Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.361
Peer-review started: August 19, 2021
First decision: November 1, 2021
Revised: November 14, 2021
Accepted: December 3, 2021
Article in press: December 3, 2021
Published online: January 7, 2022
Processing time: 132 Days and 20.7 Hours
Madelung’s disease (MD) is a chronic alcoholism-associated metabolic syndrome characterized by symmetrical subcutaneous deposition of adipose tissue in the head, neck, shoulders, back, trunk, and nerve roots of the upper and lower limbs. It is relatively rare in Asian individuals and is prone to misdiagnosis. Herein, we report a case of a patient with MD who had undergone surgical management at our hospital, and we discuss the pathogenesis, diagnosis, and treatment of MD.
We report a case of MD in a 65-year-old man of Han descent. The patient had multiple, painless progressive masses for more than five years in the neck and more than 30 years in the upper back. Because of neck mobility limitations and progressive cosmetic deformities caused by the masses, he was admitted to our hospital. He drank approximately 500 mL of liquor per day and smoked heavily for more than 30 years. Contrast-enhanced computed tomography of the neck and chest documented abundant unencapsulated, subcutaneous fatty deposits. We prepared a staged operation plan. The patient was diagnosed with MD; he was advised to abstain from alcohol and was followed up regularly. After a 3-month follow-up, no recurrence of fat accumulation was found in the surgical areas.
This report presents a case of surgical treatment for MD to improve clinicians' understanding of the disease.
Core Tip: Madelung’s disease (MD) is a rare chronic alcoholism-associated metabolic syndrome characterized by symmetrical deposition of adipose tissue subcutaneously in the head, neck, shoulders, back, trunk, and nerve roots of the upper and lower limbs. No consensus exists concerning the diagnosis, pathogenesis, and treatment of MD. Recently, a patient with MD accompanied by hypertension had undergone surgical resection and recovered well at our hospital. Herein, we present this case to improve clinicians’ diagnosis and treatment of MD while emphasizing the manifestations of MD presenting as head and neck masses.
- Citation: Yan YJ, Zhou SQ, Li CQ, Ruan Y. Diagnostic and surgical challenges of progressive neck and upper back painless masses in Madelung’s disease: A case report and review of literature. World J Clin Cases 2022; 10(1): 361-370
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/361.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.361
Madelung’s disease (MD) is a rare metabolic syndrome also called benign symmetric lipomatosis, Launois-Bensaude syndrome or multiple symmetric lipomatosis. MD is characterized by the symmetrical deposition of adipose tissue subcutaneously in the head, neck, shoulders, back, trunk, and nerve roots of the upper and lower limbs[1]. MD can be asymptomatic or can lead to reduced physical mobility, dysphagia, dysphonia, or tracheobronchial obstruction because of fatty deposits that compress the vascular, nervous, and respiratory tract structures[2-4]. This ailment is more common in middle-aged Mediterranean individuals with a history of chronic alcoholism; however, it is very rare in Asian individuals[2,5]. MD remains a clinical diagnosis, primarily based on the patient’s medical history, clinical symptoms, and examinations; thus, misdiagnosis can easily occur[3,4]. MD also has an unclear etiology, and the treatment ranges from a reduction in alcohol consumption to surgical resection.
Herein, we report a case of a patient with MD accompanied by hypertension who presented with progressive neck and upper back painless masses and who recovered well after surgical resection. We discuss the pathogenesis, diagnosis, and treatment of MD to improve clinicians' understanding of this disease.
A 65-year-old man of Han ancestry was admitted to the Department of Otorhinolaryngology, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, because of multiple, painless progressive masses for more than five years in the neck and more than 30 years in the upper back.
The patient presented with progressive soft masses over his neck and upper back for more than five years and more than 30 years, respectively, and the swellings in the anterior neck also gradually enlarged over the previous five years.
The patient drank approximately 500 mL of liquor per day and had a smoking history of approximately 20 cigarettes/day for more than 30 years. The man denied a history of glucocorticoid or drug use, but he had a history of hypertension for more than 10 years, for which he irregularly took amlodipine besylate tablets (5 mg per day) and had poorly controlled blood pressure for many years.
He denied previous drug use except for amlodipine besylate tablets and had no family history of similar diseases that caused masses.
