Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.260
Peer-review started: February 28, 2021
First decision: June 7, 2021
Revised: June 10, 2021
Accepted: July 12, 2021
Article in press: July 12, 2021
Published online: January 7, 2022
Processing time: 304 Days and 19.9 Hours
As the most common cancer in women, breast cancer is the leading cause of death. Most patients are initially diagnosed as stage I-III. Among those without distant metastases, 64% are local tumors and 27% are regional tumors. Patients in stage IIA-IIIC and those who meet the breast-conserving criterion with the exception of tumor size can consider neoadjuvant chemotherapy (NACT). It is worth noting that the status of tumor cell biomarkers is not consistently static. Endocrine-related estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) encoded by erythroblastic leukemia viral oncogene homolog 2 gene can all alter from positive to negative or vice versa, especially in luminal B subtype after NACT. In addition, determination of HER2 status currently mainly relies on immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH), but FISH is commonly used when the result of IHC is uncertain. HER2 is regarded as negative when the IHC result is 0/1+ without the addition of FISH. To the best of our knowledge, this is the first report of a case harboring HER2 status transformation and IHC1+ with positive amplification by FISH after NACT.
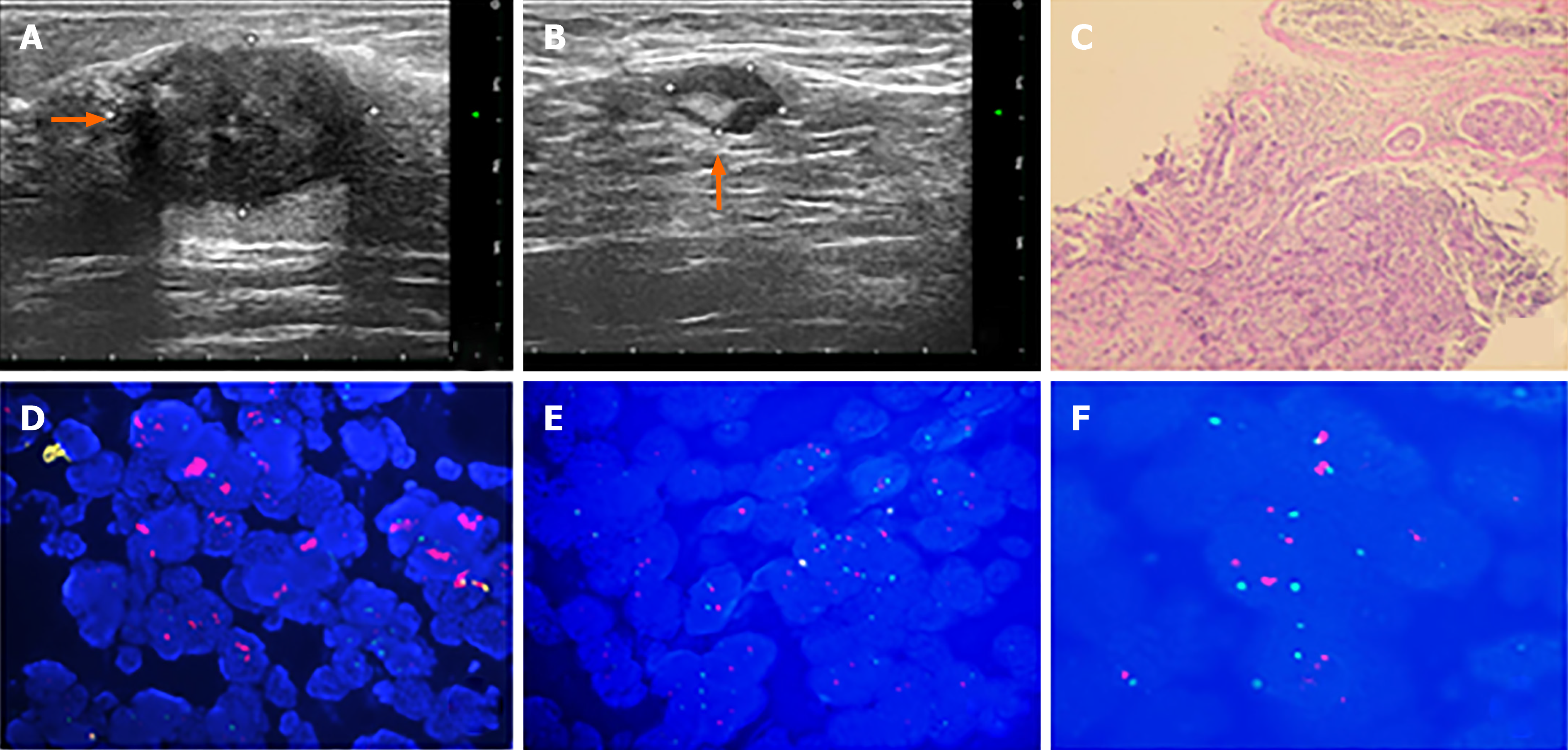
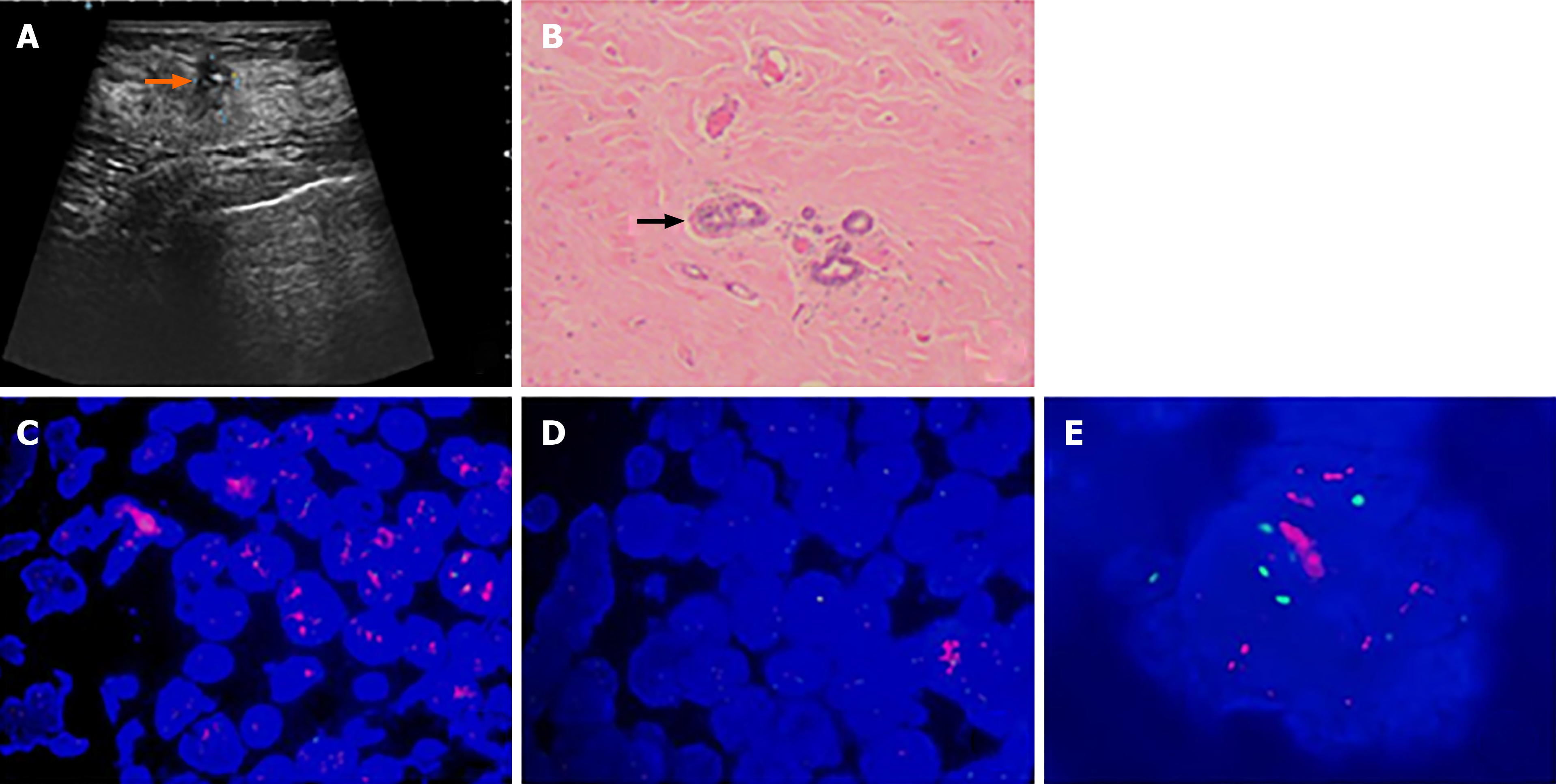
A 49-year-old woman discovered a mass in her right breast and underwent diagnostic workup. Biopsies of the right breast lesion and axillary lymph nodes were obtained. The results pointed to invasive ductal carcinoma with the IHC result for ER (80%), PR (60%), Ki-67 (20%) and ambiguous expression of HER2 (IHC 2+) with negative amplification by FISH (HER2/CEP17 ratio of 1.13). She underwent surgery after NACT. The pathological findings of the surgically resected sample supported invasive ductal carcinoma with the tumor measuring 1.1 cm × 0.8 cm × 0.5 cm and had spread to one of fifteen dissected lymph nodes. Retesting of the specimen showed that the tumor was positive for ER (2+, 85%) and PR (2+, 10%) but negative for HER2 by IHC (1+). Also Ki-67 had dropped to 2%. The patient was regularly monitored every 3 mo without evidence of recurrence.
