Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3461
Peer-review started: November 30, 2020
First decision: September 28, 2021
Revised: October 12, 2021
Accepted: February 25, 2022
Article in press: February 25, 2022
Published online: April 16, 2022
Processing time: 497 Days and 5.7 Hours
The poly (ADP-ribose) polymerase (PARP) inhibitor olaparib has displayed superior clinical effect in metastatic castration-resistant prostate cancer (mCRPC) patients with the homologous recombination repair (HRR) genes mutations. However, when a patient’s tumor tissue volume is insufficient for genomic profiling of HRR gene mutations, circulating tumor DNA (ctDNA) may be useful in helping to determine and monitor the efficacy of olaparib, as well as in abiraterone-combination treatment, and for understanding any resistance mechanism related to such mutations.
A 61-year-old man who was diagnosed with metastatic prostate adenocarcinoma was initially hormone sensitivity, showing high Gleason score (5 + 5 = 10) and absolute positive rate (14/14 biopsied specimens). Following failure of several standard therapies, the patient progressed to mCRPC. Surprisingly, the patient showed good response to olaparib-abiraterone-prednisone combination treatment (an androgen-deprivation therapy, provided as the ‘final choice’ in China). Serum total prostate-specific antigen (TPSA) level reduced and symptoms remitted for 4 months. However, thereafter, serum TPSA levels began slowly increasing, indicating development of olaparib resistance. Subsequent comprehensive genomic profiling of ctDNA, screening 508 cancer-related genes by next-generation sequencing, identified 10 somatic variants as well as 3 copy number alterations. Two identified reverse missense mutations in partner and localizer of BRCA2 (PALB2) may have recovered the reading frame, restoring function of the primary germline PALB2 mutation and causing resistance to the PARP inhibitor olaparib.
Reverse mutations in PALB2, discovered via genomic profiling of ctDNA, may represent a potential resistance mechanism against olaparib in mCRPC.
Core Tip: This case report describes the poly (ADP-ribose) polymerase inhibitor olaparib treatment response in a patient with metastatic castration-resistant prostate cancer (mCRPC). The patient’s course of response to ‘final choice’ therapy (olaparib-abiraterone-prednisone combination treatment), consisting of serum total prostate-specific antigen (TPSA) level reduction and symptom remission for 4 months followed by TPSA rise, prompted comprehensive genomic profiling of circulating tumor (ct)DNA, which revealed reverse missense mutations in the partner and localizer of BRCA2 gene. Timely multigene testing by ctDNA, especially in mCRPC, can determine the most appropriate and accurate therapeutic approach and explore the resistance mechanism.
- Citation: Yuan F, Liu N, Yang MZ, Zhang XT, Luo H, Zhou H. Circulating tumor DNA genomic profiling reveals the complicated olaparib-resistance mechanism in prostate cancer salvage therapy: A case report. World J Clin Cases 2022; 10(11): 3461-3471
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3461.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3461
The International Agency for Research on Cancer produced an update on global cancer burden using the GLOBOCAN 2020 estimates of cancer incidence and mortality[1]. With an estimated almost 1.4 million new cases and 375000 deaths worldwide, prostate cancer was ranked as the second most frequent cancer and the fifth leading cause of cancer death among men. Asia is traditionally considered a low-incidence area for this type of cancer, but the incidence and mortality rates are rapidly increasing across the continent[2]. In mainland China[3], prostate cancer is now the sixth most commonly occurring malignant tumor. According to the 2020 estimates of global burden, prostate cancer accounted for 120000 of new cases, ranking as the ninth most frequent cancer in China[1]. Patients who progress to metastatic castration-resistant prostate cancer (mCRPC) considered the most serious stage usually suffer from unfavorable clinical prognosis combined with poor quality of life[4].
Treatment options during the disease progression to mCRPC are limited and mainly consist of continued androgen-deprivation therapy (ADT) combined with one of the new endocrine drugs such as abiraterone acetate plus prednisone or with docetaxel chemotherapy plus prednisone. Poly (ADP-ribose) polymerase (PARP) inhibitors, such as olaparib, rucaparib, niraparib or talazoparib, have displayed promising clinical results, with prolonged survival duration and improved life quality in patients with mutations in the homologous recombination repair (HRR) gene who are suffering from various cancers, including ovarian[5], breast[6], pancreatic[7], and prostate[8,9]. Recent studies have also shown the potential therapeutic effect of the PARP inhibitor olaparib in mCRPC patients with deleterious mutations in genes belonging to the DNA damage repair (DDR). Thereinto, harmful mutation of BRCA2 may act as a particularly useful marker for therapeutic response to PARP inhibitor. Indeed, the published results from the study named PROfound indicate a holpful clinical effect with olaparib treatment in patients with BRCA1/2 or ATM deleterious mutations[10]. For patients without DDR deleterious mutations, it is reported that olaparib combining with abiraterone plus prednisone could be the effective treatment options[11].
The sub-population of mCRPC patients with HRR gene partner and localizer of BRCA2 (PALB2) mutation has been reported to have the good objective response and prostate-specific antigen (PSA) response[8], but due to the relatively low mutation frequency of PALB2, there are limited reports of clinical benefit. Moreover, the Food and Drug Administration has approved olaparib for adult patients with germline or somatic HRR gene-mutated mCRPC, who have progressed following prior treatment with enzalutamide, abiraterone or docetaxel chemotherapy. The evaluation of patients’ HRR gene mutation was based on tumor tissue, but in routine clinical practice, especially for mCRPC patients, it is a challenge to acquire metastatic tumor tissue biopsies and of sufficient volume for evaluative processing.
