INTRODUCTION
Chronic liver disease (CLD) is the cause of more than two million deaths each year worldwide. Together with the heavy burden of disability and demand for medical care, this makes the problem extremely relevant. Liver fibrosis (LF) is an unfavorable event in the course of CLD. As CLD progresses, diffuse excess deposition and abnormal distribution of the extracellular matrix (ECM) leads to the formation of liver cirrhosis. The severity of its clinical manifestations is mainly associated with liver failure and portal hypertension as well as characteristic complications causing a high mortality[1].
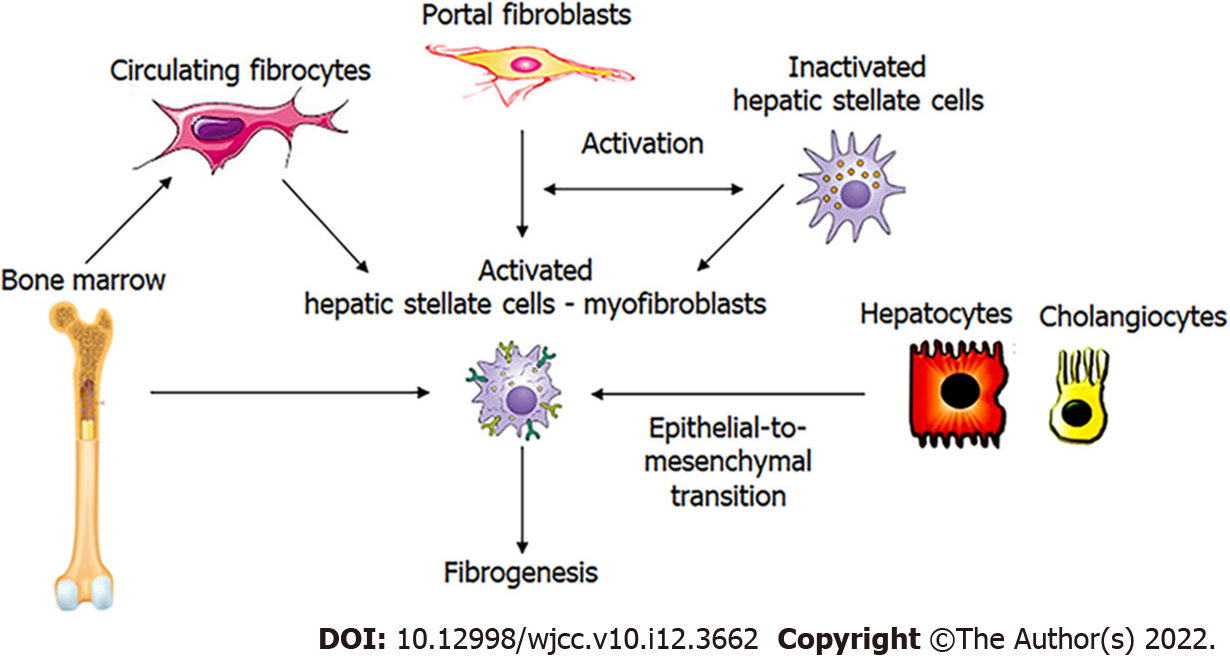
Hepatic stellate cells (HSCs) play a central role in the development of LF. Bone marrow-derived fibrocytes, epithelial-to-mesenchymal transition, and portal fibroblasts are other sources, but their contribution is much lower. Portal fibroblasts are important in the early stages of the disease, but HSCs are predominantly responsible for the progression of the disease (Figure 1). They are in a quiescent state under physiological conditions and serve as the main retinyl esters storage site in the body, which is visible as fat droplets in the cytoplasm. CLD generates a large panel of signals that stimulate the production of specific transcription factors and morphogens in quiescent HSCs. This initiates HSCs activation with the acquisition of pro-fibrogenic and pro-inflammatory properties[2].
Figure 1 Potential mediators of hepatic fibrogenesis.
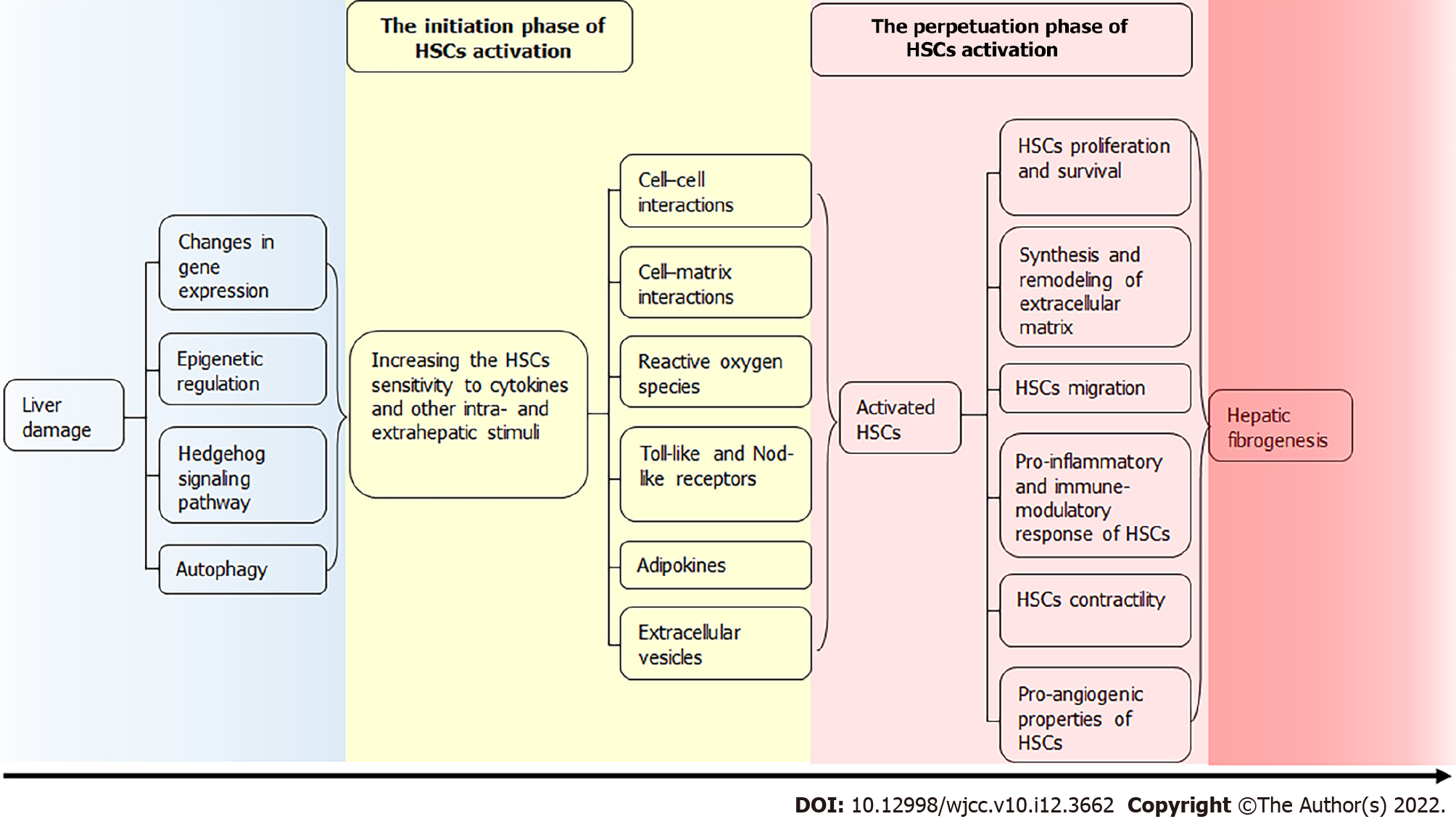
This review focuses on the pathophysiological mechanisms of HSCs activation in LF. They enter the cell cycle under the influence of various triggers. The “Initiation” phase of HSCs activation overlaps and continues with the “Perpetuation” phase, which is characterized by a pronounced inflammatory and fibrogenic reaction[3]. At this time, there are numerous changes in the transcription of genes of type I collagen, α-smooth muscle actin (α-SMA), transforming growth factor (TGF)-β1 and its receptors, matrix metalloproteinase (MMP)-2, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and 2. This is facilitated by transcription factors that are absent in quiescent HSCs, such as Ets-1, Mef2, CREB, Egr-1, JunD, vitamin D receptor, CCAAT/ enhancer-binding protein β (C/EBP-β), etc.[4].
CHANGES IN GENE EXPRESSION
The transformation of quiescent HSCs into an activated myofibroblast-like phenotype requires changes in the expression of several hundred different genes. Some of the transcription factors, which include the peroxisome proliferator-activated receptors (PPAR)γ, the retinoic acid receptors (RARs), retinoid X receptors (RXRs), the pregnane X receptor (PXR) and the LHX2 gene, control the quiescent phenotype of HSCs. On the contrary, Kruppel-like factor 6 (KLF6), Gα-interacting, vesicle-associated protein/Girdin (GIV/Girdin), and methyl-CpG-binding protein 2 (MеCP2) contribute to the transformation of quiescent HSCs into myofibroblasts.