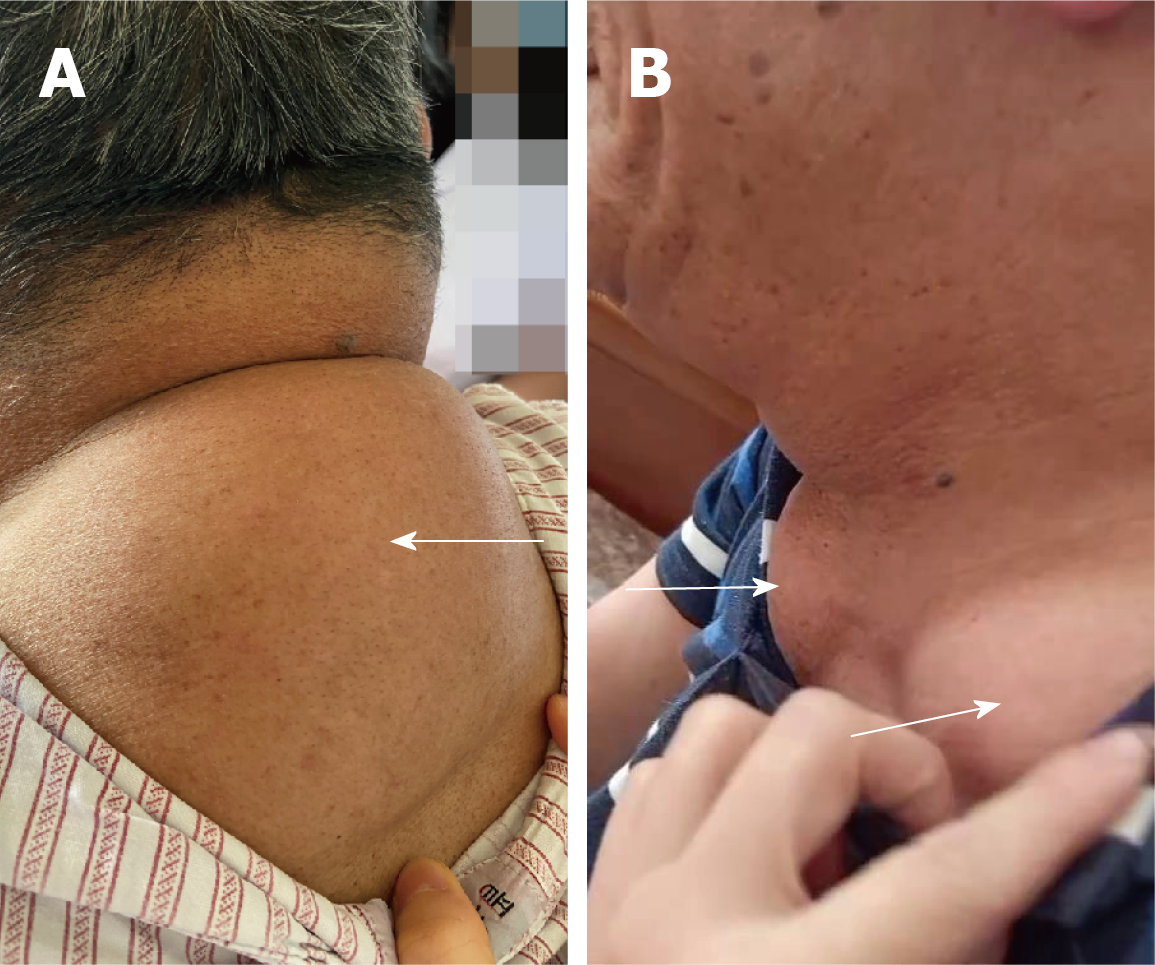
Physical examination upon admission revealed the following: his body mass index was 22.7 kg/m2, and he had no signs of moon facies, central obesity, or thin or purple striated skin. Soft masses of approximately 10 cm × 15 cm × 3 cm were easily found in the patient's upper back (Figure 1A). At each supraclavicular fossa, soft masses of 3 cm × 3 cm × 2 cm were found with clear boundaries and were fairly mobile (Figure 1B). The patient had mild limitations in his neck movements and had cosmetic deformities caused by the masses.
Laboratory tests revealed elevated serum triglycerides (2.38 mmol/L) and serum uric acid (445 μmol/L) but normal hepatic enzymes and blood glucose. The patient was negative for HBV, HCV, HIV, and syphilis. Main laboratory findings of the patient are summarized in Table 1.
