Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.91
Peer-review started: May 13, 2021
First decision: July 4, 2021
Revised: July 22, 2021
Accepted: November 26, 2021
Article in press: November 26, 2021
Published online: January 7, 2022
Processing time: 231 Days and 7.8 Hours
Early prediction of response to percutaneous catheter drainage (PCD) of necrotic collections in acute pancreatitis (AP) using simple and objective tests is critical as it may determine patient prognosis. The role of white blood cell (WBC) count and neutrophil-lymphocyte ratio (NLR) has not been assessed as a tool of early prediction of PCD success and is the focus of this study.
To assess the value of WBC and NLR in predicting response to PCD in AP.
This retrospective study comprised consecutive patients with AP who underwent PCD between June 2018 and December 2019. Severity and fluid collections were classified according to the revised Atlanta classification and organ failure was defined according to the modified Marshall Score. WBC and NLR were monitored 24 h prior PCD (WBC-0/NLR-0) and 24 h (WBC-1/NLR-1), 48 h (WBC-2/NLR-2) and 72 h (WBC-3/NLR-3) after PCD. NLR was calculated by dividing the number of neutrophils by the number of lymphocytes. The association of success of PCD (defined as survival without the need for surgery) with WBC and NLR was assessed. The trend of WBC and NLR was also assessed post PCD.
One hundred fifty-five patients [median age 40 ± 13.6 (SD), 64.5% males, 53.5% severe AP] were included in the final analysis. PCD was done for acute necrotic collection in 99 (63.8%) patients and walled-off necrosis in 56 (36.1%) patients. Median pain to PCD interval was 24 ± 69.89 d. PCD was successful in 109 patients (group 1) and 46 patients (group 2) who failed to respond. There was no significant difference in the baseline characteristics between the two groups except the severity of AP and frequency of organ failure. Both WBC and NLR showed an overall decreasing trend. There was a significant difference between WBC-0 and WBC-1 (P = 0.0001). WBC-1 and NLR-1 were significantly different between the two groups (P = 0.048 and 0.003, respectively). The area under the curve of WBC-1 and NLR-1 for predicting the success of PCD was 0.602 and 0.682, respectively. At a cut-off value of 9.87 for NLR-1, the sensitivity and specificity for predicting the success of PCD were calculated to be 75% and 65.4% respectively.
WBC and NLR can be used as simple tests for predicting response to PCD in patients with acute necrotizing pancreatitis.
Core Tip: Predicting the success of percutaneous catheter drainage (PCD) is critical for a timely decision regarding further interventions. Neutrophilia and lymphopenia are surrogate markers of systemic inflammation and physiological stress. In this study, we evaluate the performance of white blood cell (WBC) and neutrophil-lymphocyte ratio (NLR) as predictors of response to PCD in patients with acute necrotizing pancreatitis. We found a falling trend in both WBC and NLR values, with WBC values showing a significant fall on day one after PCD compared to pre-procedure value.
- Citation: Gupta P, Das GC, Bansal A, Samanta J, Mandavdhare HS, Sharma V, Naseem S, Gupta V, Yadav TD, Dutta U, Varma N, Sandhu MS, Kochhar R. Value of neutrophil-lymphocyte ratio in evaluating response to percutaneous catheter drainage in patients with acute pancreatitis. World J Clin Cases 2022; 10(1): 91-103
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/91.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.91
Acute pancreatitis (AP) is a common diagnosis in patients presenting with an acute abdomen[1]. In most patients, it is mild and recovers without any sequelae[1,2]. Necrotizing pancreatitis occurs in approximately 20%-30% of patients and is associated with significant morbidity and mortality[2]. Two-thirds of the patients with acute necrotizing pancreatitis (ANP) and sterile necrosis can be managed expectantly[3,4]. In the rest of the patients, pancreatic necrosis gets infected, usually during the third or fourth weeks of illness[3,4]. Infected pancreatic necrosis (IPN) has a mortality rate of up to 40%, particularly if associated with organ failure (OF)[5,6]. IPN needs drainage[7]. Mass effect on adjacent structures, increased intra-abdominal pressure and generalized “unwellness” for several weeks following ANP are other indications for drainage[7]. Minimally invasive techniques such as percutaneous catheter drainage (PCD), endoscopic transluminal drainage/necrosectomy, video-assisted retroperitoneal debridement (VARD), and minimally invasive surgical necrosectomy are preferred for drainage of the pancreatic collection as open surgical necrosectomy with associated with significant morbidity and mortality[8-11].
Though endoscopic ultrasound (EUS) guided interventions have become popular, PCD remains relevant as EUS has limited utility for collections away from lesser sac or gastroduodenal region and in the setting of acute necrotic collection (ANC). PCD also acts as the first step for other procedures, including VARD or percutaneous endo
Although a few reports have evaluated the role of WBC and NLR in predicting the severity of AP, there are no published reports of the utilization of WBC and NLR to predict the success of PCD in ANP[17-20]. This study aimed to assess the role of WBC and NLR in predicting response to PCD in ANP.