The PPARs belong to the nuclear hormone receptor superfamily and are transcription factors that bind DNA and regulate transcription in a ligand-dependent manner. They control gene expression after binding with RXR as a heterodimeric partner to specific DNA sequence elements termed peroxisome proliferator response element. Three main isotypes of PPARs are the following: PPARα, PPARβ/δ, and PPARγ; each one of them has a distinct, tissue-specific pattern of expression. The results of numerous studies indicate the important role of PPARγ in the regulation of quiescent and inactivated phenotypes of HSCs[5]. PPARγ has a positive effect on hepatocyte growth factor (HGF), which may reduce HSCs activation and collagen secretion, and facilitate liver recovery after injury by disrupting TGF-β1 signaling[6]. In addition, PPARγ strongly stimulates HGF promoter activity and increases HGF mRNA in fibroblasts. It was noted that the expression of PPARγ is negatively controlled in HSCs via MeCP2-dependent and EZH2-dependent transcriptional repression of the PPARγ promoter. Moreover, ETS1-PPARγ signaling pathway positively regulates PPARγ expression in quiescent HSCs[7].
The RARs and the structurally similar RXRs include three subtypes, namely α, β, and γ. Except for RXRγ, all subtypes of RARs/RXRs are well expressed in quiescent HSCs. Their expression decreases when the HSCs activate. The mechanism of this has not yet been established. Moreover, the results of various studies contradict each other[8].
The PXR is an orphan nuclear receptor (NR1I2) that has been shown to regulate the drug-induced and corticosteroid-induced expression of specific genes in hepatocytes (e.g., CYP3A subfamily genes; where CYP is cytochrome P450). The PXR is transcriptionally functional in human HSCs and can bind its activators: pregnane, bile acids, and drug ligands such as rifampicin, hyperforin, lovastatin, clotrimazole and metyrapone. As a result, it inhibits HSCs transdifferentiation and proliferation. In addition, the PXR activation suppresses the pro-fibrogenic effect of TGF-β1[9].
The LIM domain is recognized as one of the key components of the cell regulatory system, and the LIM homeobox gene 2 (Lhx2) functions in HSCs to preserve their quiescent phenotype. It was shown that the increased expression of Lhx2 reduced the levels of type I collagen and α-SMA, and its absence blocked the production of platelet-derived growth factor (PDGF), MMP-2, 3, TIMP-1, 2 and prolyl-4-hydroxylase[10].