| Main laboratory findings | Value | Normal range |
| Glucose (mmol/L) | 4.61 | 3.9–6.1 |
| Glycated albumin (%) | 14.8 | 11–16 |
| Alanine aminotransferase (U/L) | 12 | ≤ 41 |
| Aspartate aminotransferase (U/L) | 22 | ≤ 40 |
| Alkaline phosphatase (U/L) | 78 | 45–15 |
| γ-glutamyl transferase (U/L) | 46 | 10–60 |
| Creatinine (μmol/L) | 90 | 57–111 |
| Urea (mmol/L) | 3.33 | 3.6–9.5 |
| Uric acid (mmol/L) | 445 | 208–428 |
| Total cholesterol (mmol/L) | 4.62 | 2.6–5.2 |
| Triglyceride (mmol/L) | 2.38 | 0.34–1.70 |
| Treponema pallidum antibody (S/CO) | 0.08 | < 1 |
| Anti-HCV (S/CO) | 0.08 | < 1 |
| Anti-HIV (S/CO) | 0.14 | < 1 |
| HBsAg (IU/mL) | 0 | < 0.05 |
| Anti-HBs (mIU/mL) | 0.52 | < 10 |
| HBeAg (S/CO) | 0.322 | < 1 |
| Anti-HBe (S/CO) | 1.69 | > 1 |
| Anti-HBc (S/CO) | 0.46 | < 1 |
| Pulmonary function test results | ||
| Moderate obstructive pulmonary ventilation dysfunction | ||
| The maximum voluntary minute ventilation was slightly decreased | ||
| Heart color Doppler ultrasound examination | ||
| Aortic stiffness; Enlarged left atrium | ||
| Mitral regurgitation (mild) and tricuspid regurgitation (mild) |
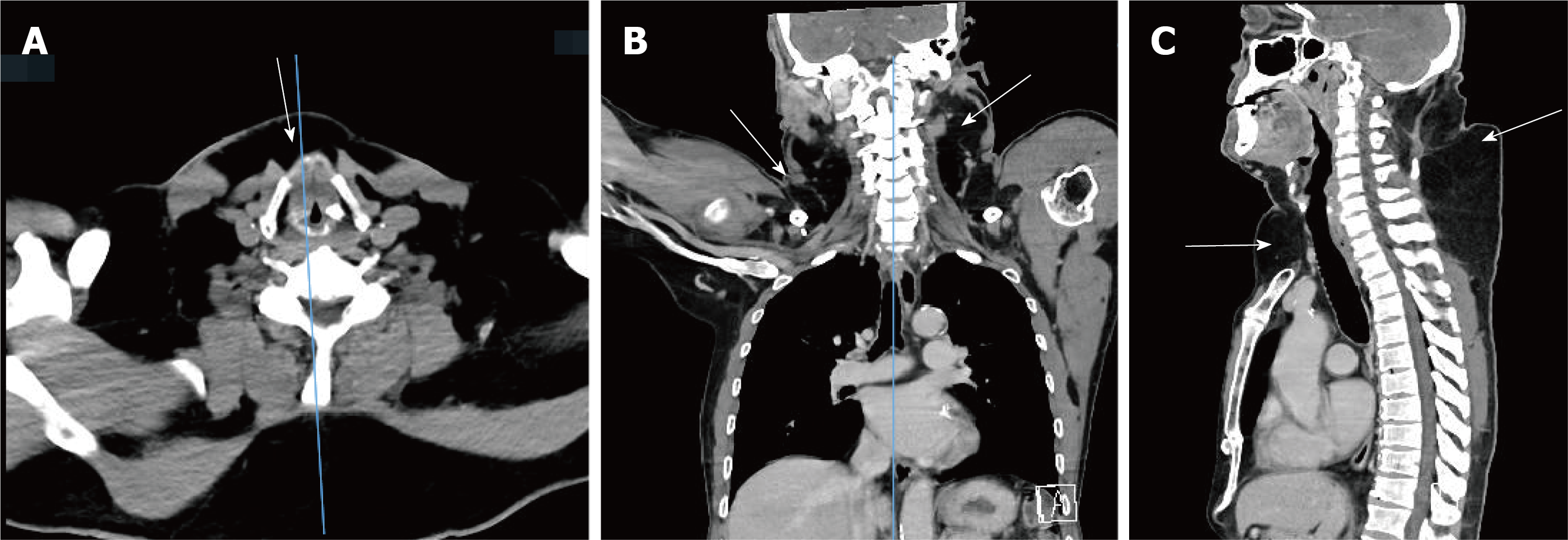
Contrast-enhanced computed tomography (CT) of the neck and chest documented abundant unencapsulated, subcutaneous fatty deposits in the anterior neck, lateral neck, supraclavicular fossa, posterior neck, and upper back (Figure 2).
The pulmonary function test showed moderate obstructive pulmonary ventilation dysfunction, and the maximum voluntary minute ventilation was slightly decreased (Table 1).
A color Doppler echocardiogram test showed aortic stiffness, an enlarged left atrium, mitral regurgitation (mild), tricuspid regurgitation (mild), and normal left ventricular systolic function (Table 1).
