Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3478
Peer-review started: July 25, 2021
First decision: December 27, 2021
Revised: January 15, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 256 Days and 19.5 Hours
Both programmed cell death-1 (PD-1) inhibitors and lenvatinib, which have a synergistic effect, are promising drugs for tumor treatment. It is generally believed that combination therapy with a PD-1 inhibitor and lenvatinib is safe and effective. However, we report a case of toxic epidermal necrolysis (TEN), a grade 4 toxicity, after this combination therapy.
A 39-year-old male presented with erythema, blisters and erosions on the face, neck, trunk and limbs 1 wk after receiving combination therapy with lenvatinib and toripalimab, a PD-1 inhibitor. The skin injury covered more than 70% of the body surface area. He was previously diagnosed with liver cancer with cervical vertebra metastasis. Histologically, prominent necrotic keratinocytes, hyperkeratosis, liquefaction of basal cells and acantholytic bullae were observed in the epidermis. Blood vessels in the dermis were infiltrated by lymphocytes and eosinophils. Direct immunofluorescence staining was negative. Thus, the diagnosis was confirmed to be TEN (associated with combination therapy with toripalimab and lenvatinib). Full-dose and long-term corticosteroids, high-dose intravenous immunoglobulin and targeted antibiotic drugs were administered. The rashes gradually faded; however, as expected, the tumor progressed. Therefore, sorafenib and regorafenib were given in succession, and the patient was still alive at the 10-mo follow-up.
Cautious attention should be given to rashes that develop after combination therapy with PD-1 inhibitors and lenvatinib. Large-dose and long-course glucocorticoids may be crucial for the treatment of TEN associated with this combination treatment.
Core Tip: Both programmed cell death-1 (PD-1) inhibitors and lenvatinib, which exhibit a synergistic effect, are promising drugs for tumor treatment. However, we encountered a patient who presented with erythema, blisters and erosions on the face, neck, trunk and limbs 1 wk after combination therapy with lenvatinib and toripalimab, a PD-1 inhibitor. Skin biopsy was performed, and the diagnosis was confirmed as toxic epidermal necrolysis (TEN). We are the first group to report the occurrence of TEN, a grade 4 toxicity, after this combination therapy. Full-dose and long-term corticosteroids were administered, and the rashes gradually faded.
- Citation: Huang KK, Han SS, He LY, Yang LL, Liang BY, Zhen QY, Zhu ZB, Zhang CY, Li HY, Lin Y. Combination therapy (toripalimab and lenvatinib)-associated toxic epidermal necrolysis in a patient with metastatic liver cancer: A case report. World J Clin Cases 2022; 10(11): 3478-3484
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3478.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3478
Toripalimab, also known as JS001 or TAB001, is a humanized immunoglobulin G4 monoclonal antibody against programmed cell death-1 (PD-1)[1,2]. PD-1 inhibitors help to enhance the ability of the immune system to defeat tumor cells, but immune-related adverse events (irAEs) occur in many cases[3]. Toxic epidermal necrolysis (TEN) is a rare type of irAE caused by PD-1 inhibitors, with a mortality rate of up to 60%[4]. Lenvatinib is an antiangiogenic tyrosine kinase inhibitor (TKI) and shows synergism with PD-1 inhibitors in solid tumors[5,6]. There have been no case reports on TEN associated with toripalimab or lenvatinib[7]. However, we encountered a patient with metastatic liver cancer who developed TEN after combination therapy with toripalimab and lenvatinib. We are the first group to report this severe cutaneous adverse event following combination therapy with a PD-1 inhibitor and lenvatinib. This case report demonstrates that cautious attention should be given to rashes that develop following this combination treatment. The successful treatment of our case also demonstrated the significance of large-dose and long-course glucocorticoid application for this special type of TEN.
Erythema, blisters and erosions appeared on the face, neck, trunk and limbs 1 wk after combination therapy with toripalimab and lenvatinib.