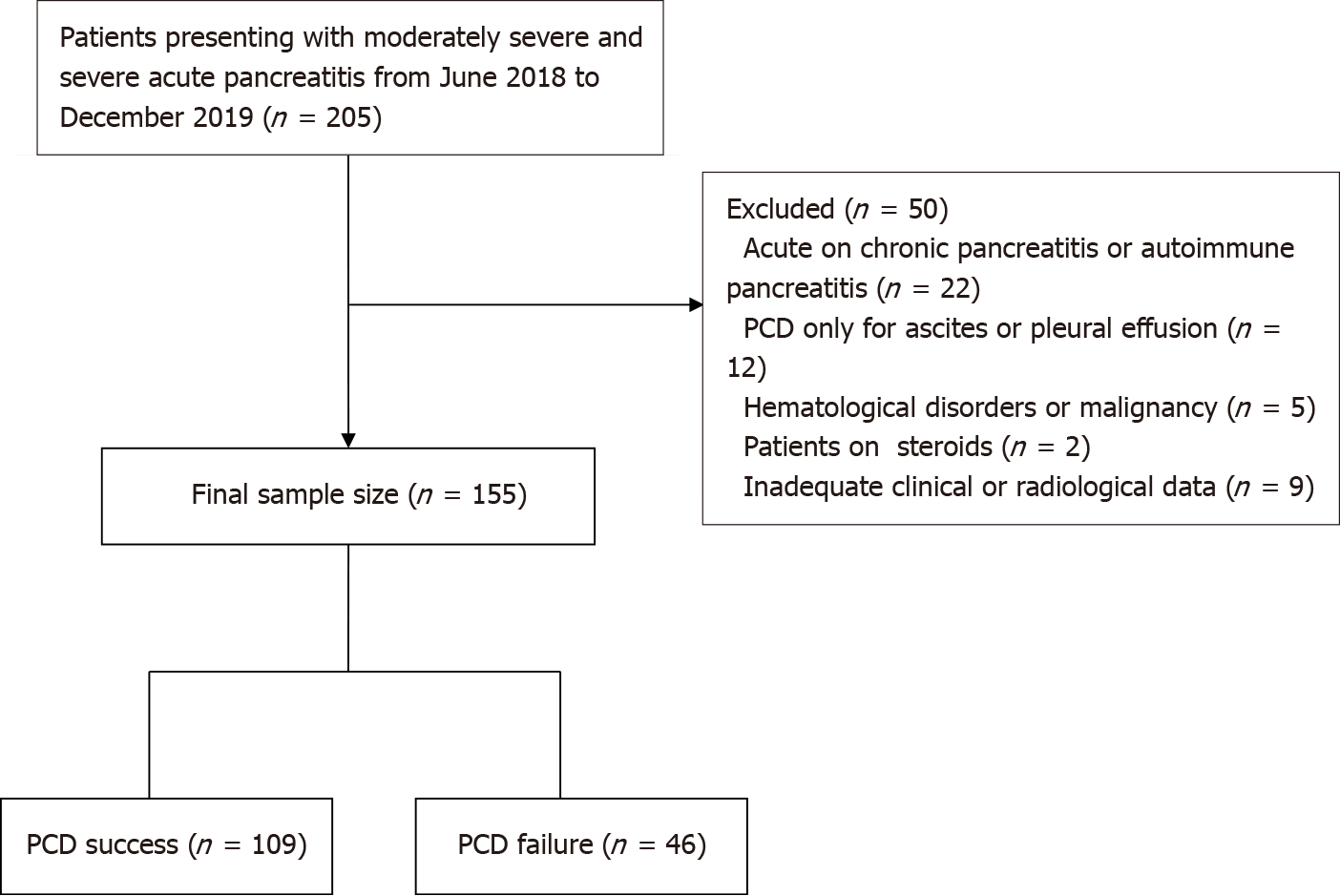
Our institutional ethics committee approved this retrospective observational study. Procedural informed written consent was obtained from all the patients. We retrospectively analyzed the data of consecutive patients with ANP undergoing PCD between June 2018 and December 2019. During the study period, 205 patients with moderately severe and severe AP were admitted. Patients who presented with acute on chronic or autoimmune pancreatitis (n = 22), patients in whom PCD was done for ascites or pleural effusion (n = 12), patients with hematological disorders or malignancy (n = 5), patients on steroids (n = 2), and who had inadequate baseline data (n = 9), were excluded. The remaining 155 patients who underwent PCD of ANC or WON comprised the final study group. Figure 1 shows the patient recruitment. The mean age was 40 ± 13.6 years (range, 15-82 years). There were 100 (64.5%) males and 55 (35.5%) females.
Co-morbidities were recorded. Severity classification of AP was done according to the revised Atlanta classification (RAC)[21]. Based on RAC, fluid collections developing ≤ 4 wk after pain onset are labeled as ANC, and those developing later are designated as walled-off necrosis (WON)[21]. A score of ≥ 2 in the modified Marshall scoring system for organ dysfunction was defined as the presence of OF. According to RAC, the presence of transient OF (OF < 48 h) or local or systemic complications without persistent OF indicates moderately severe disease. Patients with persistent OF (> 48 h, single or multiple) are classified as severe diseases. WBC and NLR values within 24 h before the first PCD (Baseline-WBC-0 and NLR-0) were recorded. WBC and differential leukocyte count were performed on an automated hematology analyzer (LH-780, Beckman coulter, United States). NLR was calculated by dividing the number of neutrophils by the number of lymphocytes. Besides, procalcitonin levels 24 h prior to the first PCD were recorded. The baseline contrast-enhanced CT (CECT) performed after 72 h of the onset of pain was reviewed for the size and collection site.