Biomarker status should be reassessed after NACT especially in luminal subtypes.
Core Tip: The precise molecular subtype of breast cancer is helpful in order to develop individualized strategies for systemic treatment; thus, more attention should be paid to the changes in tumor biomarkers before and after surgery. The conversion probability is fairly low, especially regarding HER2 status; however, it directly affects the formulation of adjuvant treatment. Importantly, anti-HER2 therapy has led to a landmark change in patients with HER2 positive breast cancer.
- Citation: Wang L, Jiang Q, He MY, Shen P. HER2 changes to positive after neoadjuvant chemotherapy in breast cancer: A case report and literature review . World J Clin Cases 2022; 10(1): 260-267
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/260.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.260
As the most common cancer in women, breast cancer is the leading cause of death. Most patients are initially diagnosed as stage I-III. Among those without distant metastases, 64% are local tumors and 27% are regional tumors[1]. Patients in stage IIA-IIIC and those who meet the breast-conserving criterion with the exception of tumor size can consider neoadjuvant chemotherapy (NACT).
It is worth noting that the status of tumor cell biomarkers is not consistently static. Endocrine-related estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) encoded by erythroblastic leukemia viral oncogene homolog 2 gene can all alter from positive to negative or vice versa, especially in luminal B subtype after NACT[2]. In addition, determination of HER2 status currently mainly relies on immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH), but FISH is commonly used when the result of IHC is uncertain. HER2 is regarded as negative when the IHC result is 0/1+ without the addition of FISH. To the best of our knowledge, this is the first report of a case harboring HER2 status transformation and IHC1+ with positive amplification by FISH after NACT.
A mass was discovered in the right breast of a 49-year-old woman during a routine examination.
The patient discovered a mass in her right breast and underwent diagnostic workup, including a mammogram which showed a nodule and ultrasound that revealed a mass measuring 2.73 cm × 2.13 cm × 2.57 cm, as well as several enlarged axillary lymph nodes with the largest measuring 1.2 cm × 0.9 cm. Biopsies of the right breast lesion and axillary lymph nodes were obtained. The results pointed to invasive ductal carcinoma with the IHC result for ER (80%), PR (60%), Ki-67 (20%) and ambiguous expression of HER2 (IHC 2+) with negative amplification by FISH (HER2/CEP17 ratio of 1.13) (Figure 1). Computed tomography (CT) of the chest, abdomen, and pelvis showed no sign of metastatic foci and emission computed tomography (ECT) showed negative results. Accordingly, the patient was classified as stage IIB. The patient received NACT with epirubicin and cyclophosphamide for 4 cycles followed by docetaxel every 3 wk for 4 cycles and she was also supported by long-acting injections to improve the quantity of leukocytes. As a result, the lesion significantly reduced in size and the patient achieved a partial remission according to the RECIST1.1 criteria, and ultrasound showed that the focus had reduced to 0.8 cm × 0.7 cm and no obvious echo of enlarged lymph nodes in the axilla. The patient subsequently underwent lumpectomy of the right breast tumor. Pathological findings of the surgically resected sample supported invasive ductal carcinoma with the tumor measuring 1.1 cm × 0.8 cm × 0.5 cm and had spread to one of fifteen dissected lymph nodes. Retesting of the specimen showed that the tumor was positive for ER (2+, 85%) and PR (2+, 10%) but negative for HER2 by IHC (1+). Also Ki-67 had dropped to 2%. However, HER2 amplified by FISH showed a HER2/CEP17 ratio of 2.46 (Figure 2). The patient completed radiotherapy after surgery. Currently, she is undergoing endocrine treatment with tamoxifen and dual targeted therapy with trastuzumab and pertuzumab. Follow-up which included breast ultrasound, abdominal ultrasound and chest CT were regularly performed every 3 mo without evidence of recurrence.