Four weeks prior to presentation, a 39-year-old male began to receive combination therapy with toripalimab and lenvatinib, as well as radiotherapy, after being diagnosed with liver cancer with cervical vertebra metastasis. The oral administration dose of lenvatinib was 12 mg once daily. Toripalimab (240 mg) was administered intravenously every two weeks. One week prior to presentation, erythema, blisters and erosions began to appear on the face and neck, along with pain and fever. Rashes soon spread to the trunk and limbs, as well as the scrotum and oral mucosa. Therefore, the patient was admitted to the Dermatology Department of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine.
The patient experienced neck pain 2 mo prior to presentation at our clinic. The diagnosis of liver cancer with cervical vertebral metastasis was ultimately confirmed by PET-CT. Hepatitis B virus (HBV) infection was diagnosed at the same time, and the patient was then treated with entecavir. The patient had no other medical history, such as diabetes or hypertension.
The patient had no history of exposure to industrial poisonous substances and reported no habit of smoking or drinking alcohol. The family history was unremarkable.
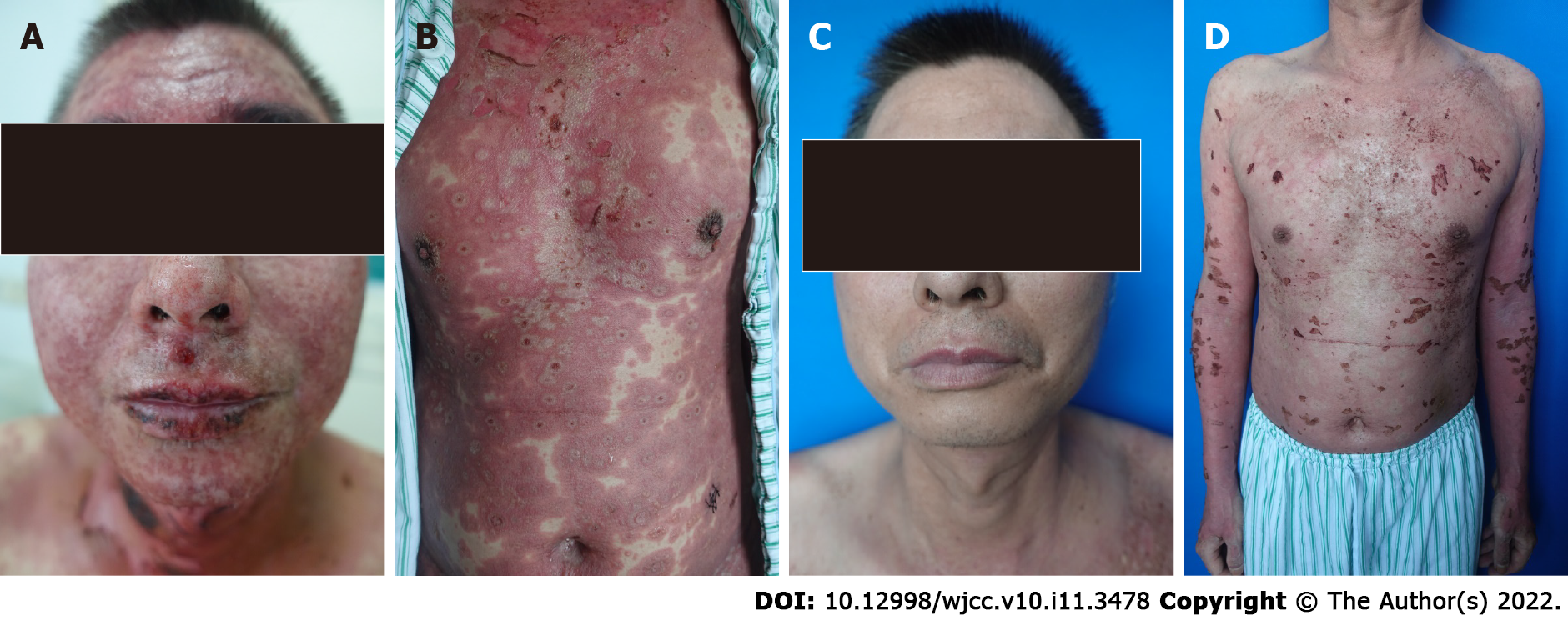
The patient presented with typical erythema multiforme, slack bullae and epidermal peeling on the face, neck, trunk and limbs. Nikolsky’s sign was positive. Scrotal and oral mucosal erosion was observed. Skin injury covered more than 70% of the body surface area (BSA) (Figure 1A and B). The patient weighed 71 kg.