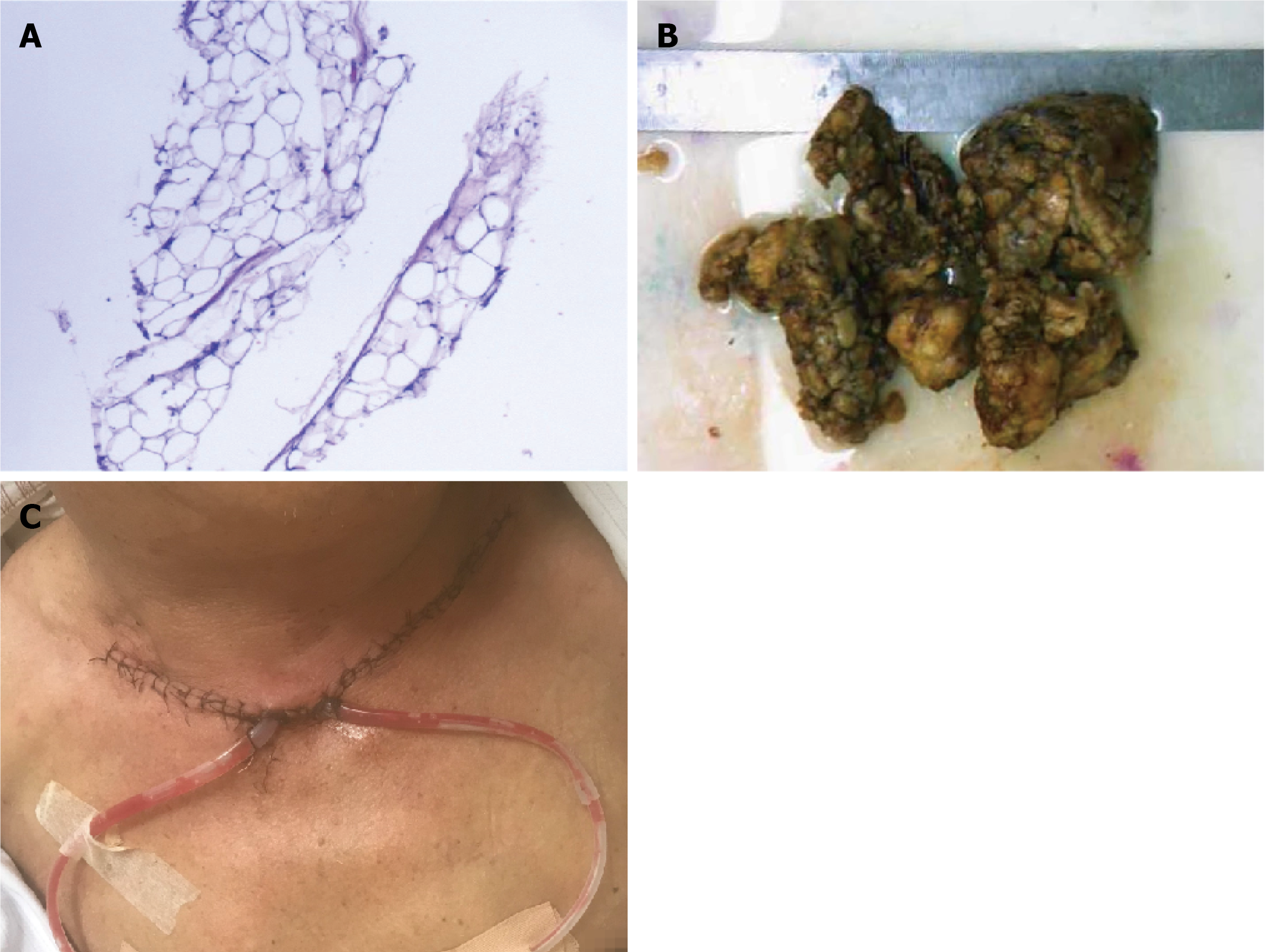
The postoperative pathological diagnosis showed (neck) lipomas (Figure 3A).
Before surgery, the patient was advised to cease drinking and keep his blood pressure normal so that he could pass the surgical safety assessment. After inducing general anesthesia, the patient had undergone cervical central and lateral mass dissections to remove the anterior neck, supraclavicular fossa, and lateral neck masses.
A neckline incision 15 mm above the superior sternal fossa was made, and when the skin, subcutaneous tissue, and platysma muscle were incised layer by layer, soft anterior cervical masses located between the platysma muscle and anterior cervical muscle were observed. These masses were completely removed along their surfaces. Soon thereafter, bilateral neck masses were found on the surface and deep to the sternocleidomastoid muscle. The sternocleidomastoid muscle was incised to expose the masses, and the lateral neck mass was carefully removed along the surface of the carotid sheath. Compressive dressings and garments were used for drainage during the postoperative period. The resected masses (Figure 3B) were sent for pathological examination. The patient was admitted to the recovery room for postoperative monitoring and care, and no complications occurred after surgery (Figure 3C) .
The patient was diagnosed with MD (type I)[6-8] based on his history, clinical features, auxiliary examinations, and pathological examination.
The patient received amlodipine besylate tablets (5 mg/d) to control his blood pressure and had undergone surgical procedures for treatment. Alcohol abstinence, a low-fat diet, and long-term follow-up were advised.
The patient’s neck movement improved after surgery. Alcohol abstinence and posterior neck mass resection after 4 mo were advised.
The earliest description of MD was by Brodie in 1846, but Madelung systematically summarized and discussed MD as a specific disease in 1888[9]. MD is characterized by the subcutaneous accumulation of adipose tissue in the head, neck, trunk, and limbs. Additionally, these fatty masses do not have distinct boundaries and are not enclosed within a membranous capsule[7]. Currently, the etiology and pathogenesis of MD remain unclear, but most scholars believe that MD is closely related to long-term heavy drinking because 95% of patients have a history of chronic alcoholism[1-3], and long-term alcohol consumption can reduce the number and activity of β-adrenergic receptors and promote fat synthesis and accumulation[10,11]. Further studies have shown that alcohol can directly affect mitochondrial activity, leading to premature oxidation of mitochondrial DNA[12,13]. Additionally, mutations in mitochondrial DNA[14,15], adipose tissue mitochondrial dysfunction[16], decreased cytochrome C oxidase activity[10,16], and fat deposition together contribute to the occurrence and development of MD[17]. The patient in this case report had several known risk factors based on the epidemiology of MD, such as a 30-year history of heavy alcohol consumption and elevated serum triglyceride and serum uric acid levels (Shown in Table 1).