Circulating tumor (ct)DNA can be obtained as a ‘liquid biopsy’ in a minimally invasive manner and as such can serve as a surrogate tumor specimen, providing a real-time snapshot of a patient’s overall tumor burden. To date, however, the impact of ctDNA on clinical decision-making for prostate cancer remains unclear and there is a lack of prospective studies in the literature to advance this important topic.
A 61-year-old man presented to Chongqing University Cancer Hospital with complaint of continuous lumbosacral pain that had lasted for 3 mo.
The patient reported a 3 mo history of lumbosacral pain.
The patient had no past illness.
The patient had no personal and family history.
The patient had stable vital signs with no edema in both lower limbs. Digital rectal examination revealed a hard prostate with many nodules and without tenderness.
The patient showed a markedly increased level of serum total (T)PSA (787 ng/mL; normal: < 4 ng/mL) but normal neuron-specific enolase (4.32 ng/mL).
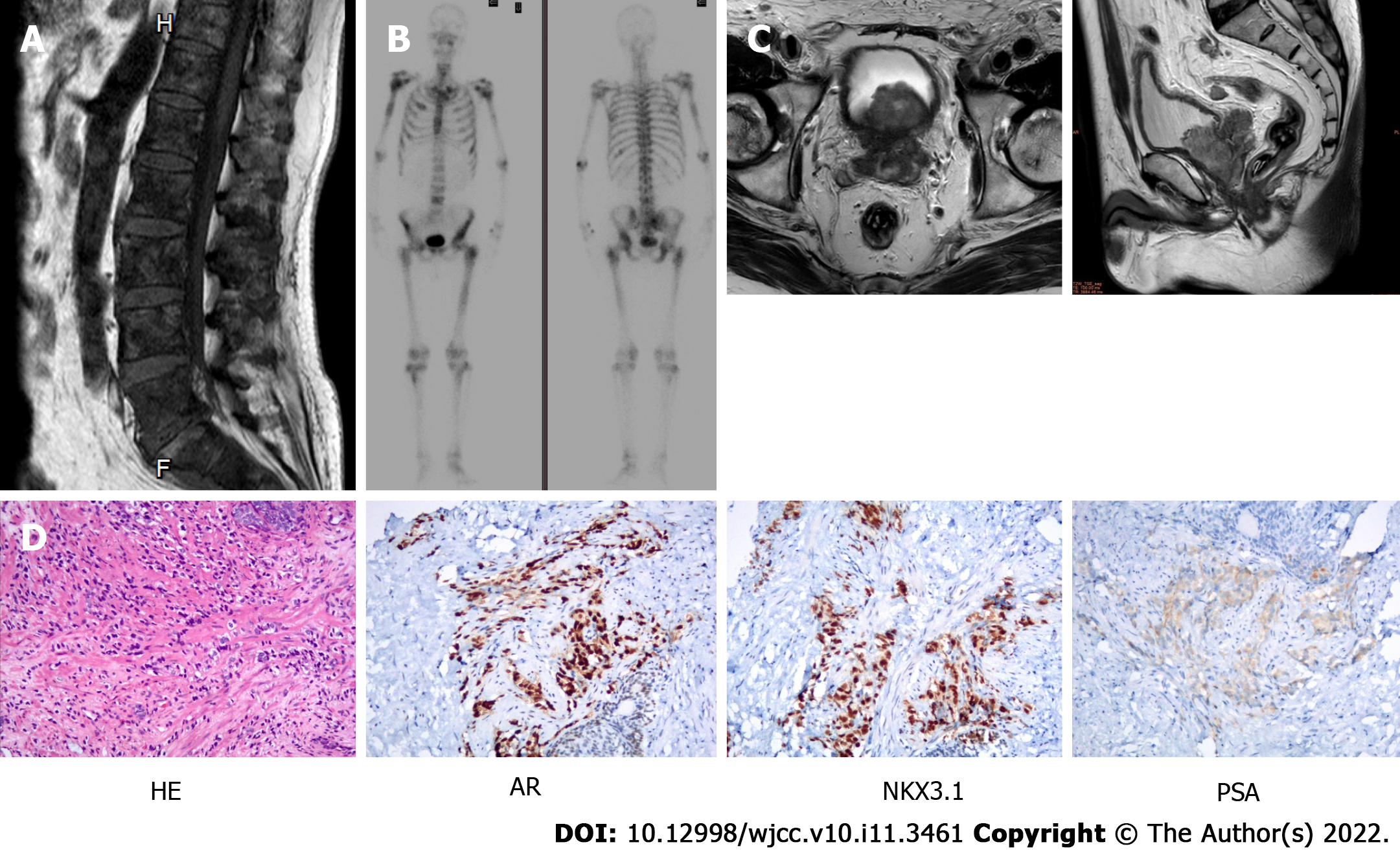
Multiple bone lesions were firstly found by the lumbar magnetic resonance imaging (MRI) (Figure 1A). Then, these lesions and other bones were revealed to have high metabolic activity by bone scanning (Figure 1B). The prostate MRI showed that the seminal vesicle and bladder wall were invaded and pelvic lymph nodes were metastasized (Figure 1C). Computed tomography showed no metastasis in lung.
Ultrasound-guided transperineal prostate needle biopsies were obtained (14 specimens in total), and histology confirmed the diagnosis of prostate adenocarcinoma. The specimens showed the Gleason score was highest (5 + 5 = 10) together with an absolute positive rate (14/14). The biopsied tissues tested through immunohistochemistry staining of serial sections excluded a neuroendocrine component [PSA (+), CK-L (+), P504S (+), AR (++) > 95%, NKX3.1 (+), Ki-67 30% (+), Syn (-), CK34βE12 (-), CgA (-), CD56 (-), P63 (-)] (Figure 1D).