The patient was healthy without a history of chronic disease or other breast diseases.
A movable mass measuring approximately 2.7 cm × 2.0 cm × 2.5 cm in the right breast and an ipsilateral enlarged axillary lymph node measuring 1.2 cm × 1.0 cm were identified. There was no evidence of disease in the contralateral breast and axillary lymph node.
All laboratory examinations were in the normal range.
A mammogram showed a nodule and ultrasound revealed a mass measuring 2.73 cm × 2.13 cm × 2.57 cm, as well as several enlarged axillary lymph nodes with the largest measuring 1.2 cm × 0.9 cm. CT of the chest, abdomen, and pelvis showed no sign of metastatic foci and ECT showed negative results.
The patient was diagnosed with HER2-positive and hormone receptor-positive invasive ductal carcinoma.
Epirubicin and cyclophosphamide for 4 cycles followed by docetaxel every 3 wk for 4 cycles and then surgery.
Follow-up including breast ultrasound, abdominal ultrasound and chest CT were regularly performed every 3 mo without evidence of recurrence.
It has been several years since NACT was recommended for invasive breast cancer patients by the National Comprehensive Cancer Network guidelines. Compared with postoperative adjuvant chemotherapy, NACT not only has the advantage of downgrading the clinical stage to make lumpectomy available for some patients, it also helps to eliminate micro-metastases. In addition, NACT also provides a novel, rapid and low-cost way to evaluate the effectiveness of systemic treatment. In contrast, observation of the efficacy of postoperative adjuvant therapy requires more time, energy and labor. With the popularization of NACT in locally advanced breast cancer, we have compiled the results over the past ten years (Table 1) and found that, compared with the biomarkers in samples obtained by fine needle aspiration or hollow needle biopsy before surgery, postoperative tissue receptors can occasionally produce completely opposite conclusions. The average conversion rate of ER is 7.3%, PR is 15.0% while HER2 is only 6.8% which is consistent with previous data, that is, the status of PR is most inclined to change while HER2 is relatively more stable[3].
| Ref. | Year | Total patients (n) | Frequency of ER alternation, n (%) | Frequency of PR alternation, n (%) | Frequency of Her-2 alternation, n (%) | Frequency of Ki-67 alternation, n (%) | ||||||||||||
| P × P | P × N | N × P | N × N | P × P | P × N | N × P | N × N | P × P | P × N | N × P | N × N | P × P | P × N | N × P | N × N | |||
| Li et al[12] | 2019 | 565 | 229 (40.5) | 48 (8.5) | 53 (9.4) | 235 (41.6) | 191 (33.8) | 76 (13.5) | 53 (9.4) | 245 (43.4) | 439 (39.7) | 117 (10.6) | 13 (1.2) | 536 (48.5) | NA | NA | NA | NA |
| Peng et al[16] | 2019 | 112 | 56 (50.0) | 18 (16.1) | 7 (6.2) | 31 (27.7) | 32 (28.6) | 22 (19.6) | 10 (9.0) | 48 (42.9) | 30 (27.8) | 17 (15.2) | 6 (5.3) | 59 (52.7) | 43 (38.4) | 42 (37.5) | 3 (2.7) | 24 (21.4) |
| Ahn et al[2] | 2018 | 442 | 305 (69.0) | 10 (2.3) | 8 (1.8) | 119 (26.9) | 201 (45.5) | 65 (14.7) | 15 (3.4) | 161 (36.4) | 109 (24.7) | 4 (0.9) | 11 (2.5) | 318 (71.9) | 113 (25.6) | 151 (34.2) | 12 (2.7) | 166 (37.6) |
| Yang et al[17] | 2018 | 231 | 173 (74.9) | NA | NA | 3 (1.3) | 158 (68.4) | NA | NA | 18 (7.8) | 26 (11.3) | NA | NA | 150 (64.9) | 128 (55.4) | NA | NA | 48 (20.8) |
| De La Cruz et al[18] | 2018 | 54 | 19 (35.2) | 2 (3.7) | 1 (1.9) | 9 (16.7) | NA | NA | NA | NA | 5 (9.6) | 1 (1.9) | 24 (44.4) | NA | NA | NA | NA | NA |
| Yoshida et al[19] | 2017 | 588 | NA | NA | NA | NA | NA | NA | NA | NA | 66 (11.2) | 33 (5.6) | 11 (1.9) | 478 (81.3) | NA | NA | NA | NA |