The KLFs are a family of zinc-finger transcription factors that regulate diverse processes such as cell proliferation, differentiation, development, regeneration and programmed cell death. The induction of KLF6 is noticeably enhanced with the beginning HSCs activation, which is accompanied by the transcription of type I collagen and TGF-β1 and stimulates some other components of this pathway, including the TGF-β type I and type II receptors (TβRI and TβRII) and the urokinase-type plasminogen activator (uPA)[11]. The KLF6 reduces the fibrogenic activity of HSCs via two distinct mechanisms: direct transcriptional repression of target profibrotic genes and increased apoptosis of activated HSCs. Following its initial induction, sustained downregulation of KLF6 in liver injury may allow de-repression of profibrotic genes and decreased HSCs clearance by inhibiting apoptosis[12].
The multidomain signaling molecule GIV/Girdin that enhances PI3K-Akt signals downstream of both G protein-coupled and growth factor receptors can be also a key regulator of HSCs activation. GIV/Girdin is located at the convergence point of several intracellular signaling pathways that regulate hepatic fibrogenesis, including PI3K-Akt-FOXO1, TGFß1-SMAD, and cAMP-PKA-pCREB[13].
The MеCP2, which binds to methylated regions of DNA to regulate transcription and participates in hepatic fibrogenesis, is expressed by HSCs. The MеCP2 helps orchestrate widespread changes in the RNA landscape that convert the quiescent HSC into fibrogenic cells and is required for genes expression that regulates entry and progression of HSC DNA replication[14].
EPIGENETIC REGULATION
It is now well-documented that epigenetic mechanisms including DNA methylation, histone modifications and non-coding RNAs (ncRNAs) orchestrate many aspects of hepatic fibrogenesis[15].
In particular, aberrant DNA methylation patterns are associated with inappropriate gene repression and the development of LF. During transdifferentiation of quiescent HSCs into myofibroblasts, HSCs express MeCP2, which can repress transcription from the promoters of methylated genes, mediating the epigenetic silence of the PPARγ gene. With the participation of histone methyltransferases EZH2 and ASH1, MeCP2 functions as a key epigenetic regulator of HSCs transdifferentiation. EZH2 is induced at the protein level in its initial stage and is recruited to the PPARγ gene causing the accumulation of the repressive signature of H3K27me3 chromatin. This is necessary for reprogramming of the quiescent HSCs transcriptome to the myofibroblast phenotype. In parallel, ASH1 is recruited to the promoter regions of the α-SMA, type I collagen (α1), TIMP-1, and TGF-β1 genes, contributing to the active state of transcription[16].
In addition, the HSCs transdifferentiation is accompanied by a change in the expression levels of DNA methylation and hydroxymethylation regulatory enzymes. For example, it was noted that the presence of LF was associated with an increase in the expression levels of DNA methyltransferases (DNMT1, DNMT3a, DNMT3b), a decrease in the expression of Ten-eleven translocation methylcytosine dioxygenase (TET) family enzymes (TET1, 2 and 3), as well as the TET-regulated conversion of 5-methylcytosine to 5-hydroxymethylcytosine, which can lead to the activation of transcription or an increase in its elongation[17].
Several studies have revealed the regulatory role of histone modification in liver damage. Most of them are associated with changes in histone acetylation as a result of pharmacological use of histone deacetylase (HDAC) inhibitors, for example, class I and II. The mechanism of an HDAC-1 influence on myofibroblasts can be explained, in particular, by anti-inflammatory and anti-fibrogenic effects due to HDAC-1 recruitment to the pro-inflammatory genes including Ccl2, Cxcl10, Gm-CSF and Mmp-13[18]. It can also influence histone acetylation through bromodomain inhibition. It was shown that JQ1, which is a small molecule inhibitor of bromodomain-containing protein 4 (BRD4), prevented liver damage and reversed or limited the progression of existing LF due to the abrogate cytokine-induced HSCs activation[19].
The cellular epigenetic landscape in LF can be also regulated by numerous ncRNAs, which are functional RNA transcripts that regulate gene expression at the level of transcription, processing and post-transcription. They are not involved in protein coding but interact in gene expression[20].
One of the most studied ncRNAs is microRNA (miRNAs). In particular, miR-21 causes HSCs activation by binding several transcripts, including PDCD4, SMAD7 and PTEN. Targeting PDCD4, in this case, leads to a vicious circle, when the enhanced production of miR-21 in HSCs inhibits the expression of the antifibrotic genes SMAD7, PTEN, SPRY2, and NHF4A, which in turn contributes to HSCs activation. Numerous miRNAs, including miR-33a, 34a, 34c, 130a and 130b, regulate the function of PPARγ in activated HSCs. miR-130a и 130b enhance HSCs activation by suppressing PPARγ expression by targeting the 3'-UTR of PPARγ mRNA, as well as an increase in the expression of profibrotic genes ACTA2, COL1A1 and TIMP-1. Several miRNAs are directly responsible for the stability of genes encoding ECM proteins and ECM processing enzymes. For example, miR-29a and miR-29b destabilize COL1A1 mRNA. MiR29b also inhibits the maturation of collagen by binding heat shock protein (HSP) 47 mRNA and lysyl oxidase with further SMAD3-mediated HSCs activation. miR-122 affects the enzyme P4HA involved in collagen processing. miR-122 also blocks the expression of COL1A1 and ACTA 2 by inhibiting serum response factor, a transcription factor that controls fibrogenic cells activation. In addition, miRNAs are involved in HSCs proliferation and migration through a variety of signal transduction pathways. For example, miR-19b, whose expression is reduced both in liver tissue and in serum of patients with LF, negatively regulates TGF-β1 signaling and inhibits HSCs proliferation by suppressing the GRB2 adapter protein. miR-146a inhibits HSCs activation and proliferation by directly affecting genes Wnt1, Wnt5a and SMAD4. miR-195 blocks HSCs proliferation due to the binding of cyclin E1. miR-155, whose expression is reduced in cirrhotic liver tissue, regulates ERK1 signaling and promotes epithelial-mesenchymal transit[21].