Suspected infection, persistent OF, and/or presence of pressure symptoms were the indications of PCD. An interventional radiologist with 3-10 years’ experience in non-vascular abdominal interventions performed PCD under ultrasonography or CT guidance. Depending on the size, location, and extent of collection on CECT, the site of PCD was determined. Coagulation parameters were normalized (platelet count of at least 50000/mL and prothrombin index > 75%) if deranged before the procedure. PCD was done with a 10F to 16F catheter using the Seldinger technique. An 18 G puncture needle and 0.035-inch stiff guidewire were used for access to the collection. The tract was adequately dilated before placing a pigtail or a malecot catheter into the collection. Finally, the catheter was sutured with skin, and a drainage bag was connected. Aspirated fluid was sent for culture and microbial sensitivity testing. Daily flushing of the catheter was done using 50-100 mL normal saline to avoid catheter blockage. In persistent OF, ongoing sepsis, or systemic inflammatory response syndrome, catheter upgradations were done under ultrasound or fluoroscopic guidance. We used a 2-4F larger catheter than the already inserted catheter for upgradation. All patients underwent ultrasound after 48 h of PCD for assessment of the size of the collection. Patients who did not improve one week after PCD or even after upgradation underwent repeat CT. The decision for additional intervention was taken accordingly. In cases where the collection had entirely resolved or drain output had decreased to less than 10-20 mL/d for three consecutive days, the catheter was removed, provided the patient was afebrile with no signs of ongoing sepsis. For this study, only the first catheter insertion was considered for assessing success.
All patients were managed initially with fluid resuscitation, pain alleviation, oxygen support, organ system support, and nutritional support (enteral or parenteral) according to standard recommendations[22,23]. CECT of the abdomen was done between 5 and 7 d of onset of symptoms. As per the protocol in our center, opioids (tramadol, fentanyl) were used for pain relief. Antibiotics were given to patients with suspicion of infected necrosis (air foci on CECT or patient’s worsening clinical course) or extrapancreatic infections (e.g., pneumonia, cholangitis), those having persistent fever beyond first week, or persistent multiple OF. Therefore, all patients were receiving antibiotics at the time of PCD. The culture of drain fluid was done to establish IPN. Pus culture and sensitivity directed choice of antibiotic regimens. The management of infected or symptomatic fluid collection was done using a step-up approach. Depending on the location of collections on CECT, percutaneous or endoscopic or a combined modality approach was chosen for drainage. If there was failure of clinical improvement after initial PCD, catheter upsizing was done. In patients having WON in the lesser sac or patients not improving with PCD alone, endoscopic necrosectomy was performed. Surgical necrosectomy was performed in patients who had necrotic collections at sites not amenable for endoscopic necrosectomy, or where endoscopic necrosectomy was not feasible or unsuccessful. The success of PCD was defined as survival (up to 6 wk after discharge from the hospital) without the need for surgery, and two groups (success vs failure) were made accordingly. The need for additional PCD or upsizing was not considered a failure of PCD.
Post-PCD WBC and NLR at 24 h (WBC-1/NLR-1), 48 h (WBC-2/NLR-2) and 72 h (WBC-3/NLR-3) were recorded.
Outcomes including the length of hospital stay, need for intensive care unit (ICU) admission, length of ICU stay, need for surgery, and mortality was recorded.
The data were analyzed using SPSS software version 23. The categorical variables were presented as proportions and percentages. The quantitative parameters were reported as the mean (with range) or median (with interquartile range), depending on the distribution. For categorical data, the Chi-square test or Fischer's exact test was used. The independent continuous variables were compared using the Student t-test or Mann-Whitney U-test, depending on the data's normality. Correlation between continuous variables was assessed using Pearson’s correlation coefficient or Spearman’s rank correlation coefficient, depending on the normality of the data. The differences in continuous variables in more than two paired groups were tested using the Wilcoxon signed rank test. Trends of WBC and NLR were evaluated over 72 h post-procedure and depicted using line diagrams, and differences in their trends were assessed. Receiver operating characteristic curves were assessed to predict the success of PCD based on WBC and NLR at different time points. The area under the curve (AUC) and cut-off values for a defined sensitivity and specificity were reported. A P value of less than 0.05 was considered to be statistically significant.
The most common etiological factor was alcohol consumption (n = 79, 51%), followed by gallstone disease (n = 63, 40.6%), and endoscopic retrograde cholangiopancreatography (ERCP) (n = 6, 3.9%). Thirty-two (20.6%) patients had comorbidities [diabetes mellitus (n = 19), hypertension (n = 21), coronary artery disease (n = 8), and chronic obstructive airway disease (n = 14)]. Thirteen patients had multiple co-morbidities. Eighteen (11.6%) patients were obese. According to RAC, seventy-two (46.5%) patients had moderately severe disease, and 83 (53.5%) patients had severe disease. Fifty-seven patients (36.7%) had infected necrosis (based on culture). Extrapancreatic infections were present in 15 (9.7%) patients. Blood cultures were positive in 10 (6.4%) patients. OF was present in 100 (64.5%) patients. Thirty-nine (25.2%) patients were on mechanical ventilation. ERCP was performed in 15 (9.7%) patients (excluding those with post-ERCP pancreatitis). The median procalcitonin level was 0.56 ng/mL (range, 0-100). PCD was done for ANC in 99 (63.8%) patients and WON in 56 (36.1%) patients. Median pain to PCD interval was 24 d. The initial catheter size ranged from 10-16F. The mean initial catheter size was 12.6 ± 1.9F. The mean number of catheters inserted per patient was 1.8 (range, 1-7). Multiple catheters were inserted in 41.9% of the patients (n = 65). Catheter upsizing was performed in 86 (55.5%) patients. Twenty-four (15.4%) patients underwent surgery, and 7 (4.5%) patients underwent endoscopic drainage. The mean hospital stay was 22 ± 19.3 d. ICU admission was recorded in 73 (47.1%) patients with a mean length of ICU stay of 6.1 ± 10.1 d. Thirty-two (20.6%) patients died during the study period. PCD was successful in 109 patients (group 1), and 46 patients (group 2) failed to respond to PCD. The baseline demographic characteristics and outcomes are highlighted in Table 1.
| Characteristic | Overall cohort |
| Age, yr (mean ± SD) | 40 ± 13.6 |
| Sex (%) | |
| Males | 64.5 |
| Females | 35.5 |
| Etiology, n (%) | |
| Alcohol | 79 (51) |
| Gallstones | 63 (40.6) |
| ERCP | 6 (3.9) |
| Idiopathic | 5 (3.2) |
| Hyperparathyroidism | 2 (1.3) |
| Co-morbidities, n (%) | 32 |
| Diabetes mellitus | 19 (59.4) |
| Hypertension | 21 (65.6) |
| Coronary artery disease | 8 (25) |
| Chronic obstructive airway disease | 14 (43.7) |
| Obesity, n (%) | 18 (11.6%) |
| Severity, n (%) | |
| Moderately severe | 72 (46.4) |
| Severe | 83 (53.5) |
| Pain to PCD interval, d (median) | 24 |
| Site of collection, n (%) | |
| Lesser sac | 94 (62.6) |
| Paracolic gutter | 28 (18.7) |
| Lesser sac and paracolic gutter | 16 (10.7) |
| Perisplenic | 4 (2.7) |
| Perihepatic | 3 (2) |
| Pelvic | 5 (3.3) |
| Infected necrosis, n (%) | 75 (36.7) |
| Organ failure, n (%) | 100 (64.5) |
| Length of hospital stay, d (mean ± SD) | 22 ± 19.3 |
| Length of ICU stay, d (mean ± SD) | 6.1 ± 10.1 |
| Surgery, n (%) | 24 (15.4) |
| Endoscopic drainage, n (%) | 7 (4.5) |
| Mortality, n (%) | 32 (20.6) |
There was no significant difference in the pain to PCD interval and procalcitonin levels between groups 1 and 2. The mean pain to PCD interval in group 1 was 39.9 ± 49.3 d, and group 2 was 54.06 ± 76.8 d (P = 0.249). The mean procalcitonin level was 3.2 ± 11.2 ng/mL in group 1 and 5.4 ± 15.3 ng/mL in group 2 (P = 0.331). According to the RAC, patients with severe AP were more likely to have PCD failure compared with the moderately severe AP (P < 0.001). The size of the collection on baseline CECT was comparable between the two groups (11.5 cm in group 1 vs 11.5 cm in group 2, P = 0.974). The most common site of the collection was a lesser sac (with variable extension to other sites) in both groups (64.2% in group 1 vs 52.2% in group 2, P = 0.464). There was no significant association between PCD success and initial catheter size (P = 0.598) or infected necrosis (P = 0.447). OF was more frequent in group 2, but the difference was not statistically significant (P = 0.076) (Table 2).
| Parameters | Overall cohort (n = 155) | |||
| Characteristics | Compared variable | Group I | Group II | P value |
| Number | 109 | 46 | ||
| Pain to PCD interval | Mean | 39.8 | 54.1 | 0.249 |
| Site of collection (%) | Lesser sac | 64.2 | 52.2 | 0.464 |
| Paracolic | 16.5 | 21.7 | ||
| Lesser sac + paracolic | 11 | 8.7 | ||
| Procalcitonin | Mean | 3.2 | 5.4 | 0.331 |
| Collection size | Mean | 11.4 | 11.5 | 0.974 |
| Catheter size | < 12F | 23 | 8 | 0.598 |
| > 12F | 86 | 38 | ||
| RAC severity | Moderately severe | 62 | 11 | < 0.001 |
| Severe | 47 | 35 | ||
| Infected necrosis | No | 71 | 27 | 0.447 |
| Yes | 38 | 19 | ||
| Organ failure | No | 43 | 11 | 0.076 |
| Yes | 66 | 34 | ||
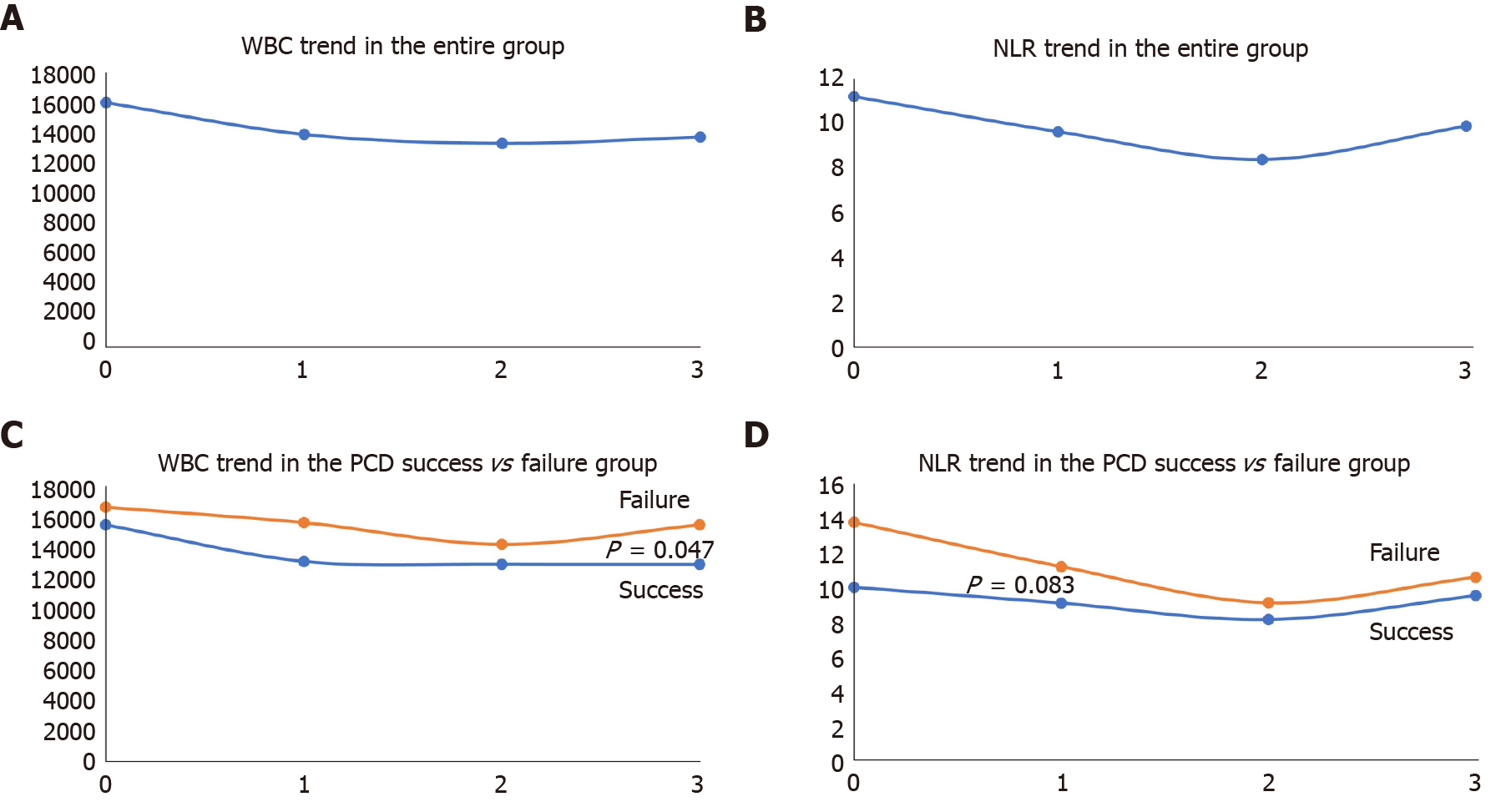
The median WBC before PCD and post PCD (day 1, 2, and 3) were as follows: WBC-0 = 14900 ± 7543.1, WBC-1 = 12320 ± 6743.4, WBC-2 = 11650 ± 6464.5 and WBC-3 = 12400 ± 8825.6. There was a significant difference between WBC-0 and WBC-1 (P = 0.0001) and WBC-1 and WBC-2 (P = 0.027). However, there was no significant difference between WBC-2 and WBC-3. The median NLR-0, NLR-1, NLR-2, and NLR-3 were 8.3 ± 9.16, 7.2 ± 10.3, 6.64 ± 5.6 and 6.6 ± 10.4, respectively. The serial NLRs were not significantly different from each other. The serial WBC and NLR are shown in Table 3 and Figure 2.
| Day | n | Median | IQR (25%-75%) |
| WBC | |||
| 0 | 155 | 14.9 | 9.9-20.7 |
| 1 | 110 | 12.3 | 8.9-17.4 |
| 2 | 151 | 11.6 | 8.3-17.6 |
| 3 | 102 | 12.4 | 8.9-16.2 |
| NLR | |||
| 0 | 140 | 8.2 | 5.2-14.1 |
| 1 | 88 | 7.2 | 4.4-12 |
| 2 | 136 | 6.4 | 4.1-11.9 |
| 3 | 89 | 6.6 | 3.8-12.1 |
Both WBC and NLR showed an overall decreasing trend (Figure 3A and B). There was a significant difference in WBC trend between groups 1 and 2 (P =0.047) (Figure 3C). The difference was also seen in NLR's trend; however, it was not statistically significant (P = 0.083) (Figure 3D). At 24 h post PCD, 71.6% of the patients had a fall in WBC while the rest had a rise in WBC. Similarly, 74.2% of the patients had a fall in NLR, and the rest had an increase in NLR at 24 h after PCD.
No significant difference was seen in the baseline WBC and NLR between the two groups. WBC-1 and NLR-1 were significantly different between the two groups (P = 0.048 and 0.003, respectively). WBC-2, NLR-2, WBC-3, and NLR-3 did not show any significant difference between the two groups. The absolute change in the WBC and NLR (at all time points) was not significantly different between the groups.
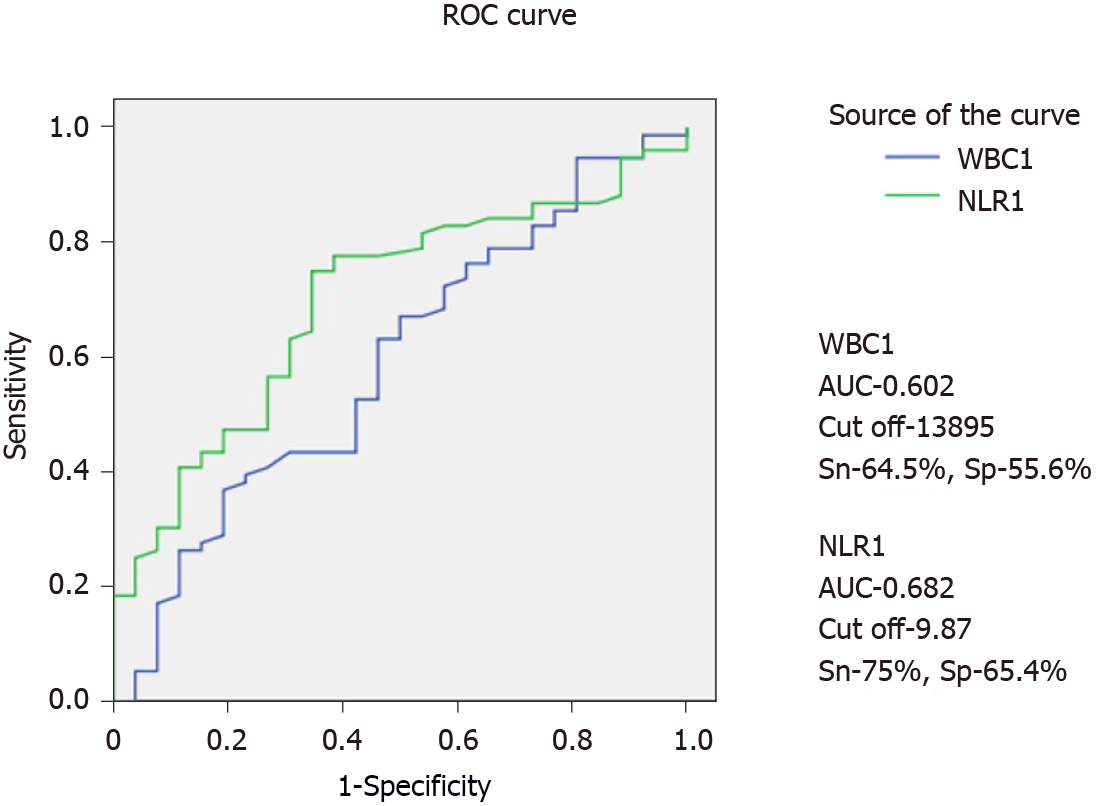
AUC for predicting the success of PCD for WBC-1 and NLR-1 was 0.602 and 0.682, respectively. Using a cut-off value of 13.9 × 103/μL for WBC-1, sensitivity and specificity were 64.5% and 55.6%, respectively. Similarly, using a cut-off value of 9.9 for NLR-1, sensitivity and specificity were calculated to be 75% and 65.4%, respectively. The AUCs of WBC and NLR at other time points are shown in Figure 4 and Table 4.
| Time point | WBC | NLR |
| Baseline (0) | 0.508 | 0.401 |
| 24 h (1) | 0.602 | 0.682 |
| 48 h (2) | 0.468 | 0.407 |
| 72 h (3) | 0.416 | 0.427 |
In this study, we evaluated the performance of WBC and NLR as predictors of response to PCD in patients with ANP. We found a significant fall in the WBC on day one following PCD in patients who responded to PCD compared with those who did not respond. NLR also showed a falling trend; however, the difference was not statistically significant. The WBC and NLR on day one post PCD were significantly lower in patients with a response to PCD than those who failed to respond to PCD. NLR performed better than WBC in predicting the success of PCD. On day one, the AUC for NLR was higher compared with the AUC for WBC on day one following PCD (0.682 vs 0.602). The day 1 NLR cut-off value of 9.9 had a sensitivity and specificity of 75% and 65.4%, respectively, for predicting response to PCD. For WBC-1, a cut-off value of 13.9 × 103/μL yielded a sensitivity and specificity of 64.5% and 55.6%, respectively.
Predicting the success of PCD is critical for a timely decision regarding further interventions. Previous studies have evaluated the role of serum inflammatory markers to predict the response to PCD[15,16]. In a study by Mallick et al[16] comprising 59 patients, serum levels of C-reactive protein (CRP), interleukin (IL)-6, and IL-10, before PCD and at 3 and 7 d following PCD were evaluated. There was a significant decrease in all three markers on day 3. Fall in IL-6 and CRP correlated with the outcomes of patients managed with PCD. Other investigators have explored the role of CT density of the collection, serial volume measurement of the collection, and resolution of OF to predict response to PCD[13,14]. WBC is a simple and inexpensive test routinely performed during the initial evaluation and follow-up of hospitalized patients with AP[24]. NLR is easily calculated from WBC. Neutrophilia and lymphopenia are surrogate markers of systemic inflammation and physiological stress[25,26]. The inflammatory cytokines generated during AP incite neutrophilia, causing increased NLR responsible for pancreatic tissue damage. Lymphocytes have been shown to decrease within 48 h of AP[20,24]. However, there are no studies evaluating the role of NLR in the prediction of response to PCD.
NLR has been shown to predict the prognosis of various benign and malignant diseases[27-31]. NLR has been shown to correlate with SOFA and APACHE-II scores in ICU patients[32,33]. A few studies have reported the prediction of the severity of AP using NLR[17-20]. It has been demonstrated that NLR is superior to WBC in predicting clinical outcomes in critically ill patients[28-31]. NLR's superiority over WBC is due to several factors, including alteration of WBC by various physiological and pathological states, including stress, pregnancy, and hydration status, and technical aspects, including withdrawal and handling of blood samples. The WBC/individual subtypes are prone to be affected by these factors while NLR remains stable. More importantly, NLR represents the contribution of two divergent immune pathways. The neutrophilic response to inflammatory mediators, e.g., myeloperoxidase, elastase, IL-1, and IL-6, leads to non-specific inflammation and tissue destruction. Lymphocyte immune response occurs later than the neutrophilic response and aims to control non-specific inflammation[34]. Persistent lymphopenia is associated with poor prognosis in critically ill patients[35,36]. Besides, there may be lymphocyte dysfunction. The association between reduced lymphocyte count and severity of pancreatitis has been reported previously[37]. NLR is dynamic, and the optimal cut-off varies with time.
We also found that NLR was superior to WBC in predicting response to PCD. WBC and NLR on day 1 were found to be significantly associated with PCD success. This can be explained by a fall in neutrophils and an increase in lymphocytes over the 1st 24 h following PCD, secondary to reducing inflammatory load. Previous studies have shown that the maximum reduction in the volume of the collection is achieved in the 1st 24 h[13,14]. Mallick et al[16] showed that a major decline in the levels of IL-6 and IL-10 occurred within 72 h.
A few other predictors of PCD outcome have been reported in the literature. Reduction in the size of the collection, decline in the inflammatory markers, and resolution of OF after PCD have been shown to predict success[13,16,38,39]. Higher CT density of collection, indicating solid debris, has been shown to be associated with poorer PCD outcomes[38,39]. Some of these factors might influence the NLR, however, the association between these factors and NLR remains to be investigated. Future studies tailored to investigate the interplay between NLR and the other predictive factors may further strengthen the rationale of the utilization of NLR as a simple robust test for PCD outcomes.
There were a few limitations to our study. As the data was analyzed retrospective, it is prone to several biases. We analyzed the WBC and NLR for the first PCD. However, patients with ANP frequently undergo multiple drainage procedures. We did not take the effect of the subsequent endoscopic or percutaneous interventions into account. We did not compare the performance of WBC and NLR with other inflammatory markers. Though we evaluated the baseline procalcitonin, the later values were not analyzed. The WBC and NLR values were missing for few patients on follow-up. The influence of co-morbidities and superadded infections on WBC and NLR could not be fully investigated.
WBC and NLR can be used as simple tests in helping to predict response to PCD in patients with ANP. The performance of NLR is superior to WBC.
Acute pancreatitis (AP) is a common diagnosis in patients presenting with an acute abdomen. Necrotizing pancreatitis occurs in approximately 20%-30% of patients and is associated with significant morbidity and mortality. Necrotic pancreatic collections are one of the most important complications that may need treatment. Minimally invasive techniques including percutaneous catheter drainage (PCD), endoscopic drainage, and minimally invasive surgery are now preferred to open necrosectomy. It is important to predict response to minimally invasive techniques to decide further interventions. The aim of this study was to predict the role of white blood cell count (WBC) and neutrophil to lymphocyte ratio (NLR) in predicting response to PCD.
Previous studies have identified computed tomography density of the collection, organ failure resolution, and volume reduction of the fluid collection after one week of PCD as significant predictors of successful PCD outcomes. A few studies have reported the utility of inflammatory markers in predicting the response to PCD, however, data on WBC and NLR is lacking in this regard. Evaluation of WBC count and NLR is simple, inexpensive, and universally available and we evaluated their role in PCD response prediction.
This was a retrospective study to evaluate the role of WBC and NLR in predicting response to PCD and clinical outcomes in terms of hospital and intensive care unit stay, need for surgery.
We retrospectively analyzed WBC and NLR values 24 h before PCD and successive values at 24, 48, and 72 h after the procedure. The success of PCD was defined as survival (up to 6 wk after discharge from the hospital) without the need for surgery, and patients were divided into two groups (success vs failure) accordingly. The association of the success of PCD with WBC and NLR was assessed. The trend of WBC and NLR was also assessed post PCD.
One hundred fifty-five patients [median age 40 ± 13.6 (SD), 64.5% males, 53.5% severe AP] were included in the final analysis. PCD was done for acute necrotic collection in 99 (63.8%) patients and walled off necrosis in 56 (36.1%) patients. PCD was successful in 109 patients (group 1) and 46 patients (group 2) failed to respond. There was no significant difference in the baseline characteristics between the two groups except severity of AP and frequency of organ failure. Both WBC and NLR showed an overall decreasing trend. There was a significant difference between WBC-0 and WBC-1 (P = 0.0001). WBC-1 and NLR-1 were significantly different between the two groups (P = 0.048 and 0.003, respectively). The area under the curve of WBC-1 and NLR-1 for predicting the success of PCD was 0.602 and 0.682, respectively. At a cut-off value of 9.87 for NLR-1, the sensitivity and specificity for predicting the success of PCD were calculated to be 75% and 65.4% respectively.
Our study has shown that WBC and NLR values and their trends can be used to predict success of PCD in a timely manner.
WBC and NLR is a simple, safe, and inexpensive tool for predicting response to PCD and can be used to decide the need for further interventions and thus improve patient outcomes.
| 1. | Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 573] [Article Influence: 31.8] [Reference Citation Analysis (5)] |
| 2. | Beger HG, Rau BM. Severe acute pancreatitis: Clinical course and management. World J Gastroenterol. 2007;13:5043-5051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 164] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | van Grinsven J, van Santvoort HC, Boermeester MA, Dejong CH, van Eijck CH, Fockens P, Besselink MG; Dutch Pancreatitis Study Group. Timing of catheter drainage in infected necrotizing pancreatitis. Nat Rev Gastroenterol Hepatol. 2016;13:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Besselink MG, van Santvoort HC, Boermeester MA, Nieuwenhuijs VB, van Goor H, Dejong CH, Schaapherder AF, Gooszen HG; Dutch Acute Pancreatitis Study Group. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH, van Ramshorst B, Schaapherder AF, van der Harst E, Hofker S, Nieuwenhuijs VB, Brink MA, Kruyt PM, Manusama ER, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, Cuesta MA, Wahab PJ, Gooszen HG; Dutch Pancreatitis Study Group. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 484] [Article Influence: 32.3] [Reference Citation Analysis (2)] |
| 6. | Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 594] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 7. | Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current Concepts in Severe Acute and Necrotizing Pancreatitis: An Evidence-Based Approach. Gastroenterology. 2019;156:1994-2007.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 8. | Hartwig W, Maksan SM, Foitzik T, Schmidt J, Herfarth C, Klar E. Reduction in mortality with delayed surgical therapy of severe pancreatitis. J Gastrointest Surg. 2002;6:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG; Dutch Pancreatitis Study Group. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2011;98:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Sharma V, Rana SS, Bhasin DK. Endoscopic ultrasound guided interventional procedures. World J Gastrointest Endosc. 2015;7:628-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | van Santvoort HC, Besselink MG, Horvath KD, Sinanan MN, Bollen TL, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford). 2007;9:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1078] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 13. | Bellam BL, Samanta J, Gupta P, Kumar M P, Sharma V, Dhaka N, Sarma P, Muktesh G, Gupta V, Sinha SK, Kochhar R. Predictors of outcome of percutaneous catheter drainage in patients with acute pancreatitis having acute fluid collection and development of a predictive model. Pancreatology. 2019;19:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Guo Q, Li A, Hu W. Predictive factors for successful ultrasound-guided percutaneous drainage in necrotizing pancreatitis. Surg Endosc. 2016;30:2929-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Kotán R, Sápy P, Sipka S, Damjanovich L, Kovács DÁ, Csiszko A, Balog K, Szentkereszty Z. Serum C-reactive protein and white blood cell level as markers of successful percutaneous drainage of acute sterile peripancreatic fluid collection. Chirurgia (Bucur). 2015;110:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Mallick B, Tomer S, Arora SK, Lal A, Dhaka N, Samanta J, Sinha SK, Gupta V, Yadav TD, Kochhar R. Change in serum levels of inflammatory markers reflects response of percutaneous catheter drainage in symptomatic fluid collections in patients with acute pancreatitis. JGH Open. 2019;3:295-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, Lesser M, Widmann WD. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Suppiah A, Malde D, Arab T, Hamed M, Allgar V, Smith AM, Morris-Stiff G. The prognostic value of the neutrophil-lymphocyte ratio (NLR) in acute pancreatitis: identification of an optimal NLR. J Gastrointest Surg. 2013;17:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Jeon TJ, Park JY. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute pancreatitis. World J Gastroenterol. 2017;23:3883-3889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 20. | Kong W, He Y, Bao H, Zhang W, Wang X. Diagnostic Value of Neutrophil-Lymphocyte Ratio for Predicting the Severity of Acute Pancreatitis: A Meta-Analysis. Dis Markers. 2020;2020:9731854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4719] [Article Influence: 363.0] [Reference Citation Analysis (48)] |
| 22. | Hasibeder WR, Torgersen C, Rieger M, Dünser M. Critical care of the patient with acute pancreatitis. Anaesth Intensive Care. 2009;37:190-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 4037] [Article Influence: 310.5] [Reference Citation Analysis (0)] |
| 24. | Pezzilli R, Billi P, Beltrandi E, Casadei Maldini M, Mancini R. Impaired lymphocyte proliferation in human acute pancreatitis. Digestion. 1997;58:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, Prasad KR. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 26. | Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362-3369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 27. | Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, Jeffrey RR, Buchan KG, El-Shafei H, Hillis GS. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 28. | Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97:993-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 302] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 29. | Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 861] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 30. | Pantzaris ND, Platanaki C, Pierrako C, Karamouzos V, Velissaris D. Neutrophil-to-lymphocyte Ratio Relation to Sepsis Severity Scores and Inflammatory Biomarkers in Patients with Community-acquired Pneumonia: A Case Series. J Transl Int Med. 2018;6:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Velissaris D, Pantzaris ND, Bountouris P, Gogos C. Correlation between neutrophil-to-lymphocyte ratio and severity scores in septic patients upon hospital admission. A series of 50 patients. Rom J Intern Med. 2018;56:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125:S3-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1516] [Cited by in RCA: 1254] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 33. | de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14:R192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 34. | Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5-14. [PubMed] |
| 35. | Takeyama Y, Takas K, Ueda T, Hori Y, Goshima M, Kuroda Y. Peripheral lymphocyte reduction in severe acute pancreatitis is caused by apoptotic cell death. J Gastrointest Surg. 2000;4:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Pezzilli R, Billi P, Beltrandi E, Maldini M, Mancini R, Morselli Labate AM, Miglioli M. Circulating lymphocyte subsets in human acute pancreatitis. Pancreas. 1995;11:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Liu WH, Wang T, Yan HT, Chen T, Xu C, Ye P, Zhang N, Liu ZC, Tang LJ. Predictors of percutaneous catheter drainage (PCD) after abdominal paracentesis drainage (APD) in patients with moderately severe or severe acute pancreatitis along with fluid collections. PLoS One. 2015;10:e0115348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 38. | Cao X, Cao F, Li A, Gao X, Wang XH, Liu DG, Fang Y, Guo DH, Li F. Predictive factors of pancreatic necrosectomy following percutaneous catheter drainage as a primary treatment of patients with infected necrotizing pancreatitis. Exp Ther Med. 2017;14:4397-4404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Ke L, Li J, Hu P, Wang L, Chen H, Zhu Y. Percutaneous Catheter Drainage in Infected Pancreatitis Necrosis: a Systematic Review. Indian J Surg. 2016;78:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gadour E, Hallac A S-Editor: Gao CC L-Editor: A P-Editor: Gao CC