The definite diagnosis and all treatments were performed by Department of Urology, Department of Tumor Radiotherapy, Department of Imaging, and Department of Pathology in Chongqing University Cancer Hospital.
Metastatic hormone-sensitive prostate cancer (mHSPC) of pT4N1M1b type and stage IV.
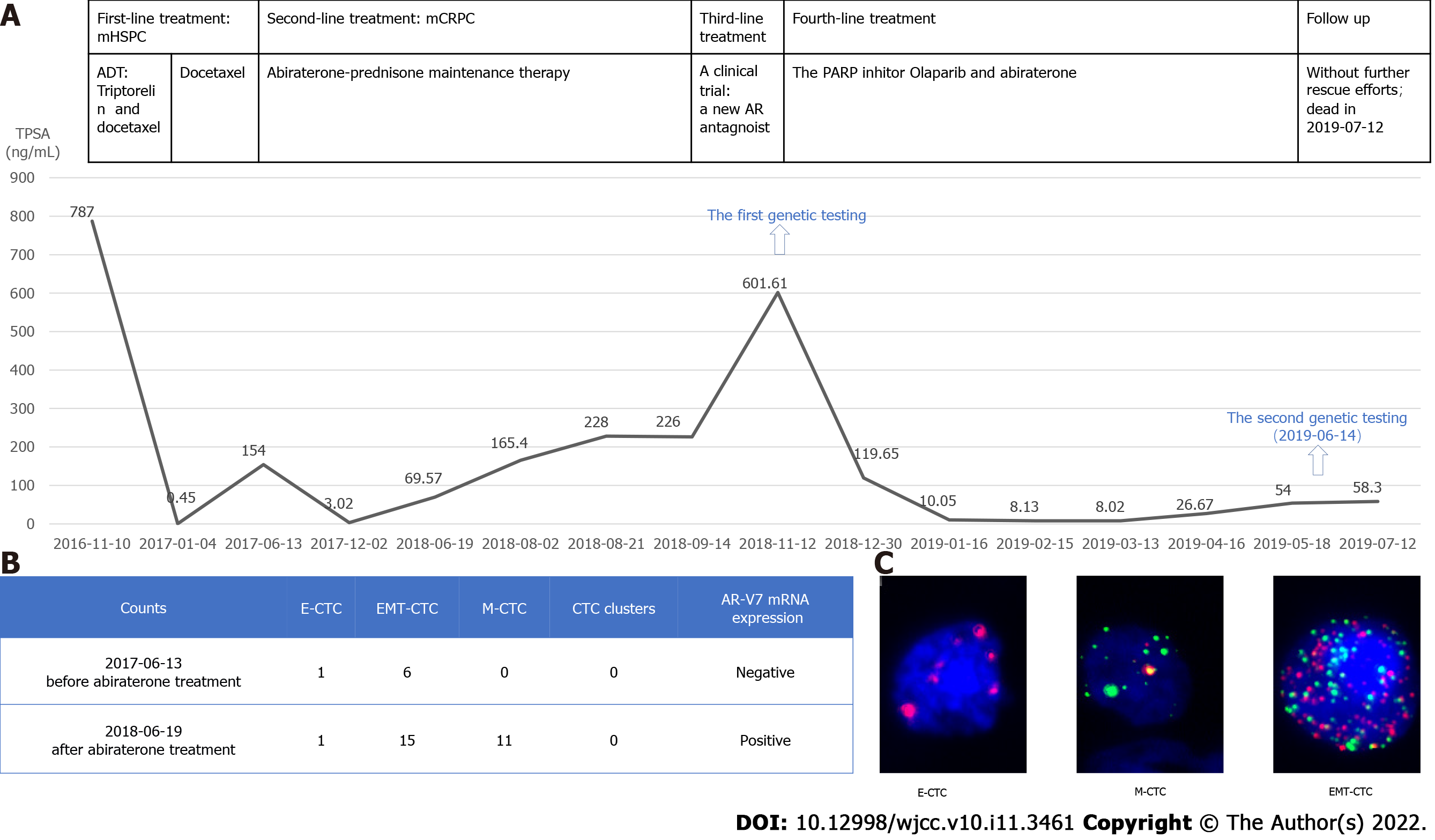
ADT therapy (triptorelin, an LHRH agonist) in combination with docetaxel (three weekly doses of 75 mg/m2) without prednisolone, was applied as the initial treatment[12-14] fitting with the patient’s mHSPC diagnosis and high tumor burden[15]. Two cycles of this therapeutic intervention led to significant relief in the patient’s self-reported pain as well as a substantial drop in TPSA level to 0.45 ng/mL (Figure 2A). However, at that point, the docetaxel had to be stopped due to severe bone marrow inhibition and liver toxicity. Therefore, ADT was continued as monotherapy.
After 5 months, the patient developed lumbosacral pain and showed a rebound of increased TPSA. Testosterone levels remained suppressed throughout entire treatment period, indicating disease progression to mCRPC. To determine the new endocrine therapy resistance or sensitivity, he was investigated the classification and respective number of circulating tumor cells (CTCs) along with the expression of AR-V7 mRNA[16]. Based on the negative findings, we had confidence in the decision for abiraterone-prednisone administration as maintenance therapy (Figure 2B and C). After 6 months of this treatment, the patient showed a decreased TPSA level (3.02 ng/mL; Figure 2A). Fortunately, the disease remained effectively controlled by this therapy for nearly 1 year.