Many studies have investigated the role of long ncRNAs (lncRNAs) in HSCs activation. For example, the lncRNA PVT1 gene contributes to this by competitively binding the inhibitory miR-152. On the contrary, the lncRNA p21, which expression decreases in liver cirrhosis, competitively binds miR-181b that regulates p27 protein and PTEN expression. The lncRNA p21 and miR-181b binding suppresses HSCs proliferation and ACTA2 and COL1A1 expression. The lncRNA GAS 5 gene suppress fibrogenesis as a result of the competitive binding of miR222. The lncRNA MAG 3 gene, which expression decreases in LF, negatively regulates the expression of ACTA2 and COL1A1 and blocks HSCs proliferation[22]. The expression of lncRNA Hotair (Hox transcript antisense intergenic RNA) was significantly increased in CCl4-induced mouse LF models, human fibrotic livers and activated HSCs by TGF-β1 stimulation[23].
The expression of lncRNA NEAT1 (nuclear paraspeckle assembly transcript 1) was significantly increased in CCl4-induced mice and activated HSCs. Loss of lncRNA NEAT1 suppressed LF in vivo and in vitro, and overexpression via the miR-122-KLF6 axis accelerated HSC activation, including increased cell proliferation and collagen expression. In addition, in human fibrotic liver samples, increased lncRNA NEAT1 levels positively correlated with LF markers[24].
Thus, increased levels of DNMT, TET3 protein of the TET family enzymes, and MеCP2 contribute to HSCs activation and transdifferentiation. Histone methyltransferase ASH1 induces the expression of profibrotic genes, including COl1A1, ACTA2 (α-SMA) and TIMP-1, which leads to ECM accumulation. A significant role in the cellular epigenetic landscape in LF is also played by ncRNAs, especially miRNAs and lncRNAs, that modulate gene expression at post-transcriptional and transcriptional levels.
HEDGEHOG SIGNALING PATHWAY
Hedgehog (Hh) signaling pathway regulates cell proliferation, apoptosis, migration and differentiation. In addition to its important role in tissue morphogenesis during fetal development, it also modulates the regenerative/repair process, for example, in CLD. At this time, most cell populations, including hepatocytes, cholangiocytes, sinusoidal endothelial cells (SECs), HSCs and natural killer T cells (NKT cells) express Hh ligands under the influence of cytotoxic or proapoptotic stress[25]. Simultaneously, activated HSCs and SECs suppress Hh-interacting proteins, providing ligand-receptor communication and stimulating the Hh signaling pathway in Hh-sensitive cells, such as NKT cells, cholangiocytes, oval cells and quiescent HSCs. Hepatic overexpression of Hh ligands occurs in parallel with an increase in the number of Hh-sensitive cells and depends on the degree of its damage and the severity of LF.
Paracrine stimulation of the Hh signaling pathway in HSCs promotes and/or supports LF in several ways. For example, Hh ligands directly lead to HSCs activation and trans differentiation, promote HSCs proliferation and enhance the ability to synthesize and release components of the ECM. The expansion of HSCs causes an increase in critical pro-fibrogenic factors produced by them in the autocrine-paracrine loop (mainly PDGF and TGF-β1), which can also exacerbate events associated with the Hh signaling pathway, stimulating further production of Hh ligands.
Therefore, the temporary activation of the Hh signaling pathway is necessary for liver recovery after acute damage to some extent. However, in the conditions of constant exposure to an aggressive agent, a steady increase in the Hh signal transduction can preserve the expansion and fibrogenic activity of critical cell types, mainly HSCs/myofibroblasts[26].
AUTOPHAGY
The role of autophagy in hepatic fibrogenesis is complex and specific for each cell type. For example, autophagy in HSCs and reactive ductal cells has a pro-fibrogenic effect. On the contrary, autophagy in hepatocytes, macrophages and SECs counteract it[27].
Upon HSCs activation, autophagy is a strictly regulated process that preserves their energy homeostasis through retinyl esters hydrolysis with the formation of fatty acids. It can be part of a broader metabolic reprogramming reaction which is facilitated by the Hh signaling pathway, the liver X receptor (LXR), a member of the Rev-ErbA family of nuclear receptors Rev-erbα, which, along with PPARγ, is especially important for preserving the adipogenic phenotype of HSCs[28].
Autophagy provides the energy that is required to support HSCs transdifferentiation through the mobilization and breakdown of lipid droplets and mitochondrial β-oxidation. This may be associated with guanine nucleotide-binding α-subunit12 (Gα12) overexpression induced by miR-16 dysregulation[29]. The protein p62, an autophagy-associated factor, is a negative regulator of liver inflammation and fibrosis through its ability to promote vitamin D receptor signaling in HSCs[30]. Hypoxia-inducible factor (HIF)-1α[31], TGF-β1[32], as well as the non-histone nuclear protein HMGB1 (High Mobility Group Box Protein 1)[33] can regulate autophagy in activated HSCs.
INITIATION PHASE OF HSCs ACTIVATION
At the early, so-called “pre-inflammatory stage” of liver damage, primary changes of gene expression and the phenotype of HSCs render them sensitive to cytokines and other intra- and extrahepatic stimuli. Some of them may be the result of a complex interplay between resident liver cells, infiltrating inflammatory cells, several locally acting signals and interactions between the ECM and cells.
Cell-cell interactions
Clinical data and animal models suggest that parenchymal epithelial cell death is the key trigger of CLD progression represented by the subsequent development of inflammation, fibrosis, cirrhosis and hepatocellular carcinoma. Different modes of cell death such as apoptosis, necrosis and necroptosis trigger specific cell death responses and promote CLD progression through distinct mechanisms[34]. Engulfment of apoptotic bodies by HSCs stimulates their fibrogenic activity and may be one of the mechanisms by which hepatocyte apoptosis promotes LF[35]. The HSCs activation, in this case, may be due to the interaction of Toll-like receptor (TLR)-9 located on their surface with the DNA of hepatocytes[36].
Damaged hepatocytes produce interleukin (IL)-33, which stimulates and accumulates type 2 innate lymphoid cells (ILC2) via ST2-dependent signaling. Activated hepatic ILC2 produces IL-13, which in turn triggers HSCs activation and transdifferentiation in an IL-4Rα- and STAT6 transcription factor-dependent fashion[37].