Clinically, MD is generally divided into three types based on the anatomical location of adipose tissue[6-8]. Type I, also known as horse collar lipoma, mainly occurs in men, and adipose tissue is mainly concentrated in the neck, upper back, and shoulders, giving it the appearance of a “horse collar”. Type II, also called the pseudoathletic type, has no gender preference. The lesions appear mainly in the upper back, deltoid area, upper arms, buttocks, and upper thighs, and some patients have upper abdominal fat accumulation. Type III is characterized by congenital fat accumulation around the trunk[8]. Other systems have classified MD into two types[10], and the difference is that types II and III are combined into one. The case reported in this article presents a patient with symmetrical, diffuse lipomatosis primarily localized to the neck region; thus, he was diagnosed with type I.
The diagnostic criteria for MD are not yet unified and are mainly based on medical history, clinical symptoms, physical signs, imaging examinations, and pathological examinations. Imagological examination in MD patients often shows an obvious thickening of subcutaneous and interfascial tissues and symmetrically distributed fat density lesions without capsules. Magnetic resonance imaging is the most commonly used imaging examination for MD[18], but CT has also been reported[2]. We conducted contrast-enhanced CT for this MD patient depending on the patient's condition, and CT is more convenient in our hospital. The pathological specimens of MD patients show that the masses comprise many smaller adipocytes, fibrous connective tissue, and vascular proliferation in the interstitium[19]. The main differential diagnosis of MD includes obesity, Cushing syndrome, familial multiple lipomatosis, liposarcoma, and the effects of medication (e.g., glucocorticoids and protease inhibitors for HIV). Obesity is characterized by diffuse adipose tissue accumulation over the whole body, and it is difficult to differentiate clinically from patients with type II MD. However, for MD patients, the increased volume of adipose tissue in the upper legs and arms and the limb and neck contrasts with the slim appearance of the lower legs and arms[17]. Additionally, obesity is characterized by an increased fat cell volume, while the fat cells of MD patients are smaller but more histologically numerous than those of obese patients[20]. Cushing syndrome is characterized by concentric obesity, hypertension, purple striations, acne, increased skin pigmentations, amenorrhea symptoms in female patients, and the elevation of 17-hydroxysteroid in 24 h urine samples. Familial multiple lipomatosis is a predominantly autosomal dominant hereditary syndrome that usually presents with multiple, discrete, encapsulated lipomas found on the trunk and extremities, while the neck and shoulder are not involved[21]. Genetic tests have demonstrated that a translocation between protein isoforms I-C on chromosome 12 and partner genes on chromosome 3 could be responsible for this condition[4]. Liposarcoma is characterized by a solitary, large, localized soft tissue mass that rarely involves the neck, and imaging appears to enhance the nodule in association with a lipoma[22], which can differentiate it from MD. Medications might also be responsible for MD. Eighty percent of HIV-1 patients who were treated with protease inhibitors develop MD[5,23]. This phenomenon indicates that protease inhibitors could induce changes in the metabolism of carbohydrates, leading to the development of MD[23]. Our MD patient denied taking medications except for hypotensive drugs, and the patient was negative for HBV, HCV, HIV, and syphilis (Shown in Table 1). Therefore, we can exclude that MD was due to a drug response in our patient. Therefore, detailed medical and medication histories are critical for the differential diagnosis of MD.
MD treatment is challenging[24]. Lifestyle adjustments, medications, and surgical treatment[4,25] are the main treatment methods, and abstinence from alcohol is one of the essential methods to prevent MD. Although sometimes a neck mass cannot be eliminated, abstinence from alcohol can slow the growth of local masses and reduce the recurrence rate[26]. Fibrate lipid-lowering drugs are PPAR-α agonists that inhibit fat growth by inhibiting the protein expression of brown fat cells[19]. A β2-adrenergic agonist (salbutamol) has been used to prevent the accumulation of fat and increase energy expenditure[27]. Local subcutaneous injections of corticosteroids, thyroxine, and deoxycholate have been reported but with unsatisfactory effects[24]. Surgical treatment, including liposuction and lipectomy, seems to be most effective for MD[26]. Liposuction refers to the aspiration of fatty tissue in small masses using local anesthesia. The advantages of this method are less trauma, fewer scars, faster recovery, cost savings, and shorter hospital stays. However, liposuction cannot be used in masses in neck areas that are around important blood vessels and anatomical structures, and the postoperative recurrence rate is high[28]. Lipectomy is the direct removal of the exposed fat tissue of the lesion under anesthesia. These measures can reduce damage to vessels and nerves, remove fatty tissue completely, and ensure that the local operative space is in a negative pressure environment so that the skin and deep tissues are closely attached, which is beneficial to the healing of the operative area. However, because most of the lesions are large and have no capsules or boundaries, radical resection is challenging; thus, most of the current literature reports have involved palliative treatments[5,18]. Lipectomy was performed in this case, and the anterior neck, supraclavicular fossa, and lateral neck masses were removed first because the fatty tissues were too large and could have compressed the vital structures in the thoracic inlet or could have caused entrapment neuropathies or difficulty breathing[10]. Additionally, surgery will be performed 4 mo later to treat the posterior neck masses.
