl Cancer Inst. 2020;112:708-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 14. | Lev S. Targeted therapy and drug resistance in triple-negative breast cancer: the EGFR axis. Biochem Soc Trans. 2020;48:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Mo H, Xu B. Progress in systemic therapy for triple-negative breast cancer. Front Med. 2021;15:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, Shi Y, Zhao X, Jing J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Aksoy SO, Sevinc Aİ, Ünal M, Balci P, Görkem İB, Durak MG, Ozer O, Bekiş R, Emir B. Management of the axilla with sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer: A single-center study. Medicine (Baltimore). 2020;99:e23538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Armer JM, Ballman KV, McCall L, Ostby PL, Zagar E, Kuerer HM, Hunt KK, Boughey JC. Factors Associated With Lymphedema in Women With Node-Positive Breast Cancer Treated With Neoadjuvant Chemotherapy and Axillary Dissection. JAMA Surg. 2019;154:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Sutton EJ, Onishi N, Fehr DA, Dashevsky BZ, Sadinski M, Pinker K, Martinez DF, Brogi E, Braunstein L, Razavi P, El-Tamer M, Sacchini V, Deasy JO, Morris EA, Veeraraghavan H. A machine learning model that classifies breast cancer pathologic complete response on MRI post-neoadjuvant chemotherapy. Breast Cancer Res. 2020;22:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Li CH, Karantza V, Aktan G, Lala M. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res. 2019;21:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Antonov A, Italy; Wells JM, United States S-Editor: Wang JL L-Editor: A P-Editor: Wang JL