Dead or dying epithelial cells and their phagocytic leukocytes release inflammatory mediators, such as TNF-α, IL-1β, IL-6, reactive oxygen species (ROS), Hh ligands, and nucleotides, which initiate and preserve a non-infectious “sterile” inflammatory response promoting HSCs activation[38].
It was shown that LY6Chi monocyte-derived macrophages can secrete pro-fibrogenic mediators, for example, TGF-β1, PDGF, CC chemokines (CCL2, CCL3, CCL5, CCL7, and CCL8) through the C-C chemokine receptor 2 (CCR2). In contrast, LY6Clo macrophages are a fibrinolytic subset that expands during LF regression[39].
CD4(+) T-helper cells, including Th cells (Th1, Th2, Th17) and regulatory (Treg) cells, play an important role in inflammation and progression of LF. For example, Th2-type responses are thought to promote alternative activation of macrophages (M2) via IL-4 and IL-13, activate HSCs, and induce their differentiation into myofibroblasts, leading to a pro-fibrogenic response[40]. IL-17 signaling in inflammatory cells, Kupffer cells and HSCs has a strong pro-fibrogenic effect through two independent mechanisms. First, IL-17 stimulates Kupffer cells to express inflammatory cytokines IL-6, IL-1β and TNF-α, as well as the major fibrogenic cytokine TGF-β1. Second, IL-17 directly stimulates HSCs to express type I collagen and promotes their activation via STAT3[41]. Intrahepatic IL-8-producing Foxp3+CD4(+) regulatory T cells activate HSCs and promote hepatic fibrogenesis in chronic hepatitis C[42]. In addition, LF occurs because of dysregulation of MyD88-dependent innate B-cell activity[43].
NK cells kill activated HSCs via retinoic acid early inducible 1/NKG2D-dependent and TNF-related apoptosis-inducing ligand-dependent mechanisms, thereby ameliorating LF[44]. IL-15 promotes preserving NK homeostasis[45], whereas CD4(+) regulatory T cells can inhibit NK cells and thereby support the survival of activated HSCs[46].
The SECs in the normal liver maintain the quiescent phenotype of HSCs and thus inhibit hepatic fibrogenesis. However, in response to liver damage, as a result of the production of fibronectin, TGF-β1 and PDGF contribute to HSCs activation[47].
Cell–matrix interactions
Progressive deposition of ECM proteins in the subendothelial Disse spaces leads to increased density and stiffness of ECM. The composition of ECM shifts from type IV collagen, heparan sulfate proteoglycan and laminin to type I and III fibrillar collagen. These disorders serve as mechanical stimuli for HSCs activation. In particular, positive feedback loops are formed through integrin-mediated signal transduction pathways which modulate hepatic fibrogenesis through the production of cytokines such as TGF-β1, PDGF, TNF-α, HGF, connective tissue growth factor (CTGF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF)[48].
A secreted phosphoprotein 1 called osteopontin is one of the cells signaling molecules associated with cell injury and LF. It serves as an important cytokine of the ECM protein network[49]. Several possible mechanisms of the involvement of osteopontin in hepatic fibrogenesis have been proposed. Pritchett et al[50] demonstrated that the transcription factor SOX9 (sex-determining region Y-box 9) is expressed on activated HSCs and is responsible for the stimulation of osteopontin production. Chen et al[51] revealed that hepatic expression of osteopontin was increased in patients with LF. In addition, hepatic osteopontin positively correlated with α-SMA and type I fibrillar collagen. Recombinant osteopontin upregulated α-SMA and type I fibrillar collagen expression in LX-2 cells via miR-129-5p suppression. The increased expression of osteopontin during hepatic fibrogenesis induces non-histone nuclear protein HMGB1, which acts as a downstream alarmin driving type I collagen synthesis in HSCs[52]. In addition, the coordinating effect of osteopontin on the pro-fibrogenic cytokine TGF-β1 is assumed[53].
The biological function of osteopontin may be also associated with its degradation products. For example, there was a positive correlation between the severity of LF and thrombin-cleaved osteopontin levels, which has an increased ability to stimulate HSCs activation, proliferation and migration compared to full-length osteopontin. Thrombin-cleaved osteopontin activated HSCs by α9 and α4 integrins that were highly expressed in LF[54].
Reactive oxygen species
ROS are thought to be the major chemicals responsible for the alteration of macromolecules, namely oxidative stress. They can activate HSCs and stimulate the progression of LF[55]. ROS may originate from hepatocytes, macrophages, cholangiocytes, inflammatory cells, and also be produced by HSCs in response to such pro-fibrogenic mediators as PDGF, TGF-β1, leptin and angiotensin Ⅱ. ROS are generated at the cellular level as a result of mitochondrial damage through the mitochondrial electron transport chain or the induction of cytochrome P450 (especially cytochrome P450 2E1), xanthine oxidase and NADPH oxidase[56]. Homologs of NADPH oxidase (NOX 1, NX2, NX4) can contribute to HSCs, Kupffer cells, and macrophages activation by inducing oxidative stress and, therefore, promote hepatic fibrogenesis[57]. Since ROS can stimulate signal transduction pathways and transcription factors, including JNK and NF-kB, they also may enhance the expression of profibrotic genes, such as COL1A1, COL1A2, MCP-1, and TIMP-1, in HSCs[58].
Toll-like receptors and Nod-like receptors
It has been established that the Toll-like receptors (TLRs) family can recognize and specifically bind unique bacterial products, launching an inflammatory signaling cascade and the mechanisms for this bacterial products clearance. It is believed that TLR-4 and TLR-9 are receptors for lipopolysaccharide (LPS) and bacterial DNA respectively, i.e. two of the most immunogenic bacterial products[59].
TLR4 ligands modulate hepatic fibrogenesis through both Kupffer cells and HSCs. The HSCs are the main targets that control this process. TLR4 mediates HSCs activation through two TGF-β–dependent mechanisms, namely the increased exposure to Kupffer cell-derived TGF-β and enhanced sensitivity to TGF-β[60].