Literature search has revealed 3 published reports of MD in this journal. One study reported that an MD patient with type 2 diabetes was treated conservatively[29]. The second study reported a superb microvascular imaging technique for suspecting and confirming MD[30], and the third case study reported that a patient had a concomitant incarcerated femoral hernia and had undergone surgical treatment for complications but did not receive surgery for the masses secondary to MD[8]. We proposed a staged operation plan to remove the masses in our patient to reduce the risk of postoperative hematomas or seromas and to prevent airway obstruction. Herein, we report a case of a patient with MD who had undergone surgical management at our hospital, and we discuss the pathogenesis, diagnosis, and treatment of MD to improve the diagnosis and treatment of MD. At the same time, this case emphasizes prompt consideration of the manifestations of MD if head and neck masses are present.
We also searched MD case reports in the last five years and summarized 13 cases (Table 2)[8,29,31-41]. Most patients were male (11/13)[8,29,31-34,36-39,41], and all patients (mean age, 58.8 years) had typical disfigurement of benign symmetric lipomatosis. Most of the masses were located on the neck, shoulders, back, limbs and trunk. Interestingly, two male patients had enlarged breasts[39,41], two patients had an enlarged tongue[31,40], and one patient had a lipoma of the posterior pharyngeal wall[33]. The features and measurements of the patients are shown in Table 2. The mean duration from symptoms to the diagnosis of MD was 9.2 years. Cases also presented MD as a metabolic syndrome. Three had hypertension[29,33,41], two had diabetes mellitus[29,37], and one patient had bronchiectasis, hypercalcemia, and high triglycerides at the same time, accompanied by impaired glucose tolerance[35]. Additionally, 10 patients had a drinking habit per day, 9 (69.2%) were heavy drinkers[8,29,31-34,36,37,39], and 1 had alcoholic fatty liver[34]. Only one explicitly denied drinking[40]. Three patients had smoking habits[29,36,41]. Alcohol abstinence, surgery, healthier lifestyle, and diet methods to improve metabolic syndrome are the most common treatments. At a mean follow-up of 7.4 mo (range from 3 to 12 mo), the mass did not recur, and local function improved.
| Patient | Age, yr | Sex | DD | Tumor site | Comorbidities | Treatment | Follow up |
| 1[29] | 61 | Male | 4 yr | Mandible, elbows and abdominal area | HD, S, H, D | Alcohol abstinence, Medications to control blood pressure and blood sugar | The patient was in stable condition at follow-up 3 mo later |
| 2[8] | 69 | Male | 15 yr | Neck and shoulders | HD, IFH | Surgery on the right groin and Alcohol abstinence | After 1 yr follow-up, no recurrence of the right inguinal femoral hernia was found and no fat accumulation was found in the neck or other areas |
| 3[31] | 87 | Male | ND | Tongue | HD, RA | An incisional biopsy, alcohol abstinence observation | On follow-up 6 mo, the tongue findings were unchanged and no new growths were observed |
| 4[32] | 45 | Male | 5 mo | Neck | HD | Alcohol abstinence | After four mo, the patient claimed to experience increased cervical mobility. The size of the cervical mass was also reduced with the extended neck circumference reduced by 3.8 cm |
| 5[33] | 64 | Male | 20 yr | Posterior pharyngeal wall, neck, torso and upper extremities | HD, H, CRLD | Surgical removal of a mass on the posterior pharyngeal wall, alcohol abstinence | During follow-up examination in 1 wk, 2 wk, and 6 mo, further improvement of his swallowing, stertor, and voice were noted |
| 6[34] | 58 | Male | ND | Supraclavicular fossa and upper back | HD, AFL | Alcohol abstinence | ND |
| 7[35] | 56 | Female | ND | Neck, parotid glands, supraclavicular region and larynx | B, HC, HT, Impaired glucose tolerance | ND | ND |
| 8[36] | 59 | Male | 20 yr | Face and neck | HD, S | Deoxycholic Acid treatment and alcohol abstinence | Although significant growth of his lipomas was noted, he also showed markedly improved compression symptoms/pain and an increased range of motion of his neck |
| 9[37] | 45 | Male | 2 yr | Anterior cervical region, pre- and postauricular regions bilaterally, and back | HD, D, ACP | Two-step surgical treatment and alcohol abstinence | After 1 yr of follow-up, the final esthetic result was satisfactory |
| 10[38] | 38 | Male | 10 yr | Shoulders, arms and upper trunk | A | Avoid alcohol intake | No further progression of the lesions was observed during the 6-mo follow-up period |
| 11[39] | 45 | Male | 2 yr | Bilateral breast, upper back, deltoid areas, hips, and thighs | HD | Abstinence, liver protection, and anti-fibrosis agents | This patient was followed up every 6 mo and did not undergo surgical treatment. The condition is stable as of this writing |
| 12[40] | 65 | Female | ND | Macroglossia | ND | Bilateral partial glossectomy in two times | Improved initial symptoms one year after surgery |
| 13[41] | 72 | Male | ND | The breasts, abdomen, and roots of thighs | S, H | Hypotensive therapy and healthier lifestyle, and diet methods to improve the metabolic syndromePlastic surgery for liposuction of inguinal lipoma | ND |
MD is a relatively rare disease but is prone to a misdiagnosis because of confusion with other conditions. The authors report a classic ethanol-related MD case with a thorough medical history, clear diagnosis, and treatment plan. Although the etiology and pathogenesis of MD remain uncertain, the clinical diagnosis can be made based on the history, clinical symptoms, physical examination, and imaging examination, and this case was carefully confirmed to be MD based on the pathological results. MD often requires alcohol abstinence and the excision of lipomas for treatment. Surgical therapy can achieve dramatic functional improvements and unquestionable benefits to the patient’s quality of life. Therefore, we developed a staged operation plan to remove the masses. This case underscores prompt consideration for the presence of MD in patients with head and neck masses.