The re-emergence of an elevated TPSA level to 69.57 ng/mL manifested disease progression again (Figure 2A). At the time, the patient’s CTCs number was increased together with AR-V7 mRNA overexpressions, which confirmed that he had developed abiraterone resistance (Figure 2B and C). Then, he was deemed eligible for a phase III double-blind, randomized, placebo-controlled, multicenter clinical trial to assess the safety and efficacy of proxalutamide (a new androgen receptor[AR] antagonist) in patients with mCRPC who failed abiraterone acetate and docetaxel therapy. Of note, as of the writing of this report, the trial is ongoing. Unfortunately, the patient’s disease progressed rapidly during this treatment, as reflected by TPSA level increased to 601 ng/mL (Figure 2A) with somnolence and severe bilateral lower limb edema.
The first genetic testing, performed with the patient’s peripheral blood leukocytes, had detected no germline BRCA1/2 deleterious mutations (Figure 2A). Therefore, we chose to re-challenge the tumor by administration of abiraterone together with the PARP inhibitor olaparib. After only 1 mo of the combined treatment, the patient’s TPSA level began to decrease rapidly (from 601.61 ng/mL to 119.65 ng/mL), and in the months thereafter he maintained a low level (8.02 ng/mL) (Figure 2A). Electrocorticography (commonly known as ECOG) findings and the patient’s mental state were significantly improved. The patient also reported decreased pain and required a reduced amount of morphine dosage (30 mg, q12h, p.o.). After 5 mo, however, the TPSA level began to rise again and the patient reported increasing carcinomatous pain.
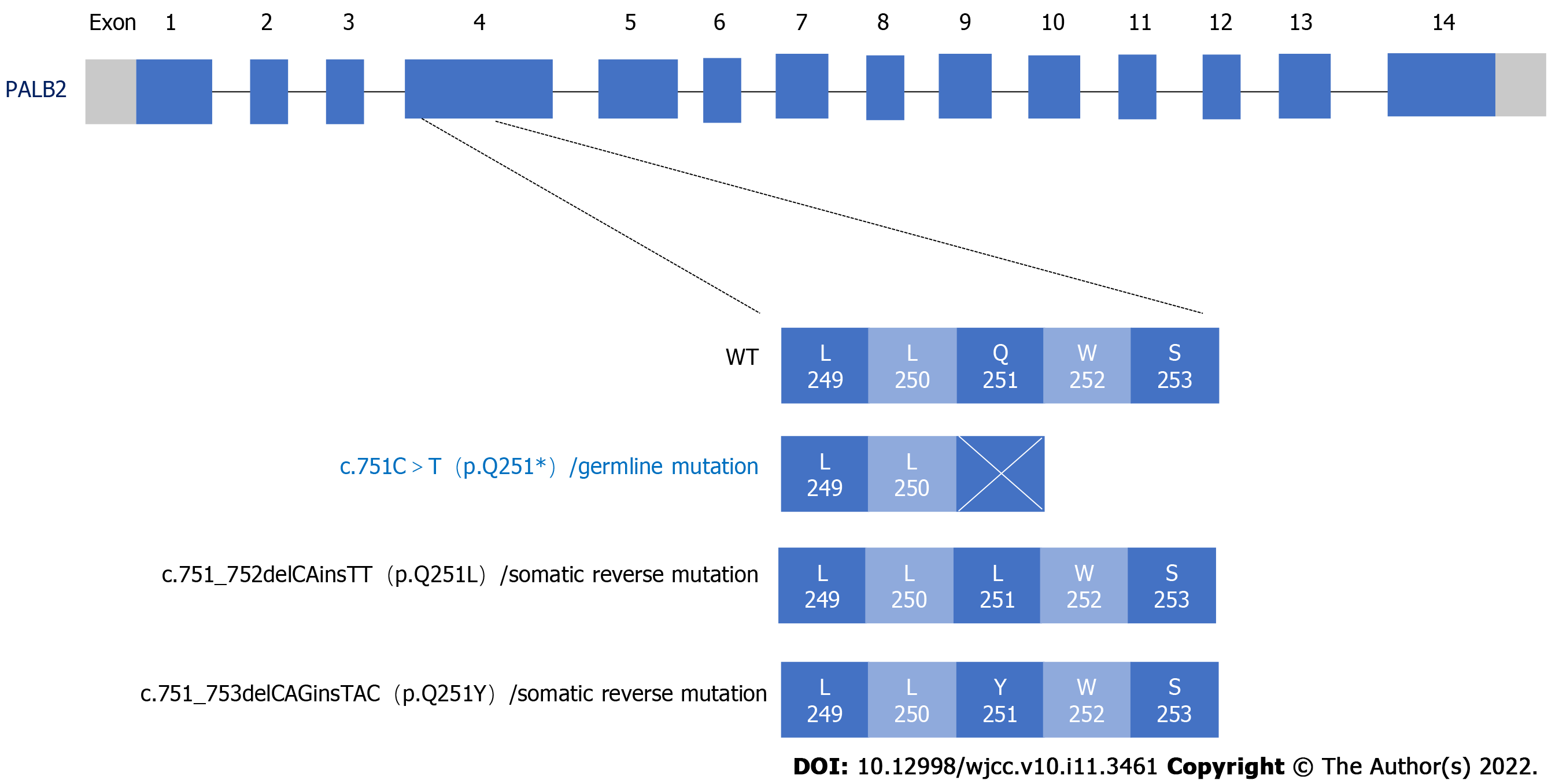
When the disease progressed the third time and we sought to reperform a second comprehensive genomic profile assessment, we chose to use peripheral ctDNA samples due to the difficulty of obtaining a tumor tissue biopsy (Figure 2A). A total of 508 cancer-related genes were sequenced by next-generation sequencing. The HRR gene PALB2 germline pathogenic mutation and two somatic mutations were discovered (Figure 3, Table 1). PALB2, as a tumor suppressor belonging to the HRR gene, physically interacts with BRCA2 leading to the subsequent recruitment of proteins to DNA breaks and plays a crucial role in repairing double-strand breaks through homologous recombination[17]. Some studies have associated PALB2 harmful mutations with therapeutic benefit attained from PARP inhibitors[8,18]. For example, the TOPARP-B study has shown that patients with mutations of PALB2 or somatic cell dysfunction can benefit from olaparib therapy[8].
| Gene | Base change | Amino acid variation | Exon | Variant frequency | Transcript |
| PTEN | c.136_137del | p.Y46Qfs*5 | EX2 | 59.47% | NM_000314.4 |
| AR | Copy number gain | ||||
| CHD1 | Copy number loss | ||||
| FGFR1 | Copy number gain | ||||
| TP53 | c.665_672*11del | - | EX6-IVS6 | 36.13% | NM_000546.5 |
| NOTCH2 | c.5311-1G>A | - | IVS29 | 1.28% | NM_024408.3 |
| PIK3C2G | c.2143A>G | p.R715G | EX15 | 40.23% | NM_004570.4 |
| LHCGR | c.143C>T | p.T48M | EX1 | 23.39% | NM_000233.3 |
| PALB2 | c.751_752delCAinsTT | p.Q251L | EX4 | 7.31% | NM_024675.3 |
| PALB2 | c.751_753delCAGinsTAC | p.Q251Y | EX4 | 3.79% | NM_024675.3 |
| PALB2 | c.751C>T | p.Q251* | EX4 | Germline | NM_024675.3 |
| CDC25C | c.1150_1151delGGinsCC | p.G384P | EX12 | 2.35% | NM_001790.3 |
| FLT4 | c.376G>A | p.A126T | EX3 | 1.15% | NM_002020.4 |
Meanwhile, 10 somatic variants genes were found: AR, PTEN, TP53, CHD1, NOTCH2, FGFR1, LHCGR, PIK3C2G, FLT4 and CDC25C (Table 1). In particular, two reverse missense mutations in PALB2 were suspected as functioning to recover a truncated polypeptide chain translated from the germline PALB2 mutation (Table 1, Figure 3). Two somatic missense mutations of PALB2 which were c.751_752delCAinsTT and c.751_753delCAGinsTAC (Figure 3) shared the same loci with the germline deleterious variant c.751C>T. Bringing about the in-frame deletion and insertion variants, the PALB2 mutations would contribute to restoring the entire reading frame and to supporting the correct functioning to repair DNA double-stranded breaks via the HRR pathway. Therefore, we reasoned that these two reversing mutations of PALB2 may represent the mechanism of resistance to olaparib and abiraterone in this patient. In the future, functional assays are needed to validate this potential drug-resistance mechanism as indicated by these ctDNA profile findings.
Finally, the patient’s ECOG scale increased and he attained a score of 4. The patient showed clouding of consciousness and developed pain throughout the body, and he died without further rescue efforts.
In the case described herein, a mCRPC patient who had undergone treatment by many differing therapy achieved surprisingly clinical response to combination therapy of olaparib and abiraterone-prednisone. Next-generation sequencing of the patient’s ctDNA revealed 13 relevant gene mutations, including in the HRR gene PALB2. Intriguingly, a subgroup of prostate cancer patients who benefit from the treatment of PARP inhibitors have been identified, and these individuals show a trend towards loss-of-function mutations in the HRR gene[8,9,18,19]. In the TOPARP-A, TOPARP-B and PROfound studies, olaparib monotherapy was shown to exert antitumor activity against mCRPC with HRR or DDR gene alterations. The PROfound study[20] indicated that radiologic progression-free survival (rPFS) was significantly longer in the olaparib group than in the group treated with the physician’s choice of enzalutamide or abiraterone (median: 7.4 mo vs 3.6 mo in patients with BRCA1/2 and ATM mutations; 5.8 mo vs 3.5 mo in patients with all 15 HRR genes’ mutations). Similarly, the TOPARP-A study showed mCRPC patients carrying the HRR gene mutation had a median rPFS of 9.8 mo, being 6.1 mo longer than that of mCRPC patients with wild-type HRR gene. The TOPARP-B study showed that the mCRPC patients with PALB2 mutation had a median rPFS of 5.3 mo in response to treatment with olaparib. The gene scope of our first genetic testing was limited to the BRCA1 and BRCA2 genes, and indicated that the patient did not carry the germline BRCA1 or BRCA2 mutation. Based on the conclusion from the Study 08 (NCT01972217)[11] that olaparib in combination with abiraterone provided efficacious clinical benefit for patients with mCRPC compared to abiraterone alone and regardless of HRR mutation status, we chose the olaparib and abiraterone-prednisone combination therapy for our patient. In detail, the Study 08 revealed that olaparib and abiraterone provided median rPFS of 13.8 mo in and intention-to-treat population and 17.8 mo in an HRR mutation-positive subgroup.
Our patient achieved rPFS of 5 mo with the PARP inhibitor olaparib and abiraterone-prednisone combination therapy. This response time was overall consistent with olaparib single-drug use previously reported in the literature but was relatively worse than that with the olaparib and abiraterone-prednisone combination therapy. The reason for this may be that our patient had received the abiraterone treatment and developed resistance to such before the olaparib. It is important to note here that Study 08 had recruited patients who had not received prior abiraterone treatment, only having received docetaxel. In addition, there is a dual model of synergy between PARP inhibitor and ADT[21,22]. PARP is involved in AR-dependent transcription and PARP inhibitor impairs this process, at the same time, the AR regulates transcription of DNA repair genes and androgen depletion impairs HRR, which might produce a so-called “BRCA-ness” phenotype that renders susceptibility to PARP inhibitor. Amplification of the AR gene, as observed via ctDNA profiling of our patient, would lead to continuous activation of downstream signaling pathway(s), overcoming the extrinsic androgen inhibition[23,24] and precluding triggering of the “BRCA-ness” phenotype. The synergy between PARP inhibitor and ADT would not be able to be established in such a patient, which would explain why our patient’s clinical response was worse.
The PALB2 reverse mutations are another important feature of our patient. There was a research about mCRPC patient who achieved 9 mo clinical effect by olaparib with germline PALB2 p.L253Ifs*2 mutation. During that patient’s disease progression, two reverse somatic mutations in PALB2 were found by ctDNA[25]. Similarly, we detected two somatic reverse mutations and a germline p.Q251* nonsense mutation in PALB2 at our patient, which may have restored the reading frame and the homologous recombinational function. Once the repair function of homologous recombination is restored, the synthetic lethality on which PARP inhibitors work will be broken, and patient will inevitably be resistant to PARP inhibitor therapy.
Our case also emphasizes the importance of blood-based liquid biopsies and genomic profiling by means of ctDNA. Tumor tissue can be much more difficult to obtain for this evaluative purpose, particularly from advanced cancer patients who are at higher risk in or counter-indicated for surgery or patients who cannot tolerate biopsy for other reasons; another complication is that tumor tissues, in general, may show negative pathological results if the patient has already started or undergone treatment. CtDNA is thus an attractive, minimally invasive alternative, which can used as a practical tool to profile tumor dynamics over time, elucidating features with tumor progression and overcoming spatial heterogeneity of tumors. For our patient, the genetic testing of ctDNA indicated a complicated mechanism of disease response and progression. First, the discovered HRR gene PALB2 germline mutation could explain the rapid response of to the PARP inhibitor olaparib. Second, the two PALB2 somatic reverse mutations and AR gene amplification could underlie the relative shorter response time. Gogola et al[26] had reported on activation of the PI3K-AKT–mTOR signal transduction pathway as a mechanism of resistance. These activated oncogenic pathways may cause the expression of homologous recombination genes, which were compensating for DNA double-breaks. By the way,the activated signaling pathways can accelerate the progression of cell cycle or allow cells to evade apoptosis[27]. Because our patient carried mutations in the PTEN and FGFR1 genes, it is intriguing to consider that FGFR1 amplification with a loss-of-function mutation in PTEN can directly or indirectly activate the RAS-RAF-MAPK/ERK signaling pathway as well as that of PI3K-AKT-mTOR[28]. Further validation at the functional level is warranted. According to the patient’s ctDNA sequencing results, we were able to evaluate the patient’s treatment and response course thoroughly.
Several limits to this clinical evaluative approach still need to be addressed. First, with full respect to the patient’s willingness, comprehensive genomic profiling is typically performed only when resistance to olaparib presents. Owing to our patient’s rapid disease progression, we were unable to adjust the treatment strategy based on the ctDNA sequencing result. Second, we were unable to distinguish whether the two somatic PALB2 mutations discovered had been present originally or resulted from the abiraterone combination treatment. If the ctDNA comprehensive sequencing (of 508 genes) had been performed earlier, in lieu of sequencing only the BRCA1 and BRCA2 genes, then the abiraterone combination treatment may have been started earlier. In that situation, the detection of germline PALB2 aberration at a relatively early stage in the disease course may have allowed for the patient to receive olaparib monotherapy, while not being enrolled into the proxalutamide clinical trial, or the abiraterone combination treatment. Third, PALB2 as a cancer susceptibility gene increases the hazard of breast cancer (absolute risk: 41%-61%). The National Comprehensive Cancer Network (commonly known as NCCN) Genetic/Familial High-Risk assessment: Breast, Ovarian and Pancreatic (Version 1.2022) recommends that the PALB2 germline mutation carrier should start annual mammograms, with consideration of tomosynthesis and breast MRI with contrast, at age of 30 years and that the healthcare team open discussions into the option of risk-reducing mastectomy. PALB2 gene mutations are also associated with susceptibility to cancers of the ovary (absolute risk: 3%-5%), pancreas (absolute risk: 5%-10%), and breast in males. The NCCN: Prostate cancer (Version 1.2022) also recommends germline multigene testing that includes (at least) BRCA1/2 and PALB2 in its testing panel. Our patient carried a PALB2 germline pathogenic variation, so that his offspring would carry a 50% likelihood of harboring the same variation. As such, we would suggest that first- and second-degree relatives visit a genetic counselor to further evaluate whether they carry the proband’s same PALB2 mutation; if so, the relative should receive genetic counseling to gain a sufficient understanding of the correlative cancer risk, further screening options and risk-reduction strategies. This will allow the positive carrier to better protect against the onset of related cancers or at least promote their ability to suspect and seek timely assessment to detect a cancer much earlier. Although we made such suggestions to our patient’s relatives, none have accepted our suggestion as of the writing of this case report; nonetheless, this is part of our routine strategy of care and we will continue such efforts in the future.
Herein, we have described a comprehensive genomic profiling of ctDNA in a mCRPC patient which revealed HRR gene germline PALB2 mutation, including reverse mutations and others affecting known cancer-related signaling pathways. Using the ctDNA sequencing results, we were able to analyze the intrinsic mechanism underlying the patient’s rapid response and resistance to the PARP inhibitor and abiraterone combination therapy. On one hand, our case demonstrated that a patient with HRR gene PALB2 mutation can benefit from PAPR inhibitor treatment. On the other hand, the case showed the feasibility of ctDNA sequencing to guide treatment, indicate prognosis and analyze resistance and its underlying mechanisms, with ctDNA serving as a surrogate for limited or unavailable tumor tissue. Overall, though, the case provides a real-world example of how timely multigene testing can be of great importance for selecting the most precise therapeutic approach for cancer patients, especially for those with mCRPC.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68631] [Article Influence: 13726.2] [Reference Citation Analysis (201)] |
| 2. | Zhu Y, Mo M, Wei Y, Wu J, Pan J, Freedland SJ, Zheng Y, Ye D. Epidemiology and genomics of prostate cancer in Asian men. Nat Rev Urol. 2021;18:282-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 3. | Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, Gu XY, Wei WW, He J. [Report of cancer epidemiology in China, 2015]. ZhonghuaZhong Liu ZaZhi. 2019;41:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 282] [Reference Citation Analysis (0)] |
| 4. | Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H, Abida W, Bradley RK, Vinson J, Cao X, Vats P, Kunju LP, Hussain M, Feng FY, Tomlins SA, Cooney KA, Smith DC, Brennan C, Siddiqui J, Mehra R, Chen Y, Rathkopf DE, Morris MJ, Solomon SB, Durack JC, Reuter VE, Gopalan A, Gao J, Loda M, Lis RT, Bowden M, Balk SP, Gaviola G, Sougnez C, Gupta M, Yu EY, Mostaghel EA, Cheng HH, Mulcahy H, True LD, Plymate SR, Dvinge H, Ferraldeschi R, Flohr P, Miranda S, Zafeiriou Z, Tunariu N, Mateo J, Perez-Lopez R, Demichelis F, Robinson BD, Schiffman M, Nanus DM, Tagawa ST, Sigaras A, Eng KW, Elemento O, Sboner A, Heath EI, Scher HI, Pienta KJ, Kantoff P, de Bono JS, Rubin MA, Nelson PS, Garraway LA, Sawyers CL, Chinnaiyan AM. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2400] [Cited by in RCA: 2656] [Article Influence: 241.5] [Reference Citation Analysis (0)] |
| 5. | DiSilvestro P, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, Gourley C, Banerjee S, Oza A, González-Martín A, Aghajanian CA, Bradley WH, Mathews CA, Liu J, Lowe ES, Bloomfield R, Moore KN. Efficacy of Maintenance Olaparib for Patients With Newly Diagnosed Advanced Ovarian Cancer With a BRCA Mutation: Subgroup Analysis Findings From the SOLO1 Trial. J Clin Oncol. 2020;38:3528-3537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 2424] [Article Influence: 269.3] [Reference Citation Analysis (0)] |
| 7. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1766] [Article Influence: 252.3] [Reference Citation Analysis (0)] |
| 8. | Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, Syndikus I, Ralph C, Jain S, Varughese M, Parikh O, Crabb S, Robinson A, McLaren D, Birtle A, Tanguay J, Miranda S, Figueiredo I, Seed G, Bertan C, Flohr P, Ebbs B, Rescigno P, Fowler G, Ferreira A, Riisnaes R, Pereira R, Curcean A, Chandler R, Clarke M, Gurel B, Crespo M, Nava Rodrigues D, Sandhu S, Espinasse A, Chatfield P, Tunariu N, Yuan W, Hall E, Carreira S, de Bono JS. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 507] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 9. | Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A'Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373:1697-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1642] [Cited by in RCA: 1806] [Article Influence: 164.2] [Reference Citation Analysis (0)] |
| 10. | de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Mehra N, Goessl C, Kang J, Burgents J, Wu W, Kohlmann A, Adelman CA, Hussain M. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382:2091-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 1683] [Article Influence: 280.5] [Reference Citation Analysis (0)] |
| 11. | Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, Chiuri VE, Jassem J, Fléchon A, Redfern C, Goessl C, Burgents J, Kozarski R, Hodgson D, Learoyd M, Saad F. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19:975-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 12. | Lavaud P, Gravis G, Foulon S, Joly F, Oudard S, Priou F, Latorzeff I, Mourey L, Soulié M, Delva R, Krakowski I, Laguerre B, Théodore C, Ferrero JM, Beuzeboc P, Habibian M, Rolland F, Deplanque G, Pouessel D, Zanetta S, Berdah JF, Dauba J, Baciuchka M, Platini C, Linassier C, Tubiana-Mathieu N, Machiels JP, Kouri CE, Ravaud A, Suc E, Eymard JC, Hasbini A, Bousquet G, Culine S, Boher JM, Tergemina-Clain G, Legoupil C, Fizazi K. Anticancer Activity and Tolerance of Treatments Received Beyond Progression in Men Treated Upfront with Androgen Deprivation Therapy With or Without Docetaxel for Metastatic Castration-naïve Prostate Cancer in the GETUG-AFU 15 Phase 3 Trial. EurUrol. 2018;73:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard G, de Bono J, Cross W, Jones RJ, Thalmann G, Amos C, Matheson D, Millman R, Alzouebi M, Beesley S, Birtle AJ, Brock S, Cathomas R, Chakraborti P, Chowdhury S, Cook A, Elliott T, Gale J, Gibbs S, Graham JD, Hetherington J, Hughes R, Laing R, McKinna F, McLaren DB, O'Sullivan JM, Parikh O, Peedell C, Protheroe A, Robinson AJ, Srihari N, Srinivasan R, Staffurth J, Sundar S, Tolan S, Tsang D, Wagstaff J, Parmar MK; STAMPEDE investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1374] [Cited by in RCA: 1617] [Article Influence: 161.7] [Reference Citation Analysis (0)] |