Additionally, to LPS, there are also endogenous substrates for TLR4 that bind and activate TLR4. These include low molecular weight hyaluronic acid, free fatty acids, fibrinogen, fibronectin, HSPs 60 and 70, and non-histone nuclear protein HMGB1, etc. For example, in vitro studies indicate that HMGB1 stimulates HSCs proliferation and α-SMA expression. In vivo, damage signals and intact ECM degradation also activate TLR4[61].
Most of these endogenous ligands are released and/or increased during tissue injury and matrix degradation and are collectively referred to as damage-associated molecular patterns (DAMPs). Release of DAMPs into the extracellular space is achieved by several mechanisms, including leakage from necrotic cells, increased synthesis and post-translational modification in response to inflammation, and degradation of inactive precursors into TLR-mimetic degradation products in inflammatory environments. DAMPs mediate non-sterile inflammation by activating TLR4 signaling[62].
Extracellular histones are the most abundant DAMPs. These are well-conserved proteins that are essential for DNA packaging and gene regulation. During tissue damage and cell death, nuclear chromatin is cleaved into nucleosomes that are released extracellularly and further degraded into individual histones. Although histones are rapidly cleared by the liver and rarely detected in blood, liver cell death leads to the local release of histones, which stimulate adjacent cells, including HSCs. Histones can activate TLR2, TLR4 and TLR9 in the early stage of chronic liver injury and hepatic fibrogenesis[63].
It was shown that the specificity of TLR9 for viral and bacterial DNA is not absolute. The concept of apoptotic mammalian DNA activating TLR9 on HSCs has experimental support from immunological studies. According to this, B cells and plasmacytoid dendritic cells are activated by apoptotic DNA via TLR9. Furthermore, Watanabe et al[64] have demonstrated that DNA from apoptotic hepatocytes induces TLR9-dependent changes consistent with the late stages of HSCs differentiation, with up-regulation of ECM components, changes in HSCs morphology and inhibition of PDGF-mediated chemotaxis.
Pathogen-associated molecular patterns (PAMP) are molecules with conserved motifs that are associated with pathogen infection that serve as ligands for host pattern recognition molecules such as TLRs[65]. The interaction between PAMP and TLRs activate intracellular molecular pathways, either MyD88-dependent or MyD88-independent, resulting in the NF-κB activation pathway and the expression of inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-12, IL-18), chemokines (CXCL1, CXCL2, CCL2, CCL5, CCL3, CCL4), vasoactive factors [nitric oxide (NO)] and ROS. This local inflammatory storm leads to the recruitment of systemic leukocytes, such as neutrophils, CD4(+) T cells and monocytes, that perpetuate liver inflammation. Net result of this process is the induction of hepatocyte apoptosis and necrosis. Both inflammatory cytokines and cell death cause HSCs activation and proliferation as well as the development of LF under TGF-β1 stimulation[66].
Nod-like receptors (NLRs) are intracellular pattern recognition receptors that activate downstream signaling cascades leading to activation of inflammasome components. NLRs include positive regulators of inflammation, such as the NOD-LRR and pyrin domain-containing protein 3 (NLRP3), and anti-inflammatory ones such as NLRX1 and NLRP12. Upon activation, the majority of NLRs oligomerize to form multiprotein inflammasome complexes, which are high-molecular-weight cytosolic multiprotein complexes in the cytosol of host cells that regulate pathogen recognition, host defense, and inflammatory processes[67]. NLRP3 is the most studied inflammasome to date. NLRP3 becomes activated after interaction with caspase-1. Upon activation, caspase-1 promotes the maturation of the pro-inflammatory cytokines IL-1β and IL-18 following cleavage of their precursors, such as Pro-IL-1β and Pro-IL-18[68].
NLRP3 inflammasome components, which are expressed in HSCs, have been identified as essential for inflammatory and fibrotic rearrangements[69]. It was demonstrated that activation of the NLRP3-inflammasome pathway was associated with the IL-1R/MyD88 signaling in the development of experimental LF[70]. In addition, it was noted that hepatocyte pyroptosis leads to the release of NLRP3 inflammasome particles from inside hepatocytes into the extracellular space. Then they are taken up by other cells, e.g., HSCs, and thereby mediate pro-inflammatory and pro-fibrogenic stress signals[71].
Adipokines
Some adipokines also have pro-fibrogenic activity. In particular, the product of the obesity gene (ob) leptin can directly affect the HSCs, Kupffer cells and SECs. Its pro-fibrogenic role is to transmit signals by activating the leptin receptor (Ob-Rb) and the JAK-STAT signaling pathway of this receptor. The expression of Ob-Rb in quiescent HSCs is quite low and increases during their transdifferentiation into an activated myofibroblast-like phenotype, which occurs with the participation of the Wnt/β-catenin signaling pathway. This causes HSCs proliferation, prevents apoptosis, mediates collagen secretion and increases the production of pro-inflammatory cytokines in the liver. Leptin can induce HSCs phagocytosis, activate macrophages and stimulate SECs to produce TGF-β1. Its pro-fibrogenic properties mainly depend on NADPH oxidase and ROS and can be also mediated by suppression of PPARγ expression and a decrease in the expression levels of sterol regulatory element-binding protein-1c (SREBP-1c). In addition, leptin mediates HSCs activation and promotes LF through indirect effects on Kupffer cells. These effects are partly mediated by TGF-β1[72].
Apelin is an endogenous peptide identified as a ligand of the G protein-coupled receptor APJ. Apelin mediates some of the fibrogenic effects triggered by angiotensin II and endothelin 1 (ET-1), which contribute to HSCs activation occurring in LF. Apelin has also demonstrated a significant potential to promote LF in vitro. It acts directly on LX-2 cells through ERK signaling, stimulating cell survival and synthesis of PDGF-β receptor (PDGFβR) and type I collagen in these cells. In turn, PDGFβR and LPS can stimulate APJ expression, expanding and perpetuating HSCs activation. Therefore, apelin may, in theory, induce HSCs to a pro-fibrogenic profile and prolong its stimulation autocrinally during all stages of LF in CLD[73].
Extracellular vesicles
Extracellular vesicles are membrane fragments that can be produced by all cell types. Their influence on the HSCs depends on the specific pathological situation. For example, extracellular vesicles released by hepatocytes carry miR-128-3p, a known inhibitor of PPARγ expression. The transfer of this miR-128-3p to HSCs is central for the PPARγ inhibition and HSCs activation. miR-192 has a similar effect. SECs-derived extracellular vesicles induce HSCs migration through the S1P pathway activation and transfer of pro-fibrogenic growth factors such as CTGF.
HSCs are also able to produce extracellular vesicles, thereby amplifying the fibrosis through action on HSCs or other non-parenchymal cells. It was noted that primary and lineage HSCs (LX-2 cells) sequestrated PDGF-α receptor (PDGFRα) into extracellular vesicles after cell incubation with its ligand PDGF-BB, a cytokine implicated in LF. Such extracellular vesicles promoted HSCs migration in vitro but also LF in vivo and were isolated from the blood of patients with LF. Activation of HSCs (LX-2 cells) in vitro with TGF-β reduced the production of extracellular vesicles containing miR-30a, an inhibitor of LF, which is acting through the autophagy pathway[74].
It is also assumed that the activation-associated changes in production, function and protein content of extracellular vesicles from HSCs contribute to the regulation of HSCs function in vivo and the fine-tuning of fibrogenic pathways in the liver[75].
PERPETUATION PHASE OF HSCs ACTIVATION
In the perpetuation phase of HSCs activation, discrete changes occur in their behavior which is manifested by proliferation and survival, synthesis and remodeling of ECM, migration in response to chemoattractants and ROS, pro-inflammatory and immune-modulatory response, contractility and pro-angiogenic properties[76].
HSCs proliferation and survival
During the progression of CLD activated HSCs acquire well-expressed proliferative properties, which is a result of the high availability of pro-fibrogenic growth factors released by neighboring cells and increased expression of related receptors. It is believed that the most powerful mitogen for HSCs is PDGF, in particular the PDGF-BB isoform that binds to specific PDGFαR or PDGFβR receptors, which expression is stimulated by TGF-β1. In addition, TGF-α, EGF, CTGF, bFGF, thrombin, ET-1, keratinocyte growth factor (also known as FGF7) and leptin have been described to have this capacity. Interacting with related receptors, most of them transmit proliferative stimuli mainly through the ERK1/2 signaling pathway and recruitment of adaptive proteins to intracellular domains of the different receptors involved. The mitogenic stimuli described for activated HSCs, in addition to TGF-β1 and possibly other mediators, act as significant survival factors in the potentially hostile pro-fibrogenic environment of on-going CLDs[77].
Synthesis and remodeling of ECM
Stably activated HSCs can increase ECM synthesis and deposition because of a disorder in the regulation of genes responsible for ECM remodeling. This is aggravated by the concomitant ineffective removal of excess fibrillar collagen by MMP and increased expression of TIMP. Type I collagen and CTGF are typical TGF-β1-dependent target genes that are activated through the canonical pathway (involving TβRI and TβRII receptors containing serine/threonine kinase domains, phosphorylation of SMAD2 and SMAD3, the interaction of SMAD2/3 with SMAD4 to form a transcriptional complex). It is believed that the canonical pathway, in addition to enhanced synthesis and remodeling of ECM, contributes to the activation/transdifferentiation of progenitor cells into activated myofibroblasts. However, TGF-β1 is also able to act through non-canonical or SMAD-independent pathways that include small GTPases (RhoA and Cdc42) leading to the MAPK signaling pathways activation, in particular, ERK1/2, JNK and p38. It was reported that the ERK1/2 signaling pathway activates type I collagen and CTGF synthesis. In addition to TGF-β1, at least CTGF, FGF and leptin, as well as some other growth factors, peptide ligands and signaling pathways can support this specific response of activated myofibroblasts. Furthermore, the literature data indicate the participation of ROS and mediators associated with oxidative stress in these and other phenotypic responses of myofibroblasts. ROS involvement follows activation of NADPH-oxidase isoforms occurring in parallel with the interaction of growth factors, cytokines and other active peptides with their respective receptors. This is important since ROS can activate MAPK signaling pathways in myofibroblasts[78].
HSCs migration
The ability of activated HSCs to migrate is associated with the response to polypeptide chemoattractants released by activated macrophages and HSCs themselves, as well as by surrounding hepatocytes, SECs, platelets and other immune cells or cells trapped in the ECM. The most potent chemoattractant is PDGF. Chemokine CCL2, angiotensin II and VEGF-A are also important. The chemotaxis caused by them requires the involvement of ligand/receptor-related and NADPH-oxidase-dependent increases in intracellular ROS levels, which in turn leads to the activation of the ERK1/2 and JNK1/2 signaling pathways and increased HSCs migration[79].
Although adenosine blunts chemotaxis and fixes HSCs at the sites of damage through the loss of actin fibers, it enhances signal transduction and can stimulate hepatic fibrogenesis[80].
Pro-inflammatory and immune-modulatory response of HSCs
The ability of activated HSCs to express receptors of certain cytokines and other inflammatory mediators, synthesize and release chemokines CCL2, CCL21 and cytokine IL-1β following activation of the NLRP3 inflammasome, is extremely important. In the pro-fibrogenic scenario of chronic liver injury, activated HSCs can then actively contribute either to perpetuate inflammatory response as well as to regulate and/or modulate interactions with cells of innate and adaptive immunity[81].
HSCs contractility
Activated HSCs and their paracrine interaction with SECs play an important role in hepatic sinusoidal microcirculation in LF[82]. Activated HSCs begin to perform the functions of pericytes. This is confirmed by the expression of its phenotypic markers such as α-SMA, desmin, NG2 and glial fibrillary acidic protein, as well as emergence or increase of receptors for growth factors, cytokines and ET-1, and many cell adhesion molecules on its surface[83].
HSCs located in the subendothelial Disse spaces between SECs and hepatocytes are in contact due to the long branching cytoplasmic processes with nerve endings that contain various neurotransmitters such as substance P, vasoactive intestinal peptide, somatostatin, cholecystokinin, neurotensin, NO, calcitonin gene related peptide and neuropeptide Y. Some vasoactive substances can regulate HSCs contractility. For example, ET-1, substance P, angiotensin Ⅱ, norepinephrine, prostaglandin F2, thromboxane A2, platelet-activating factor (PAF) and thrombin can trigger HSCs contraction. In contrast, acetylcholine, vasoactive intestinal peptide, NO, carbon monoxide, hydrogen sulfide, prostaglandin E2 and adrenomedullin are known for the ability to relax HSCs[84].
Myosin Ⅱ is involved in HSCs contractility, and Ca2+dependent and Ca2+-independent pathways mediate this process. In a Ca2+-dependent pathway, myosin light chains are phosphorylated by activated myosin light chain kinase, which activation is induced in response to an increase in intracellular Ca2+ concentration [(Са2+)i] and subsequent formation of the Ca2+/calmodulin complex. In a Ca2+independent pathway, Rho kinase and protein kinase C inhibit the activity of myosin light chain phosphatase, an enzyme that dephosphorylates phosphorylated myosin light chains and induces relaxation[85].
ET is a powerful endogenous vasoconstrictor that modulates HSCs contraction. ET1 is the most studied. The main site of its synthesis in LF is activated HSCs. Stimulation of endothelin A receptors leads to its proliferation[86], whereas the farnesoid receptor X (FXR) counteracts this[87].
Angiotensin Ⅱ has a similar effect. In LF, activated HSCs increase its synthesis because of increased expression of angiotensin converting enzyme. HSCs constriction may be also caused by decreased NO production and/or bioavailability in the fibrotic liver[88]. In contrast, carbon monoxide overproduction by Kupffer cells causes a dilation of the sinusoids and a decrease in hepatic vascular resistance because of paracrine impact on HSCs and SECs[89].
Pro-angiogenic properties of HSCs
There are two main ways for new blood vessel formation in LF. On the one hand, hypoxia caused by HIF1α stimulation activates the HSCs and leads to the production of various angiogenic and fibrogenic factors (placental growth factor (PIGF), VEGF, NO, HGF, PDGF), promoting angiogenesis and progression of LF. On the other hand, diffuse fibrosis, regenerative liver nodules and sinusoidal capillarization cause an increase of hepatic vascular resistance and impair the oxygen supply to the liver cells[90].
Accumulation of HIFs, in particular, HIF1α, increases the VEGF, angiopoietin1, and the expression of their receptors on activated HSCs. This leads to the involvement and stimulation of SECs and stabilization of the newly formed vessels. In turn, SECs generate PDGF and TGFβ, participating in the attraction and migration of HSCs[91].
Activated HSCs exhibit their proangiogenic properties in response to several stimuli either in a hypoxia-dependent or hypoxia-independent manner. On the one hand, they are sensitive to hypoxia and act as pro-angiogenic cells, reacting to hypoxia in a HIF1α-dependent manner by increasing VEGF, angiopoietin1 and their VEGFR-2 and Tie-2 receptors[92]. On the other hand, activated HSCs can demonstrate their pro-angiogenic role in a hypoxia-independent manner, responding to pro-fibrogenic polypeptide mediators such as PDGF and leptin. The angiogenic phenotype of HSCs stimulated by PDGF regulates the formation of vascular tubules, increases the caliber of sinusoids and blood flow through them. In addition, HSCs together with PDGF contribute to the modulation and dynamics of the hepatic microvascular structure[93]. Leptin can directly stimulate VEGF, angiopoietin1 and MCP-1 or CCL2 in human HSCs[94].
The cross-interaction between HSCs and other liver cells, especially SECs and macrophages, plays a crucial role in angiogenesis. Indeed, during persistent liver damage, SECs acquire a pro-vasoconstrictor, pro-inflammatory, pro-angiogenic and profibrotic phenotype. The loss of endothelial fenestration leads to a disorder of oxygen metabolism, the development of hypoxia and the accumulation of HIF. These disturbances stimulate the production of angiogenic growth factors, including VEGF, PDGF, FGF, angiopoietins and the formation of new blood vessels. In addition, SECs contribute to HSCs activation through the secretion of PDGF, TGF-β, etc.[95].
Activated HSCs recruit macrophages through chemokine CCL2. In turn, infiltrating macrophages additionally activate HSCs. Moreover, monocyte-derived C-C chemokine receptor 2 (CCR2)+ macrophages are responsible for angiogenesis in LF[96]. During the initiation and progression of LF, activated HSCs express various angiogenesis-stimulating chemokines, in particular, CC (CCL2, CCL3, CCL5) and CXC (CXCL8, CXCL9, CXCL10, CXCL12). The CXC chemokines containing the ELR motif (ELR+) contribute to the induction of angiogenesis, and those lacking this motif (ELR-) inhibit it[97].
CONCLUSION
It is obvious that LF is a complex pathological process controlled by a variety of cells, mediators and signaling pathways, and HSCs play a central role in its development. In CLD, they undergo dramatic phenotypic activation with the acquisition of fibrogenic properties (Figure 2). Knowledge of these pathophysiological mechanisms paved the way for drugs aimed at preventing the development and progression of liver fibrosis. This is highly important, since the formation of liver cirrhosis is an unfavorable event in the natural history of CLD and serves as one of the leading causes of death in adults worldwide. Currently, the only radical approach to treat liver cirrhosis is liver transplantation which can be performed in only a limited number of countries.
Figure 2 Phases of hepatic stellate cells fibrogenic activation.
HSCs: Hepatic stellate cells.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ullah K, Pakistan S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