We thank the patient for granting permission to publish this information.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Carvalho JF, Seskute G S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Nisi G, Sisti A. IMAGES IN CLINICAL MEDICINE. Madelung's Disease. N Engl J Med. 2016;374:572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Calarco R, Rapaccini G, Miele L. Fat Deposits as Manifestation of Alcohol Use Disorder: Madelung's Disease. Clin Gastroenterol Hepatol. 2019;17:A26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Kohli DR, Luppens DP, Matherly SC. Rare Case of Madelung's Disease. Clin Gastroenterol Hepatol. 2018;16:e17-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Tadisina KK, Mlynek KS, Hwang LK, Riazi H, Papay FA, Zins JE. Syndromic lipomatosis of the head and neck: a review of the literature. Aesthetic Plast Surg. 2015;39:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Brea-García B, Cameselle-Teijeiro J, Couto-González I, Taboada-Suárez A, González-Álvarez E. Madelung's disease: comorbidities, fatty mass distribution, and response to treatment of 22 patients. Aesthetic Plast Surg. 2013;37:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Carlsen A, Thomsen M. Different clinical types of lipomatosis. Case report. Scand J Plast Reconstr Surg. 1978;12:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Suresh Chandran CJ, Godge YR, Oak PJ, Ravat SH. Madelung's disease with myopathy. Ann Indian Acad Neurol. 2009;12:131-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Li B, Rang ZX, Weng JC, Xiong GZ, Dai XP. Benign symmetric lipomatosis (Madelung's disease) with concomitant incarcerated femoral hernia: A case report. World J Clin Cases. 2020;8:5474-5479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Lee MS, Lee MH, Hur KB. Multiple symmetric lipomatosis. J Korean Med Sci. 1988;3:163-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Ray S, Chakraborty PP, Pramanik S, Chowdhury S. Bilateral breast enlargement in a chronic alcoholic: do not miss Madelung's disease. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Ruzicka T, Vieluf D, Landthaler M, Braun-Falco O. Benign symmetric lipomatosis Launois-Bensaude. Report of ten cases and review of the literature. J Am Acad Dermatol. 1987;17:663-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 92] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Ozderya A, Temizkan S, Aydin Tezcan K, Ozturk FY, Altuntas Y. A case of Madelung's disease accompanied by Klinefelter's syndrome. Endocrinol Diabetes Metab Case Rep. 2015;2015:140119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kodish ME, Alsever RN, Block MB. Benign symmetric lipomatosis: functional sympathetic denervation of adipose tissue and possible hypertrophy of brown fat. Metabolism. 1974;23:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Lee YC, Wei YH, Lirng JF, Lee HC, Tso DJ, Lin KP, Wu ZA, Liu HC. Wernicke's encephalopathy in a patient with multiple symmetrical lipomatosis and the A8344G mutation of mitochondrial DNA. Eur Neurol. 2002;47:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Holme E, Larsson NG, Oldfors A, Tulinius M, Sahlin P, Stenman G. Multiple symmetric lipomas with high levels of mtDNA with the tRNA(Lys) A-->G(8344) mutation as the only manifestation of disease in a carrier of myoclonus epilepsy and ragged-red fibers (MERRF) syndrome. Am J Hum Genet. 1993;52:551-556. [PubMed] |
| 16. | Plummer C, Spring PJ, Marotta R, Chin J, Taylor G, Sharpe D, Athanasou NA, Thyagarajan D, Berkovic SF. Multiple Symmetrical Lipomatosis-a mitochondrial disorder of brown fat. Mitochondrion. 2013;13:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Heike Z, Gudrun UM, Frank RD, Vetter H, Walger P. Multiple benign symmetric lipomatosis--a differential diagnosis of obesity: is there a rationale for fibrate treatment? Obes Surg. 2008;18:240-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Sharma N, Hunter-Smith DJ, Rizzitelli A, Rozen WM. A surgical view on the treatment of Madelung's disease. Clin Obes. 2015;5:288-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 19. | Ardeleanu V, Chicos S, Georgescu C, Tutunaru D. Multiple benign symmetric lipomatosis - a differential diagnosis of obesity. Chirurgia (Bucur). 2013;108:580-583. [PubMed] |