| Xian et al[20] | 2017 | 77 | NA | NA | 2 (3.0) | NA | NA | NA | 2 (3.0) | NA | NA | NA | 1 (1.0) | NA | NA | NA | NA | NA |
| Niikura et al[21] | 2016 | 16580/16515/16271 | 10474 (63.2) | 499 (3.0) | 519 (3.1) | 5088 (30.7) | 6735 (40.8) | 1545 (9.4) | 766 (4.6) | 7469 (45.9) | 2210 (13.6) | 601 (3.7) | 340 (2.1) | 9607 (59.0) | NA | NA | NA | NA |
| Gahlaut et al[22] | 2016 | 133 | NA | 7 (5.3) | 9 (6.8) | NA | NA | 13 (9.8) | 5 (3.8) | NA | NA | 5 (3.8) | 2 (1.5) | NA | NA | NA | NA | NA |
| Lim et al[23] | 2016 | 290 | 189 (65.2) | 23 (7.9) | 29 (10.0) | 49 (16.9) | NA | NA | NA | NA | 65 (22.4) | 17 (5.9) | 0 (0.0) | 208 (71.7) | NA | NA | NA | NA |
| Parinyanitikul et al[10] | 2015 | 398 | 188 (47.2) | 23 (5.8) | 39 (9.8) | 148 (37.2) | 105 (26.4) | 57 (14.3) | 28 (7.0) | 207 (52.0) | 43 (10.8) | 29 (7.3) | 11 (2.8) | 308 (77.4) | NA | NA | NA | NA |
| Zhou et al[24] | 2015 | 107 | 66 (61.7) | 11 (10.3) | 4 (3.7) | 31 (29.0) | 50 (46.7) | 13 (12.1) | 13 (12.1) | 31 (29.0) | 39 (36.4) | 3 (2.8) | 2 (1.9) | 63 (58.9) | NA | NA | NA | NA |
| Jin et al[25] | 2015 | 423 | 202 (47.8) | 55 (13.0) | 23 (5.4) | 143 (33.8) | NA | NA | NA | NA | 55 (13.0) | 27 (6.4) | 13 (3.1) | 328 (77.5) | NA | NA | NA | NA |
| Tan et al[26] | 2014 | 267 | 87 (32.6) | 57 (21.3) | 27 (10.1) | 123 (46.1) | 78 (29.2) | 33 (12.4) | 21 (7.9) | 135 (50.6) | NA | NA | NA | NA | NA | NA | NA | NA |
| Yang et al[27] | 2013 | 113 | NA | 6 (5.3) | 8 (7.1) | NA | NA | 8 (7.1) | 10 (8.8) | NA | NA | 1 (0.9) | 1 (0.9) | NA | NA | NA | NA | NA |
| Cockburn et al[28] | 2013 | 133 | 67 (50.4) | 11 (8.3) | 1 (0.8) | 54 (40.6) | 40 (30.1) | 16 (12.0) | 8 (6.0) | 69 (51.9) | 24 (18.0) | 9 (6.8) | 7 (5.3) | 93 (69.9) | NA | NA | NA | NA |
| Lee et al[29] | 2013 | 120 | 58 (48.3) | 11 (9.2) | 4 (3.3) | 47 (39.2) | 26 (21.7) | 19 (15.8) | 3 (2.5) | 72 (60.0) | 18 (16.8) | 6 (5.6) | 5 (4.7) | 78 (72.9) | NA | NA | NA | NA |
| Dede et al[30] | 2013 | 63 | 29 (46.0) | 2 (3.2) | 0 (0.0) | 4 (6.3) | 17 (27.0) | 7 (11.1) | 3 (4.8) | 6 (9.5) | NA | NA | NA | NA | NA | NA | NA | NA |
| Kumaki et al[31] | 2011 | 53 | 30 (56.7) | 3 (5.7) | 2 (3.8) | 18 (34.0) | 15 (28.3) | 4 (7.5) | 3 (5.7) | 31 (58.5) | 9 (18.4) | 5 (10.2) | 0 (0.0) | 35 (71.4) | NA | NA | NA | NA |
Before 2010, the status of HER2 was only determined by FISH, and since then, the results of IHC analysis have been combined. According to the ASCO/CAP guidelines, if the IHC result is 3+-, it can be diagnosed as HER2 positive, and if the IHC result is 0/1+-, it is regarded as HER2 negative. In an equivocal situation (IHC2+-), that is, the complete membrane staining of > 10% of tumor cells is weak to moderate intensity, in situ hybridization (ISH) must be performed to determine whether HER2 is amplified or not. Therefore, it is not necessary to supplement FISH to further confirm the status of HER2 in cases with an IHC score of 0 or 1+-. However, as luminal subtypes are more likely to reveal biomarker conversion and limited therapeutic efficiency which is attributed to the decreased expression of Ki-67 in luminal cases after NACT, we chose to perform FISH on the postoperative specimens of this patient. The results suggested that although the IHC score was 1+-, HER2 was actually proved to be amplified. When reviewing previous literature, we found that despite the low positive rate of gene amplification in IHC0/1+- cases, there was always a small discrepancy between IHC and FISH. Only 2% of the gene was amplified in negative (0/1+-) expression cases by FISH among Chinese patients in the study by Shui et al[4] and was approximately 4% in other populations[5].