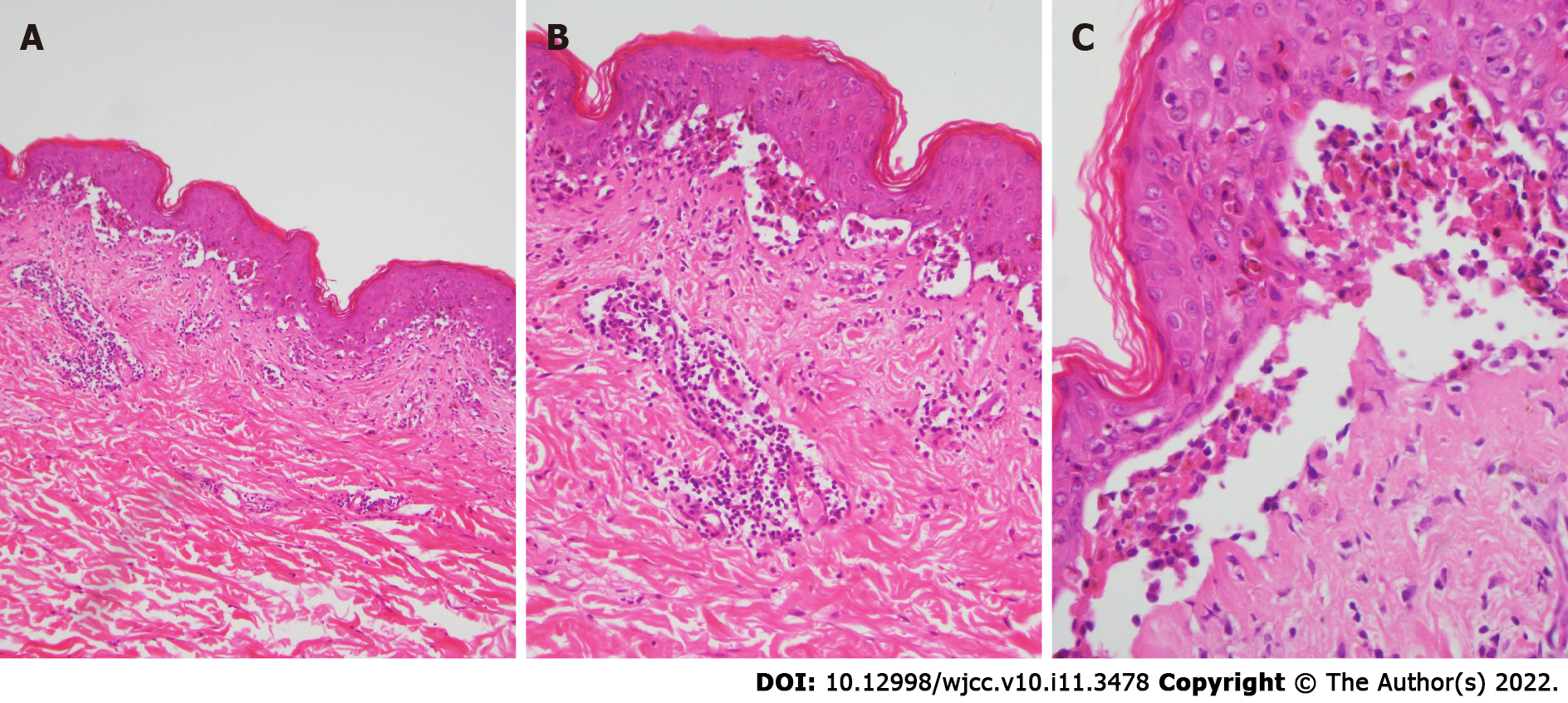
Skin biopsy was performed. Prominent necrotic keratinocytes, hyperkeratosis, liquefaction of basal cells and acantholytic bullae were observed in the epidermis. Blood vessels in the dermis were infiltrated by lymphocytes and eosinophils (Figure 2A-C). Direct immunofluorescence (DIF) staining was negative.
Serum albumin decreased to 33.6 g/L. CRP and procalcitonin slightly increased to 20.9 mg/L and 0.09 ng/mL, respectively. Random blood glucose was 11.23 mmol/L. Quantitative analysis of HBV DNA yielded a value of 3.57 × 103 IU/mL. The AFP value was 1497 ng/mL. The results of routine blood, blood coagulation function, liver and kidney function, routine stool, and routine urine tests, as well as of electrocardiography and chest radiography, were normal. Staphylococcus aureus, Escherichia coli, and Klebsiella aerogenes were cultured from the sites of skin erosion.
The diagnosis was confirmed to be TEN (associated with combination therapy with toripalimab and lenvatinib) according to the patient’s medical history, typical lesion morphologies, and typical pathological findings.
The patient ceased treatment with toripalimab and lenvatinib. Methylprednisolone was administered at an initial dose of 80 mg/d. The rashes continued to worsen; thus, 3 days later, the dosage of methylprednisolone was increased to 120 mg/d. Blood pressure and blood glucose were monitored, and insulin was used to control secondary hyperglycemia. The patient received high-dose intravenous immunoglobulin (IVIG) at 0.4 g/kg/d for 5 days. Targeted antibiotic drugs, such as piperacillin-tazobactam and cefuroxime, were chosen in succession based on the drug sensitivity tests in pathogenic bacteria. A potassium permanganate solution bath and compound polymyxin B ointment were administered for external use. The ocular, scrotal and oral mucosa were also carefully treated with topical medication. Oxycontin was administered to relieve the cancer-related pain.
Interestingly, the rashes began to improve on the face and neck and progressed on the trunk and edge of the limbs at a much lower speed when the methylprednisolone dose was adjusted to 120 mg/d. This course lasted for 2 wk, and we did not reduce the dosage of methylprednisolone until we observed remarkable improvements in erythema, blisters and erosions (Figure 1C and D).
At the 2-mo follow-up for glucocorticoid therapy, the dosage of methylprednisolone was gradually reduced to 8 mg/d, and the rashes did not recur. Enhanced computed tomography (CT) scans showed that the size of the primary liver cancer focus increased, and rib metastasis and portal vein tumor thrombosis were noticed. The patient was then treated by transcatheter arterial chemoembolization (TACE).
At the 4-mo follow-up, enhanced CT scans showed that portal vein tumor thrombosis improved, while rib metastasis progressed. Thus, ultrasound-guided microwave ablation and radiotherapy were conducted in succession. Oral sorafenib was also administered, and no cutaneous adverse drug reactions were observed.
At the 6-mo follow-up, the patient reported paraplegia, which was possibly due to the intraspinal metastatic tumors revealed on the contrast-enhanced magnetic resonance imaging scan. Emission computed tomography (ECT) revealed metastasis of the occipital bone, cervical vertebra, ribs and femur. The patient was experiencing severe pain and agreed to take the risk of the rash recurring to receive additional combination treatment with immunotherapy and targeted therapy. Due to the poor therapeutic effect of sorafenib, the therapeutic regimen was adjusted to 120 mg regorafenib once daily in combination with sintilimab, another PD-1 inhibitor, in addition to toripalimab[8]. A maculopapular rash developed on the trunk 2 wk later; thus, sintilimab was stopped. The rashes were not as severe as the initial rashes and vanished after a small dose of methylprednisolone was administered.
The patient continued taking regorafenib, and disease progression seemed to decrease. At the 10-mo follow-up, the patient was still alive.
TEN and Stevens Johnson syndrome (SJS) are two ends of a spectrum of rare severe adverse cutaneous drug reactions, typically with a clinical presentation of erythema multiforme, slack bullae and epidermal peeling[9]. They are distinguished only by their extent of skin detachment (< 10% BSA: SJS, 10%-30% BSA: SJS/TEN, > 30% BSA: TEN)[9]. Histopathologically, widespread necrosis in the epidermis aids in the diagnosis. DIF staining should be negative to rule out certain autoimmune blistering diseases. Specific drugs, such as allopurinol, carbamazepine, phenytoin, phenobarbital and some antibiotics, increase the risk of developing SJS/TEN[9]. The average mortality rate of TEN is 25-35%[9].
Immune checkpoint inhibitors, including monoclonal antibodies targeting PD-1, programmed death ligand 1 (PD-L1) or cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), represent a major breakthrough in tumor therapy[10]. Physiologically, the CTLA-4 and PD-1 immune checkpoint pathways play a central role in maintaining peripheral tolerance by downregulating T cell activation[3]. However, tumor cells may take advantage of this peripheral tolerance to evade the host immune system[3]. Immune checkpoint inhibitors can restore antitumor immune responses, achieving long-term benefits for tumor treatment[3]. Pruritic maculopapular rashes, the majority of which are self-limiting, represent the most frequent cutaneous irAE, occurring in more than 1/3 of patients who receive immunotherapy[10]. There are few cases of TEN associated with PD-1 inhibitors, such as nivolumab and pembrolizumab[11-13]. Toripalimab was introduced into practice in recent years and widely adopted, especially in China, while no cases of TEN associated with toripalimab have been reported in association with any cancer condition.
Recently, a systematic review summarized 5 cases of TEN-like reactions associated with checkpoint inhibitors and reported a median time to onset of 4 wk (average of 5.38 wk) from checkpoint inhibitor initiation[4]. Two patients also developed morbilliform rashes that gradually progressed for 2-4 wk before evolving into TEN, and the mortality rate reached up to 60%[4]. The incubation period in our case was approximately 3 wk, in accordance with previously reported cases. Interestingly, we found that the rashes in our case progressed/improved at different sites at the same time. This characteristic may be explained by the pharmacokinetic patterns and long half-lives of checkpoint inhibitors. For example, nivolumab and pembrolizumab have half-lives of 25 and 23 days, respectively; thus, their peak concentrations are not reached until late in the course of treatment[4]. The PD-1/PD-L1 interaction plays an important role in peripheral tolerance by sustaining Tregs and inhibiting T cell activation. Anti-PD-1 treatment allows autoreactive CD8+ T cells targeting keratinocytes to become activated and proliferate, contributing to the apoptosis of epidermal keratinocytes[14].
Lenvatinib is an antiangiogenic TKI that is widely used in multiple solid tumors[5]. Both lenvatinib and sorafenib are recommended as the first-line treatment for unresectable hepatocellular carcinoma in the guidelines[15]. In a global randomized phase 3 trial, lenvatinib was demonstrated to be non-inferior to sorafenib for overall survival, and it led to greater improvements in progression-free survival, time to progression, objective response and quality-of-life assessments compared with sorafenib[16]. In addition, a synergistic effect has been found between lenvatinib and immune checkpoint inhibitors[6]. The combination of lenvatinib/pembrolizumab is promising in several solid tumors, such as endometrial, lung, hepatocellular and gastrointestinal malignancies[5]. Thus, the patient received combination therapy with toripalimab and lenvatinib. Some dermatological adverse events associated with the application of lenvatinib have been noted, the most common of which are hand-foot skin reactions[7]. SJS/TEN induced by TKIs is rather rare, and no case of TEN associated with lenvatinib has been reported in association with any cancer condition[7,17]. However, we are the first group to report TEN, a grade 4 toxicity, after combination therapy with a PD-1 inhibitor and lenvatinib. Since this was a single case, there is still not sufficient evidence to conclude that combination therapy with a PD-1 inhibitor and lenvatinib increases the risk of TEN compared with a PD-1 inhibitor alone.
Despite the high mortality rate of the limited cases of TEN associated with checkpoint inhibitors, whether they should be managed differently from classic cases of TEN[4] (e.g., the application of corticosteroids[11]) is controversial. In our case, the rashes continued to worsen with the initial methylprednisolone dose of 80 mg/d but improved as the dosage increased later. Considering the long half-life of toripalimab, methylprednisolone was sustained for a long time, and the rashes did not recur. Successful treatment of the case above demonstrates the importance of full-dose and long-term corticosteroids in TEN associated with checkpoint inhibitors. High-dose IVIG may help to boost the immune system and prevent opportunistic infection caused by corticosteroids. Other treatments, such as anti-infection regimens, mucosal protection, anti-HBV drugs and maintaining homeostasis of the internal environment (e.g., blood glucose), also contributed to the patient’s recovery.
It is understandable that a tumor would progress rapidly after ceasing antineoplastic drugs due to severe adverse effects. Thus, it is important for patients to restart antitumor therapy as soon as the rashes are controlled. Based on the follow-up of our case, we found that different types of PD-1 inhibitors or targeted drugs do not necessarily cause the same dermatological adverse events. After weighing the pros and cons, other types of PD-1 inhibitors or targeted drugs are still worthy of investigation.
In conclusion, we are the first group to report TEN following combination therapy with a PD-1 inhibitor and lenvatinib. Cautious attention should be given to rashes that develop after this combination treatment. Large-dose and long-course glucocorticoid application may be crucial for the treatment of this special type of TEN.
| 1. | Tang B, Yan X, Sheng X, Si L, Cui C, Kong Y, Mao L, Lian B, Bai X, Wang X, Li S, Zhou L, Yu J, Dai J, Wang K, Hu J, Dong L, Song H, Wu H, Feng H, Yao S, Chi Z, Guo J. Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients. J Hematol Oncol. 2019;12:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 2. | Sheng X, Yan X, Chi Z, Si L, Cui C, Tang B, Li S, Mao L, Lian B, Wang X, Bai X, Zhou L, Kong Y, Dai J, Wang K, Tang X, Zhou H, Wu H, Feng H, Yao S, Flaherty KT, Guo J. Axitinib in Combination With Toripalimab, a Humanized Immunoglobulin G4 Monoclonal Antibody Against Programmed Cell Death-1, in Patients With Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial. J Clin Oncol. 2019;37:2987-2999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 3. | Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39:98-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1427] [Cited by in RCA: 1831] [Article Influence: 183.1] [Reference Citation Analysis (0)] |
| 4. | Maloney NJ, Ravi V, Cheng K, Bach DQ, Worswick S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: a systematic review. Int J Dermatol. 2020;59:e183-e188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Hao Z, Wang P. Lenvatinib in Management of Solid Tumors. Oncologist. 2020;25:e302-e310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Makker V, Rasco D, Vogelzang NJ, Brose MS, Cohn AL, Mier J, Di Simone C, Hyman DM, Stepan DE, Dutcus CE, Schmidt EV, Guo M, Sachdev P, Shumaker R, Aghajanian C, Taylor M. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 383] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 7. | Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: Improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: A Promising Anti-Tumor PD-1 Antibody. Front Oncol. 2020;10:594558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 9. | Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 10. | Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors : Skin Toxicities and Immunotherapy. Am J Clin Dermatol. 2018;19:345-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 468] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 11. | Griffin LL, Cove-Smith L, Alachkar H, Radford JA, Brooke R, Linton KM. Toxic epidermal necrolysis (TEN) associated with the use of nivolumab (PD-1 inhibitor) for lymphoma. JAAD Case Rep. 2018;4:229-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Logan IT, Zaman S, Hussein L, Perrett CM. Combination Therapy of Ipilimumab and Nivolumab-associated Toxic Epidermal Necrolysis (TEN) in a Patient With Metastatic Melanoma: A Case Report and Literature Review. J Immunother. 2020;43:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Robinson S, Saleh J, Curry J, Mudaliar K. Pembrolizumab-Induced Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis in a Patient With Metastatic Cervical Squamous Cell Carcinoma: A Case Report. Am J Dermatopathol. 2020;42:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Goldinger SM, Stieger P, Meier B, Micaletto S, Contassot E, French LE, Dummer R. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin Cancer Res. 2016;22:4023-4029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 15. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3144] [Article Influence: 393.0] [Reference Citation Analysis (3)] |
| 16. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4112] [Article Influence: 514.0] [Reference Citation Analysis (5)] |
| 17. | Doesch J, Debus D, Meyer C, Papadopoulos T, Schultz ES, Ficker JH, Brueckl WM. Afatinib-associated Stevens-Johnson syndrome in an EGFR-mutated lung cancer patient. Lung Cancer. 2016;95:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bhargava S, Nath L S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