| 14. | Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, Shevrin DH, Dreicer R, Hussain M, Eisenberger M, Kohli M, Plimack ER, Vogelzang NJ, Picus J, Cooney MM, Garcia JA, DiPaola RS, Sweeney CJ. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36:1080-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 823] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 15. | Gravis G, Boher JM, Chen YH, Liu G, Fizazi K, Carducci MA, Oudard S, Joly F, Jarrard DM, Soulie M, Eisenberger MJ, Habibian M, Dreicer R, Garcia JA, Hussain MHM, Kohli M, Vogelzang NJ, Picus J, DiPaola R, Sweeney C. Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. EurUrol. 2018;73:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 16. | Armstrong AJ, Halabi S, Luo J, Nanus DM, Giannakakou P, Szmulewitz RZ, Danila DC, Healy P, Anand M, Rothwell CJ, Rasmussen J, Thornburg B, Berry WR, Wilder RS, Lu C, Chen Y, Silberstein JL, Kemeny G, Galletti G, Somarelli JA, Gupta S, Gregory SG, Scher HI, Dittamore R, Tagawa ST, Antonarakis ES, George DJ. Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer: The PROPHECY Study. J Clin Oncol. 2019;37:1120-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 293] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 17. | Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, Liu X, Jasin M, Couch FJ, Livingston DM. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 659] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 18. | Abida W, Campbell D, Patnaik A, Shapiro JD, Sautois B, Vogelzang NJ, Voog EG, Bryce AH, McDermott R, Ricci F, Rowe J, Zhang J, Piulats JM, Fizazi K, Merseburger AS, Higano CS, Krieger LE, Ryan CJ, Feng FY, Simmons AD, Loehr A, Despain D, Dowson M, Green F, Watkins SP, Golsorkhi T, Chowdhury S. Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin Cancer Res. 2020;26:2487-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 19. | Niraparib Shrinks. BRCA-Mutated Prostate Tumors. Cancer Discov. 2019;9:OF7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | M Hussain JM, K Fizazi, F Saad, ND Shore, S Sandhu, KN Chi, O Sartor, N Agarwal, D Olmos, A Thiery-Vuillemin, P Twardowski, N Mehra, C Goessl, J Kang, J Burgents, W Wu, A Kohlmann, CA Adelman, J de Bono. Profound: Phase 3 Study of Olaparib Versus Enzalutamide or Abiraterone for Metastatic Castration-Resistant Prostate Cancer (Mcrpc) with Homologous Recombination Repair (Hrr) Gene Alterations. Annals of Oncology. 2019;851-934. |
| 21. | Asim M, Tarish F, Zecchini HI, Sanjiv K, Gelali E, Massie CE, Baridi A, Warren AY, Zhao W, Ogris C, McDuffus LA, Mascalchi P, Shaw G, Dev H, Wadhwa K, Wijnhoven P, Forment JV, Lyons SR, Lynch AG, O'Neill C, Zecchini VR, Rennie PS, Baniahmad A, Tavaré S, Mills IG, Galanty Y, Crosetto N, Schultz N, Neal D, Helleday T. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017;8:374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 22. | Li L, Karanika S, Yang G, Wang J, Park S, Broom BM, Manyam GC, Wu W, Luo Y, Basourakos S, Song JH, Gallick GE, Karantanos T, Korentzelos D, Azad AK, Kim J, Corn PG, Aparicio AM, Logothetis CJ, Troncoso P, Heffernan T, Toniatti C, Lee HS, Lee JS, Zuo X, Chang W, Yin J, Thompson TC. Androgen receptor inhibitor-induced "BRCAness" and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 23. | Seitz AK, Thoene S, Bietenbeck A, Nawroth R, Tauber R, Thalgott M, Schmid S, Secci R, Retz M, Gschwend JE, Ruland J, Winter C, Heck MM. AR-V7 in Peripheral Whole Blood of Patients with Castration-resistant Prostate Cancer: Association with Treatment-specific Outcome Under Abiraterone and Enzalutamide. EurUrol. 2017;72:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | De Laere B, Rajan P, Grönberg H, Dirix L, Lindberg J; CORE-ARV-CTC and ProBIO Investigators. Androgen Receptor Burden and Poor Response to Abiraterone or Enzalutamide in TP53 Wild-Type Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2019;5:1060-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Goodall J, Mateo J, Yuan W, Mossop H, Porta N, Miranda S, Perez-Lopez R, Dolling D, Robinson DR, Sandhu S, Fowler G, Ebbs B, Flohr P, Seed G, Rodrigues DN, Boysen G, Bertan C, Atkin M, Clarke M, Crespo M, Figueiredo I, Riisnaes R, Sumanasuriya S, Rescigno P, Zafeiriou Z, Sharp A, Tunariu N, Bianchini D, Gillman A, Lord CJ, Hall E, Chinnaiyan AM, Carreira S, de Bono JS; TOPARP-A investigators. Circulating Cell-Free DNA to Guide Prostate Cancer Treatment with PARP Inhibition. Cancer Discov. 2017;7:1006-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 345] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 26. | Gogola E, Rottenberg S, Jonkers J. Resistance to PARP Inhibitors: Lessons from Preclinical Models of BRCA-Associated Cancer. Annual Review of Cancer Biology. 2019;3:235-254. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Pilié PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019;16:81-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 877] [Article Influence: 125.3] [Reference Citation Analysis (1)] |
| 28. | Corn PG, Wang F, McKeehan WL, Navone N. Targeting fibroblast growth factor pathways in prostate cancer. Clin Cancer Res. 2013;19:5856-5866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Chinese Anti-Cancer Association - Medical Ethics Professional Committee; the Chinese Society of Clinical Oncology (CSCO) Big Data Expert Committee.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hazafa A, Pakistan; Yamaguchi K, Japan S-Editor: Wang LL L-Editor: A P-Editor: Wang LL