At present, there is no concensus on the mechanism of NACT on HER2 status. Some researchers believe that the small tissue samples obtained by fine needle aspiration are insufficient to represent the phenotypic characteristics of the entire tumor as different molecular expressions may be displayed in specimens. Some studies have taken heterogeneity within the tumor into account. It has been speculated that NACT kills cells that are sensitive to chemotherapy and the remaining cells gradually dominate during the treatment process resulting in a different appearance with subsequent unfavorable characteristics and composition[6]. Another group assumed that a low level of estrogen in the body after NACT down-regulates the expression of ERs in tumor cells[7]. The management of HER2 expression is partly dependent on ER and the status transition also affects each of these parameters[8]. Therefore, NACT indirectly affects the status of HER2. One study has recently discovered that HER2 targeted therapy can also result in differential expression of genes[9]; thus, we predict that NACT can induce subtle changes in gene stability. In addition, it is worth noting that drugs that target cell microtubules such as paclitaxel can lead to polyploidization of cells, that is, all chromosomes multiply, including those that carry HER2. This is followed by increased copy number of the HER2 gene and the outcome is not equal to the actual amplification of HER2, which seems to explain why some patients are resistant to drugs even if the copy number of HER2 increases[10]. Although statistical and staining biases are rare and the criteria for defining IHC ambiguity (IHC 2+-) varies among trials, they should not be ignored. On the contrary, Parinyanitikul et al[11] analyzed HER2 mRNA level after treatment and the results indicated that the level of HER2 expression in most patients remained stable.
The prognosis of these receptor discordances after NACT are multifarious. For patients with locally advanced breast cancer, HER2 overexpression is an independent risk factor regarding 5-year disease-free survival (DFS). In the multivariate analysis by Tural et al[12], clinical stage of the tumor, transformation of HER2 from positive to negative and triple negative receptor status significantly influenced DFS. Li et al[13] included 2847 patients from eight studies and found that patients with hormone receptors (HR) which changed from positive to negative had worse DFS. Moreover, compared with patients who maintained negative HR status after NACT, those with negative HR which changed to positive tended to have longer DFS and overall survival. However, there are a variety of cut-off values to define HR positivity including 1%, 5% and 10% with few employing the Allred score, therefore they came to a contradictory conclusion regarding the prognosis of negative conversion of ER and PR status after NACT[2,14]. We cannot simply attribute this to different definitions as the total number of patients and their characteristics may also play a role. Additionally, the level of the protein encoded by the MKI67 gene (Ki67) is another independent predictive factor. A high Ki-67 index before surgery is associated with achieving a complete clinical response to NACT[15], whereas a high Ki-67 proliferation index in post-NACT samples is related to shorter DFS.
Nowadays, the status of HER2 can easily be influenced due to the combination of NACT and HER2-targeted therapy. Therefore, verification procedures should routinely be performed pre- and post-NACT. The decision whether or not to administer HER2-targeted therapy or endocrine therapy is largely based on the result. The estimation of rates of recurrence and outcome can also be affected. We expect the patient in this report to benefit from the use of trastuzumab and pertuzumab in the days to come.