| 20. | Kan Y, Yao P, Xin W, Chen Q, Wang J, Yue J, Zhu J. [Recent progress on diagnosis and treatment of benign symmetric lipomatosis]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;24:105-107. [PubMed] |
| 21. | Keskin D, Ezirmik N, Celik H. Familial multiple lipomatosis. Isr Med Assoc J. 2002;4:1121-1123. [PubMed] |
| 22. | Crago AM, Dickson MA. Liposarcoma: Multimodality Management and Future Targeted Therapies. Surg Oncol Clin N Am. 2016;25:761-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (5)] |
| 23. | Bornhövd E, Sakrauski AK, Brühl H, Walli R, Plewig G, Röcken M. Multiple circumscribed subcutaneous lipomas associated with use of human immunodeficiency virus protease inhibitors? Br J Dermatol. 2000;143:1113-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Räßler F, Goetze S, Elsner P. Abdominal variant of benign symmetric lipomatosis (Launois-Bensaude syndrome) imitating obesity. J Eur Acad Dermatol Venereol. 2016;30:460-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Noguchi H, Masuda R, Hisaoka M. Multiple symmetric lipomatosis with spindle cell proliferation. Pathol Int. 2016;66:311-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Chen CY, Fang QQ, Wang XF, Zhang MX, Zhao WY, Shi BH, Wu LH, Zhang LY, Tan WQ. Madelung's Disease: Lipectomy or Liposuction? Biomed Res Int. 2018;2018:3975974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Leung NW, Gaer J, Beggs D, Kark AE, Holloway B, Peters TJ. Multiple symmetric lipomatosis (Launois-Bensaude syndrome): effect of oral salbutamol. Clin Endocrinol (Oxf). 1987;27:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | da Costa JN, Gomes T, Matias J. Madelung Disease Affecting Scrotal Region. Ann Plast Surg. 2017;78:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Chen KK, Ni LS, Yu WH. Madelung disease: A case report. World J Clin Cases. 2021;9:1983-1988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Seskute G, Dapkute A, Kausaite D, Strainiene S, Talijunas A, Butrimiene I. Multidisciplinary diagnostic dilemma in differentiating Madelung's disease - the value of superb microvascular imaging technique: A case report. World J Clin Cases. 2021;9:6145-6154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Bastos DB, Figueira JA, Furuse C, Biasoli ÉR, Miyahara GI, Bernabé DG. Benign symmetric lipomatosis in the tongue: an uncommon case. Dermatol Online J. 2020;26. [PubMed] |
| 32. | Luo ZY, Yuan Y, Lu CY, Yang YJ, Li W, Yan W. A case of Madelung disease improved by alcohol abstinence. Australas J Dermatol. 2020;61:e449-e451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Chrysovitsiotis G, Papanikolaou V, Kyrodimos E, Giotakis E. Symptomatic retropharyngeal space lipoma. A patient with Madelung disease. Hippokratia. 2020;24:91-93. [PubMed] |
| 34. | Karashima S, Yoneda T. Madelung disease in a 58-year-old man. CMAJ. 2019;191:E48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Hoxha M, Cakoni R, Basho M. Rapidly progressive case of type I Madelung disease with bilateral parotid and minor salivary glands involvement. Br J Biomed Sci. 2020;77:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Rodriguez M, Beal BT, Khetarpal S, Vidimos A. Madelung Disease Treated With Deoxycholic Acid. Dermatol Surg. 2021;47:879-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Maximiano LF, Gaspar MT, Nakahira ES. Madelung disease (multiple symmetric lipomatosis). Autops Case Rep. 2018;8:e2018030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Mohanty P, Vivekanandh K, Dash G, Mohapatra L. Madelung's disease: A benign symmetric lipomatosis. Indian J Dermatol Venereol Leprol. 2018;84:190-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Gao H, Xin ZY, Yin X, Zhang Y, Jin QL, Wen XY. Madelung disease: A case report. Medicine (Baltimore). 2019;98:e14116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Mayo Yáñez M, González Poggioli N, Álvarez-Buylla Blanco M, Herranz González-Botas J. Benign symmetric lipomatosis with lingual involvement: Case report and literature review. J Stomatol Oral Maxillofac Surg. 2018;119:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | El Ouahabi H, Doubi S, Lahlou K, Boujraf S, Ajdi F. Launois-bensaude syndrome: A benign symmetric lipomatosis without alcohol association. Ann Afr Med. 2017;16:33-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |