Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3593
Peer-review started: December 6, 2021
First decision: January 18, 2022
Revised: January 26, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 122 Days and 22.2 Hours
Apatinib is an orally bioavailable small-molecule receptor tyrosine kinase inhibitor. In December 2014, the China Food and Drug Administration made it the first anti-angiogenic therapy to be approved for treating metastatic gastric cancer. It was specifically designated as a third-line or later treatment for metastatic gastric cancer.
Here, we present a case of advanced renal cell carcinoma (RCC) with multiple metastases (Stage IV) in a 48-year-old male with an extremely poor general status (Karnofsky 30%). He was initially given pazopanib as a targeted therapeutic. However, he experienced severe adverse reactions within two weeks, including grade IV oral mucositis. We, thus, tried switching his targeted treatment to an apatinib dose of 250 mg once daily since April 2018. The patient demonstrated striking benefits from this switch to the apatinib palliative treatment. Nearly one month later, his pain and other associated symptoms were alleviated. The patient was able to move freely and had an excellent general status (Karnofsky 90%). His progress has been followed up with regularly, allowing for a documented progression-free survival interval of approximately 32 mo.
This case suggests that, like other multi-target drugs, apatinib may be a useful first-line therapeutic drug for advanced RCC. It may be a particularly helpful curative option when patients are found to be intolerant of other targeted drugs.
Core Tip: Apatinib, an orally bioavailable small-molecule receptor tyrosine kinase inhibitor, is typically used in the treatment of partial solid tumours. Here, we report a case of advanced renal cell carcinoma (RCC) with multiple metastases (Stage IV) in a 48-year-old man with an extremely poor general status (Karnofsky 30%). Due to his intolerance to pazopanib (patient developed oral mucositis), we attempted targeted treatment with apatinib, at a dose of 250 mg once daily (from April 2018). This led to an excellent recovery response, realising an approximately 32 mo progression-free survival. This case suggests that apatinib, like other multi-target drugs, may be used as a first-line therapeutic treatment for advanced RCC.
- Citation: Wei HP, Mao J, Hu ZL. Successful apatinib treatment for advanced clear cell renal carcinoma as a first-line palliative treatment: A case report. World J Clin Cases 2022; 10(11): 3593-3600
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3593.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3593
Renal cell carcinoma (RCC) derives from malignant tumours originating in the renal parenchymal urotubular epithelium. Although it only accounts for approximately 2% of all malignant tumours, its morbidity and mortality rates are increasing annually[1,2]. Clear cell RCC (ccRCC) is the most common subtype of RCC, accounting for 80% of all cases[3]. Although surgical excision can cure localised RCC, recurrence is common. In addition, many RCC patients do not present clinical symptoms until they have developed unresectable, locally progressive, or metastatic lesions. Consequently, many patients miss the window for surgery. Moreover, RCC is unresponsive to standard radiotherapy and chemotherapy, and its incidence and mortality rates are increasing with each passing year. Thus, targeted therapy is the most effective treatment for ccRCC patients. Currently, the main targeted drugs for ccRCC include sorafenib, sunitinib, pazopanib, and cabozantinib; all of which achieve anti-tumour effects by impacting vascular endothelial growth factor receptor (VEGFR) expression and inhibiting blood pressure angiogenesis and cell proliferation[2,4].
Apatinib (Hengrui Pharmaceutical Co, Ltd, Shanghai, China) is a highly selective inhibitor of VEGFR-2 tyrosine kinase. In December 2014, it became the first China Food and Drug Administration approved anti-angiogenic therapy for treating metastatic gastric cancer. Its official approved use was as a third-line or later treatment[5]. It acts to specifically inhibit the VEGFR tyrosine kinase activity, thereby inhibiting tumour angiogenesis. Since its approval, given its effectiveness in treating gastric cancer, it has also been used to treat other tumours. Hence, apatinib has shown to be a strikingly effective new option against multifarious malignancies, including advanced non-small cell lung cancer, metastatic triple-negative breast cancer, angiosarcoma, desmoplastic small round cell tumour, advanced intrahepatic cholangiocarcinoma, and myxoid/round cell liposarcoma[6-10].
However, to the best of our knowledge, cases have yet to be reported regarding apatinib’s usefulness in both the treatment of RCC and controlling tumour growth. Thus, in the current report, we present an advanced ccRCC case with multiple metastases (Stage IV) in a 48-year-old male with an extremely poor general status (Karnofsky 30%).
In March 2010, a middle-aged (48-year-old) man was admitted to Dongyang Hospital Affiliated to Wenzhou Medical University complaining of soreness and swelling on his waist and back.
The patient’s symptoms of persistent pain had started one year prior. However, two weeks before admission, it had worsened to the point that he could not lie down on his back at night. Oral analgesics did not control his pain, which he reported suffering from in frequent bouts.
In May 2017, the patient was admitted to the Fourth Affiliated Hospital of Zhejiang University’s Medical College. Urinary system and bladder ultrasounds showed the presence of a parenchymal space-occupying tumour of the left kidney. Chest and abdomen computed tomography (CT) scans suggested that the tumour had triggered implantation metastasis at multiple sites, including parapyloric nodules, lumbar areas, multiple nodules in both lungs, left pleura, left breast, and the abdominal wall. Realising the seriousness of his condition, the patient immediately visited the Cancer Hospital affiliated with Fudan University, where he was advised to undergo a left nephrectomy, followed by adjuvant therapy. However, the patient was resistant and refused the suggested treatment. Instead, he consumed traditional Chinese medicine orally at home. Unfortunately, by March 2018, the tumours had progressed and the mass on his back had grown more.
The patient had no history of smoking or drinking alcohol. There was no relevant family history.
The patient’s vital signs were stable in March 2018. However, the clinical examination showed tenderness in the lower back and localised skin swelling.
Blood tests showed increased leukocytes and tumour markers. The neutrophil, hemoglobin, platelets and biochemistry was normal.
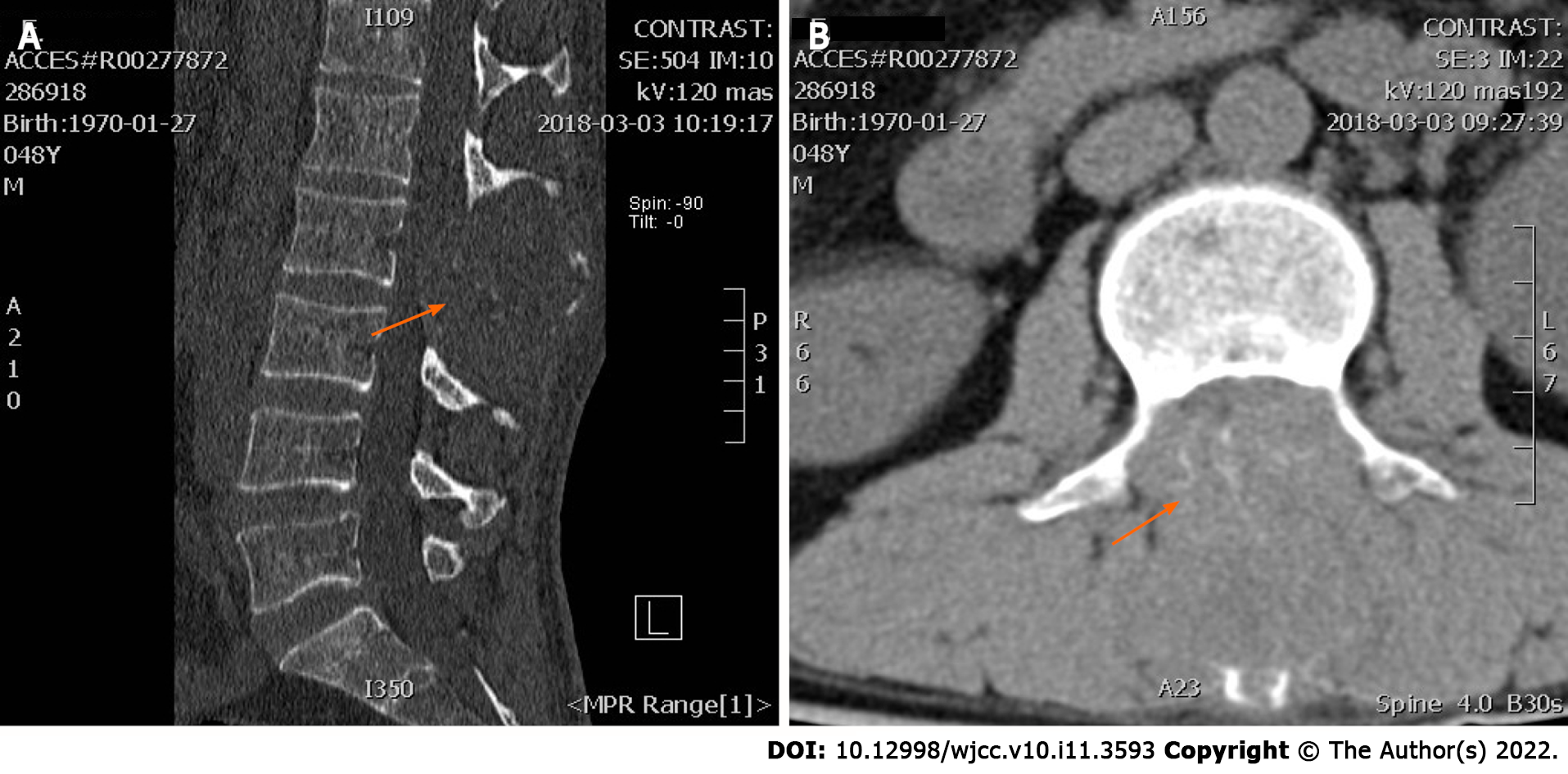
Lumbar CT scans, taken on March 5, 2018, showed that the patient’s lumbar spinous process level space had been blocked by the deterioration and absorption of lumbar 2 appendages and the invasion of the spinal canal (Figure 1).
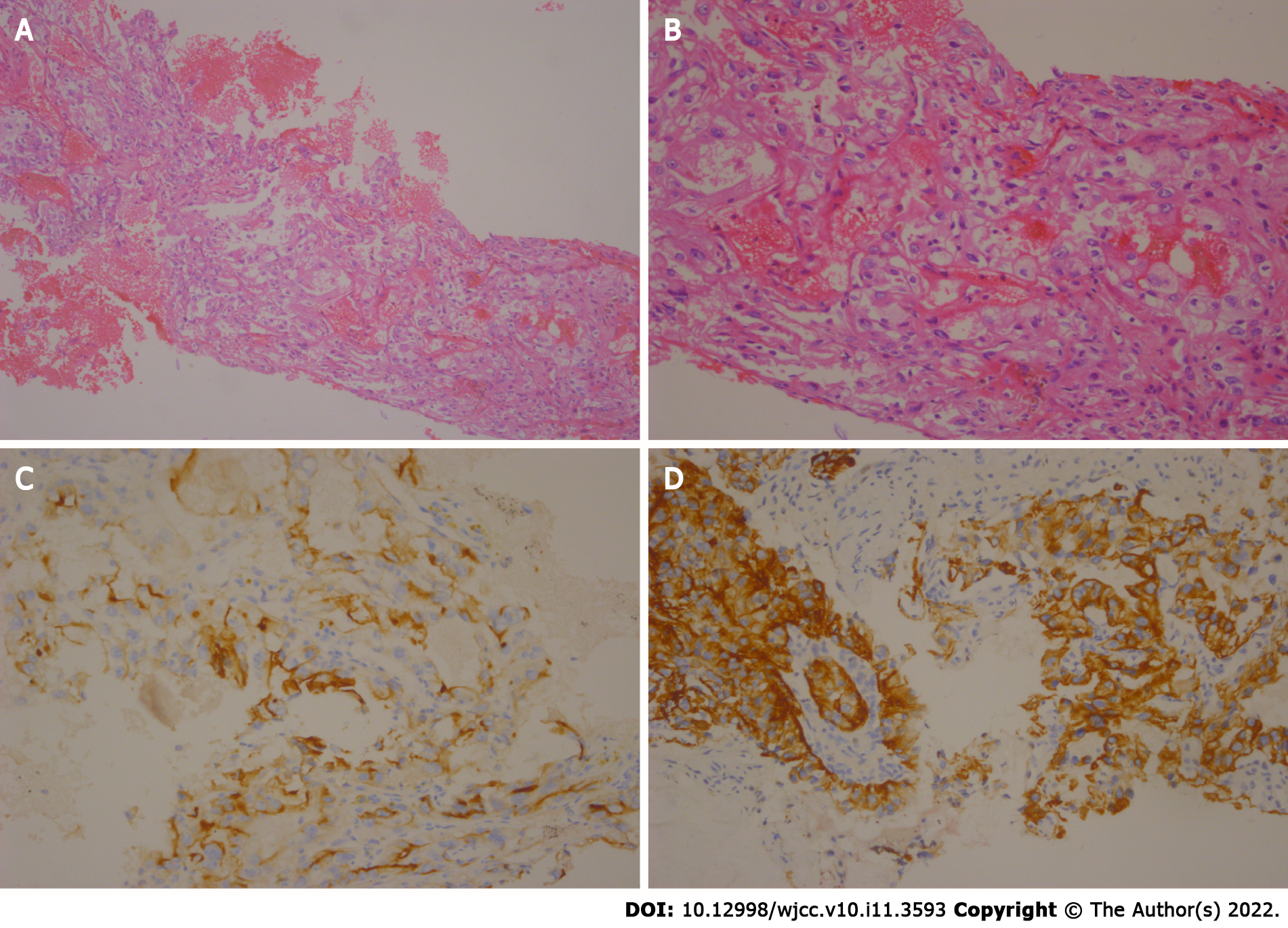
Considering the medical history and follow-up treatments in this case, a puncture biopsy of the mass was recommended. Thus, on March 17, 2018, a puncture biopsy was performed on the back tumour, with guided colour Doppler ultrasound. The observed malignant tumours were consistent with ccRCC according to their morphology and histology; CK+, CK7−, CK20−, cam5.2+, CD10+, EMA+, 34beta E12−, Ki-67+ (10%) (Figure 2).
This patient had a clear diagnosis of ccRCC with multiple metastases (Stage IV; IMDC mid-risk). Given the multiple metastases, surgery was not advisable. Instead, medical treatment was considered.
The patient was diagnosed with ccRCC with multiple metastases (Stage IV; IMDC mid-risk) in various organs, including the lungs (contralateral), bones, pleura, abdominal cavities, and lymph nodes. The patient had an extremely poor general status (Karnofsky 30%) and was informed on his poor prognosis.
Initially, the patient was administered pazopanib targeted treatment. However, two weeks later he experienced severe adverse reactions in the form of grade IV oral mucositis. Considering his intolerance to pazopanib, we attempted targeted treatment with apatinib, at a dose of 250 mg daily since April 2018. There were no grade III or higher adverse reactions during apatinib treatment.
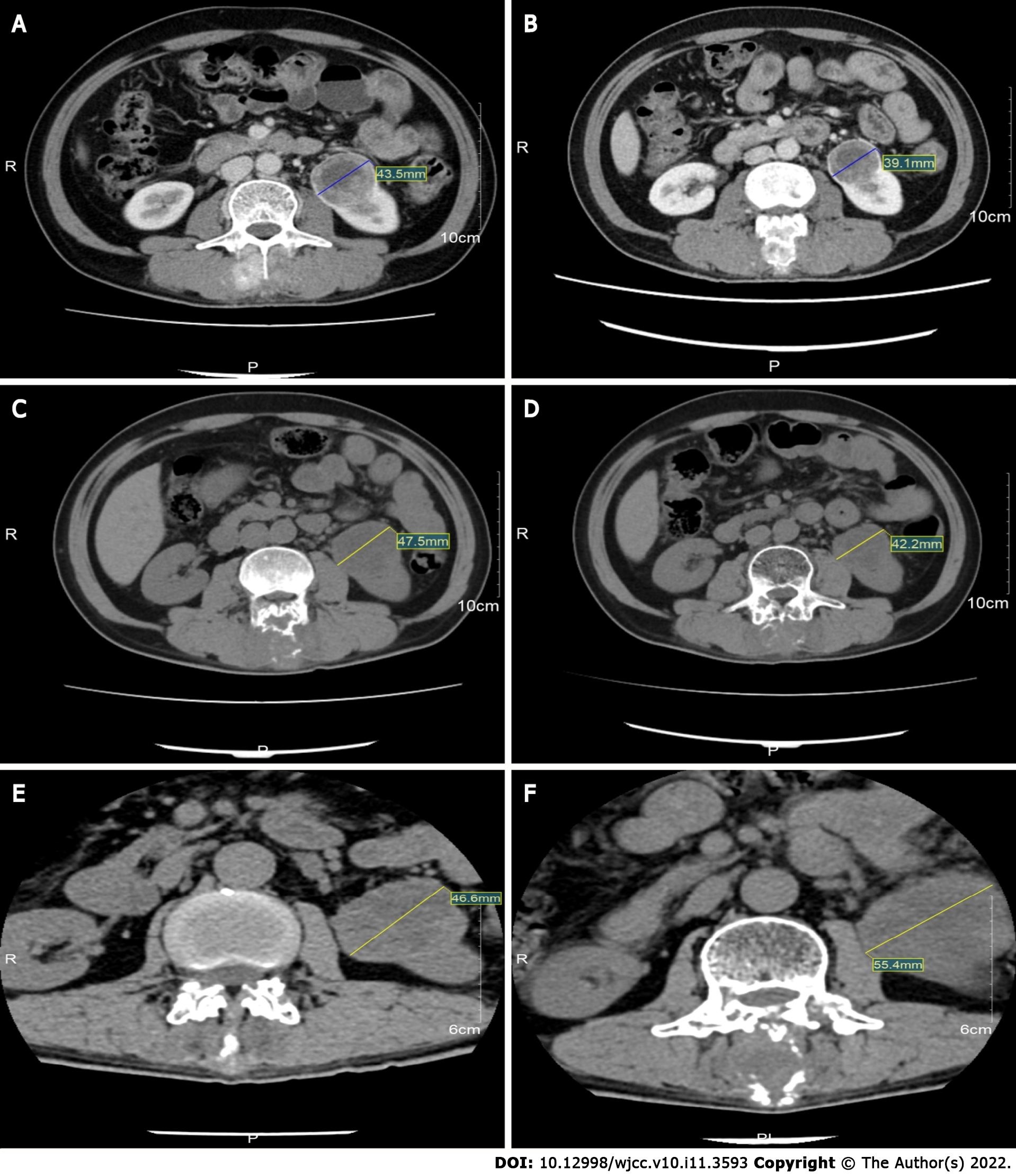
In response to apatinib treatment, the lump in his back shrank after one week. The patient was thus able to lie down, and his pain in so doing was alleviated. He was able to move freely and his performance status improved drastically (Karnofsky 90%). CT taken on July 26, 2018, revealed left inferior pole RCC (diameter: 4.5 cm), L2 spinous process metastatic bone tumours secondary to corresponding plane spinal stenosis, and multiple metastatic tumours in both the lungs (both the length and diameter of the lesion were approximately 1.0 cm), and he had a stable disease (SD), according to the RECIST 1.1 criteria (Figure 3). After several months, a follow-up CT taken on October 20, 2018, and compared with the prior scan (July 26, 2018), suggested that the left inferior pole RCC had reduced in size (diameter: 3.9 cm). Moreover, the rest of the metastatic tumours did not demonstrate any noticeable change (in terms of growth) (Figure 3). Furthermore, the patient showed a SD, according to the RECIST 1.1 criteria. The follow-up CT taken on March 5, 2019 showed that the left inferior pole RCC had enlarged (diameter: Approximately 4.7 cm). However, the progression was slow, according to the RECIST 1.1 criteria (Figure 3). During this period, combination immunotherapy was recommended due to the increase in tumour size. However, the patient rejected it for economic reasons. Thereafter, he was followed up with several times and was reported to be stable till December 2020. The follow-up CT, taken on December 14, 2020, showed that the left inferior pole RCC had increased in size (diameter: Approximately 5.5 cm). Considered together with the other lesions, this indicated that the disease had resumed progression (Figure 3). Strikingly, until this point, the patient had experienced 32 mo of progression-free survival (PFS).
RCC is considered a serious disease worldwide because of the extent to which it endangers human life and health. It demonstrates insidious onset, low survival rate, and high morbidity and mortality. RCC is the second most common urinary tumour, accounting for 2%–3% of all adult malignant tumours[2]. Currently, the best treatment for RCC is surgical resection. In 2010, radical nephrectomy was proposed as a viable option for advanced RCC patients in sufficiently good general condition such that they could tolerate surgery[11]. However, approximately one third of RCC patients demonstrate distant metastasis at the time of initial diagnosis. Thus, they have missed the window for surgery. Statistically, RCC patients showing a 2-year survival rate amount to less than 20% of all cases, whereas the 5-year survival rate is approximately 10%[2]. Therefore, pursuit of medical treatment for RCC is also extremely important. Luckily, over the past decade, a range of therapeutic options have been approved for mRCC, including the targeted, immune checkpoint, and combined immune checkpoint treatments[12]. In the mid to late 2000s, targeted therapies, including inhibitors of VEGF and the mammalian target of rapamycin pathway have entered into the clinical toolkit, thus expanding the range of standard ccRCC therapy options[13]. Consequently, the 5-year survival rate for advanced RCC has increased to nearly 20%[2]. Recent studies have shown that immunotherapy and target therapy combined with immunotherapy have led to positive results in the PFS of advanced RCC patients[14,15].
Researchers have shown that targeted therapies, such as bevacizumab, sunitinib, and axitinib, have a 25%–35% overall response rate among treatment-naive patients, a disease control rate of 45%–80%, and a median PFS ranging from 6.5–11 mo[16]. Currently, immunotherapy has also been used as a first-line treatment[17]. The median PFS for advanced first-line RCC immunotherapy is between 11.2 and 15.1 mo[18-20]. However, immunotherapy, being very expensive and not yet insured, is not an economic first-line RCC treatment[21].
Apatinib is an orally bioavailable small-molecule VEGFR2 tyrosine kinase inhibitor. It inhibits VEGF-mediated endothelial cell migration and proliferation, thereby arresting angiogenesis in tumours[22]. In addition, this agent mildly inhibits c-Kit and c-Src tyrosine kinases. Studies have shown that apatinib can arrest the growth of tumour cells in advanced gastric cancer patients. It has been shown to similarly affect advanced epithelial ovarian cancer, recurrent malignant glioma, and metastatic breast cancer[5,6,8,23,24].
In the present case, the patient was diagnosed with ccRCC with multiple metastases (Stage IV) and was in deplorable physical condition. Therefore, targeted therapy was recommended. Given the patient’s intolerance to pazopanib and our uncertainty with regards to his response to sunitinib, we chose apatinib as this drug had been reported to be successful in treating various malignancies. The patient showed a positive clinical response to apatinib. His general status improved drastically, and nearly 32 mo of PFS was realised, which was longer than any previously reported case. Although, the patient showed a slow disease progression, apatinib was still effective in reversing disease severity. Thus, we posit an ideal treatment combination of apatinib and immunotherapy. Unfortunately, the patient did not receive immunotherapy concurrently for financial reasons.
However, this study has some limitations. Because case reports cannot replace randomised controlled trials, it is unclear whether apatinib is suitable for all RCC patients. Further prospective studies are required to optimise the use of apatinib in the treatment of RCC and identify patients that can benefit from this agent.
In conclusion, this case report indicates that apatinib significantly augmented the quality of life for an RCC patient. It also markedly improved his PFS. Thus, apatinib, like other multi-target drugs, may be useful as a first-line therapeutic drug for advanced RCC. It may be a particularly helpful curative option when patients are found to be intolerant of other targeted drugs.
| 1. | Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 2001] [Article Influence: 222.3] [Reference Citation Analysis (1)] |
| 2. | Xia L, Song YY, Hao RM. Current status and drug development consideration of treatment for advanced renal cell carcinoma. Chin J Clin Pharmacol. 2020;318:2571-2577. |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15626] [Article Influence: 2232.3] [Reference Citation Analysis (11)] |
| 4. | Singh D. Current updates and future perspectives on the management of renal cell carcinoma. Life Sci. 2021;264:118632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 767] [Article Influence: 76.7] [Reference Citation Analysis (1)] |
| 6. | Deng L, Wang Y, Lu W, Liu Q, Wu J, Jin J. Apatinib treatment combined with chemotherapy for advanced epithelial ovarian cancer: a case report. Onco Targets Ther. 2017;10:1521-1525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Du Z, Yu Y, Wu D, Zhang G, Wang Y, He L, Meng R. Apatinib for salvage treatment of advanced malignant pleural mesothelioma: A case report. Medicine (Baltimore). 2018;97:e13105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Fang SC, Zhang HT, Zhang YM, Xie WP. Apatinib as post second-line therapy in EGFR wild-type and ALK-negative advanced lung adenocarcinoma. Onco Targets Ther. 2017;10:447-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Kou P, Zhang Y, Shao W, Zhu H, Zhang J, Wang H, Kong L, Yu J. Significant efficacy and well safety of apatinib in an advanced liver cancer patient: a case report and literature review. Oncotarget. 2017;8:20510-20515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Wang LY, Gong S, Gao LP, Hou LX, He W. Apatinib for treating advanced intrahepatic cholangiocarcinoma after failed chemotherapy: A case report and literature review. Medicine (Baltimore). 2018;97:e13372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Yang F, Zhou X, Du S, Zhao Y, Ren W, Deng Q, Wang F, Yuan J. LIM and SH3 domain protein 1 (LASP-1) overexpression was associated with aggressive phenotype and poor prognosis in clear cell renal cell cancer. PLoS One. 2014;9:e100557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Ghatalia P, Zibelman MR, Geynisman DM, Plimack ER. Evolving landscape of the treatment of metastatic clear cell renal cell carcinoma. Clin Adv Hematol Oncol. 2018;16:677-686. [PubMed] |
| 13. | Lee CH, Chung J, Kwak C, Jeong CW, Seo SI, Kang M, Hong SH, Song C, Park JY, Hwang EC, Lee H, Ku JY, Seo WI, Choi SH, Ha HK; Korean Renal Cancer Study Group. Targeted therapy response in early versus late recurrence of renal cell carcinoma after surgical treatment: A propensity score-matched study using the Korean Renal Cancer Study Group database. Int J Urol. 2021;28:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Perego G, Barzaghi P, Vavassori I, Petrelli F. Treating metastatic clear-cell renal cell carcinoma: beyond immunotherapy. Med Oncol. 2020;37:81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Caruso M, Romeo V, Stanzione A, Buonerba C, Di-Lorenzo G, Maurea S. Current imaging evaluation of tumor response to advanced medical treatment in metastatic renal-cell carcinoma: Clinical implications. Applied Sciences. 2021;11:6930. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, Feldman DR, Olencki T, Picus J, Small EJ, Dakhil S, George DJ, Morris MJ. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 576] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 17. | Bando Y, Furukawa J, Terakawa T, Harada K, Hinata N, Nakano Y, Fujisawa M. Treatment outcomes of molecular targeted therapy following nivolumab in metastatic renal cell carcinoma. Jpn J Clin Oncol. 2021;51:1313-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T; KEYNOTE-426 Investigators. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 2561] [Article Influence: 365.9] [Reference Citation Analysis (0)] |
| 19. | Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, Lee JL, Vasiliev A, Miller WH Jr, Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1103-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1956] [Article Influence: 279.4] [Reference Citation Analysis (0)] |
| 20. | Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B; CheckMate 214 Investigators. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378:1277-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2567] [Cited by in RCA: 3592] [Article Influence: 449.0] [Reference Citation Analysis (7)] |
| 21. | Pipitone S, Vitale MG, Baldessari C, Dominici M, Sabbatini R. Long survival of a young patient with Xp11.2 translocation metastatic clear cell renal carcinoma: case report. Tumori. 2021;107:NP131-NP135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 435] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 23. | Lin Y, Wang C, Gao W, Cui R, Liang J. Overwhelming rapid metabolic and structural response to apatinib in radioiodine refractory differentiated thyroid cancer. Oncotarget. 2017;8:42252-42261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Zhang H, Chen F, Wang Z, Wu S. Successful treatment with apatinib for refractory recurrent malignant gliomas: a case series. Onco Targets Ther. 2017;10:837-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maurea S, Italy; Rioja Viera PE, Peru S-Editor: Fan JR L-Editor: A P-Editor: Fan JR