The conversion of the status of biomarkers including ER, PR, HER2 and Ki-67 is important. Reassessment of the status of these biomarkers after NACT is recommended, especially in patients with luminal subtypes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ni W S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Xing YX
| 1. | DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 2076] [Article Influence: 296.6] [Reference Citation Analysis (0)] |
| 2. | Ahn S, Kim HJ, Kim M, Chung YR, Kang E, Kim EK, Kim SH, Kim YJ, Kim JH, Kim IA, Park SY. Negative Conversion of Progesterone Receptor Status after Primary Systemic Therapy Is Associated with Poor Clinical Outcome in Patients with Breast Cancer. Cancer Res Treat. 2018;50:1418-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Hariri N, Roma AA, Hasteh F, Walavalkar V, Fadare O. Phenotypic alterations in breast cancer associated with neoadjuvant chemotherapy: A comparison with baseline rates of change. Ann Diagn Pathol. 2017;31:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Shui R, Liang X, Li X, Liu Y, Li H, Xu E, Zhang Z, Lian Y, Guo S, Yao M, Yang H, Xu F, Liu J, Guo D, Wang K, Li J, Ma Y, Wang J, Shi J, Bu H, Yang W. Hormone Receptor and Human Epidermal Growth Factor Receptor 2 Detection in Invasive Breast Carcinoma: A Retrospective Study of 12,467 Patients From 19 Chinese Representative Clinical Centers. Clin Breast Cancer. 2020;20:e65-e74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Bahreini F, Soltanian AR, Mehdipour P. A meta-analysis on concordance between immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) to detect HER2 gene overexpression in breast cancer. Breast Cancer. 2015;22:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Almendro V, Cheng YK, Randles A, Itzkovitz S, Marusyk A, Ametller E, Gonzalez-Farre X, Muñoz M, Russnes HG, Helland A, Rye IH, Borresen-Dale AL, Maruyama R, van Oudenaarden A, Dowsett M, Jones RL, Reis-Filho J, Gascon P, Gönen M, Michor F, Polyak K. Inference of tumor evolution during chemotherapy by computational modeling and in situ analysis of genetic and phenotypic cellular diversity. Cell Rep. 2014;6:514-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 540] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Dati C, Antoniotti S, Taverna D, Perroteau I, De Bortoli M. Inhibition of c-erbB-2 oncogene expression by estrogens in human breast cancer cells. Oncogene. 1990;5:1001-1006. [PubMed] |
| 9. | Brasó-Maristany F, Griguolo G, Pascual T, Paré L, Nuciforo P, Llombart-Cussac A, Bermejo B, Oliveira M, Morales S, Martínez N, Vidal M, Adamo B, Martínez O, Pernas S, López R, Muñoz M, Chic N, Galván P, Garau I, Manso L, Alarcón J, Martínez E, Gregorio S, Gomis RR, Villagrasa P, Cortés J, Ciruelos E, Prat A. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade. Nat Commun. 2020;11:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Valent A, Penault-Llorca F, Cayre A, Kroemer G. Change in HER2 (ERBB2) gene status after taxane-based chemotherapy for breast cancer: polyploidization can lead to diagnostic pitfalls with potential impact for clinical management. Cancer Genet. 2013;206:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Parinyanitikul N, Lei X, Chavez-MacGregor M, Liu S, Mittendorf EA, Litton JK, Woodward W, Zhang AH, Hortobagyi GN, Valero V, Meric-Bernstam F, Gonzalez-Angulo AM. Receptor status change from primary to residual breast cancer after neoadjuvant chemotherapy and analysis of survival outcomes. Clin Breast Cancer. 2015;15:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Tural D, Karaca M, Zirtiloglu A, M Hacioglu B, Sendur MA, Ozet A. Receptor discordances after neoadjuvant chemotherapy and their effects on survival. J BUON. 2019;24:20-25. [PubMed] |
| 13. | Li C, Fan H, Xiang Q, Xu L, Zhang Z, Liu Q, Zhang T, Ling J, Zhou Y, Zhao X, Cui Y. Prognostic value of receptor status conversion following neoadjuvant chemotherapy in breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;178:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Hirata T, Shimizu C, Yonemori K, Hirakawa A, Kouno T, Tamura K, Ando M, Katsumata N, Fujiwara Y. Change in the hormone receptor status following administration of neoadjuvant chemotherapy and its impact on the long-term outcome in patients with primary breast cancer. Br J Cancer. 2009;101:1529-1536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Jain P, Doval DC, Batra U, Goyal P, Bothra SJ, Agarwal C, Choudhary DK, Yadav A, Koyalla VPB, Sharma M, Dash P, Talwar V. Ki-67 Labeling index as a predictor of response to neoadjuvant chemotherapy in breast cancer. Jpn J Clin Oncol. 2019;49:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Peng JH, Zhang X, Song JL, Ran L, Luo R, Li HY, Wang YH. Neoadjuvant chemotherapy reduces the expression rates of ER, PR, HER2, Ki67, and P53 of invasive ductal carcinoma. Medicine (Baltimore). 2019;98:e13554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Yang L, Zhong X, Pu T, Qiu Y, Ye F, Bu H. Clinical significance and prognostic value of receptor conversion in hormone receptor positive breast cancers after neoadjuvant chemotherapy. World J Surg Oncol. 2018;16:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | De La Cruz LM, Harhay MO, Zhang P, Ugras S. Impact of Neoadjuvant Chemotherapy on Breast Cancer Subtype: Does Subtype Change and, if so, How? Ann Surg Oncol. 2018;25:3535-3540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Yoshida A, Hayashi N, Suzuki K, Takimoto M, Nakamura S, Yamauchi H. Change in HER2 status after neoadjuvant chemotherapy and the prognostic impact in patients with primary breast cancer. J Surg Oncol. 2017;116:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Xian Z, Quinones AK, Tozbikian G, Zynger DL. Breast cancer biomarkers before and after neoadjuvant chemotherapy: does repeat testing impact therapeutic management? Hum Pathol. 2017;62:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Niikura N, Tomotaki A, Miyata H, Iwamoto T, Kawai M, Anan K, Hayashi N, Aogi K, Ishida T, Masuoka H, Iijima K, Masuda S, Tsugawa K, Kinoshita T, Nakamura S, Tokuda Y. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol. 2016;27:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Gahlaut R, Bennett A, Fatayer H, Dall BJ, Sharma N, Velikova G, Perren T, Dodwell D, Lansdown M, Shaaban AM. Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression - Implications for the practising oncologist. Eur J Cancer. 2016;60:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Lim SK, Lee MH, Park IH, You JY, Nam BH, Kim BN, Ro J, Lee KS, Jung SY, Kwon YM, Lee ES. Impact of Molecular Subtype Conversion of Breast Cancers after Neoadjuvant Chemotherapy on Clinical Outcome. Cancer Res Treat. 2016;48:133-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Zhou X, Zhang J, Yun H, Shi R, Wang Y, Wang W, Lagercrantz SB, Mu K. Alterations of biomarker profiles after neoadjuvant chemotherapy in breast cancer: tumor heterogeneity should be taken into consideration. Oncotarget. 2015;6:36894-36902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Jin X, Jiang YZ, Chen S, Yu KD, Shao ZM, Di GH. Prognostic value of receptor conversion after neoadjuvant chemotherapy in breast cancer patients: a prospective observational study. Oncotarget. 2015;6:9600-9611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Tan QX, Qin QH, Yang WP, Lian B, Wei CY. Prognostic value of hormone receptor status conversion following neoadjuvant chemotherapy in a series of operable breast cancer patients. Int J Clin Exp Pathol. 2014;7:4086-4094. [PubMed] |
| 27. | Yang YF, Liao YY, Li LQ, Xie SR, Xie YF, Peng NF. Changes in ER, PR and HER2 receptors status after neoadjuvant chemotherapy in breast cancer. Pathol Res Pract. 2013;209:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Cockburn A, Yan J, Rahardja D, Euhus D, Peng Y, Fang Y, Rumnong Sarode V. Modulatory effect of neoadjuvant chemotherapy on biomarkers expression; assessment by digital image analysis and relationship to residual cancer burden in patients with invasive breast cancer. Hum Pathol. 2014;45:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Lee HC, Ko H, Seol H, Noh DY, Han W, Kim TY, Im SA, Park IA. Expression of Immunohistochemical Markers before and after Neoadjuvant Chemotherapy in Breast Carcinoma, and Their Use as Predictors of Response. J Breast Cancer. 2013;16:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Dede DS, Gumuskaya B, Guler G, Onat D, Altundag K, Ozisik Y. Evaluation of changes in biologic markers ER, PR, HER 2 and Ki-67 index in breast cancer with administration of neoadjuvant dose dense doxorubicin, cyclophosphamide followed by paclitaxel chemotherapy. J BUON. 2013;18:366-371. [PubMed] |
| 31. | Kumaki N, Umemura S, Tang X, Saito Y, Suzuki Y, Tokuda Y. Alteration of immunohistochemical biomarkers between pre- and post-chemotherapy: hormone receptors, HER2 and Ki-67. Breast Cancer. 2011;18:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |