Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.236
Peer-review started: December 12, 2020
First decision: July 8, 2021
Revised: July 21, 2021
Accepted: November 26, 2021
Article in press: November 26, 2021
Published online: January 7, 2022
Processing time: 382 Days and 17.1 Hours
Rheumatoid arthritis (RA) is a common chronic inflammatory autoimmune disease with the main clinical feature of progressive joint synovial inflammation, which can lead to joint deformities as well as disability. RA often causes damage to multiple organs and systems within the body, including the blood hemostasis system. Few reports have focused on acquired coagulation dysfunction resulting from vitamin K-dependent coagulation factor deficiency associated with RA.
A 64-year-old woman with a history of RA presented to our hospital, complaining of painless gross hematuria for 2 wk. Blood coagulation function tests showed increased prothrombin time, international normalized ratio, and activated partial thromboplastin time. Abnormal blood coagulation factor (F) activity was detected (FII, 7.0%; FV, 122.0%; and FX, 6.0%), indicating vitamin K-dependent coagulation factor deficiency. Thromboelastography and an activated partial thromboplastin time mixed correction experiment also suggested decreased coagulation factor activity. Clinically, the patient was initially diagnosed with hematuria, RA, and vitamin K-dependent coagulation factor deficiency. The patient received daily intravenous administration of vitamin K1 20 mg, etamsylate 3 g, and vitamin C 3000 mg for 10 d. Concurrently, oral leflunomide tablets and prednisone were administered for treatment of RA. After the treatment, the patient's symptoms improved markedly and she was discharged on day 12. There were no hemorrhagic events during 18 mo of follow- up.
RA can result in vitamin K-dependent coagulation factor deficiency, which leads to acquired coagulation dysfunction. Vitamin K1 supplementation has an obvious effect on coagulation dysfunction under these circumstances.
Core Tip: Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that frequently involves multiple organs and systems, potentially leading to coagulation dysfunction. In this paper, we report the rare case of a patient who was diagnosed with acquired coagulation dysfunction resulting from vitamin K-dependent coagulation factor deficiency associated with RA, and subsequently benefited from vitamin K1 supplementation treatment. This case report may provide some references for diagnosis and treatment of RA patients with coagulation dysfunction symptoms.
- Citation: Huang YJ, Han L, Li J, Chen C. Acquired coagulation dysfunction resulting from vitamin K-dependent coagulation factor deficiency associated with rheumatoid arthritis: A case report . World J Clin Cases 2022; 10(1): 236-241
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/236.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.236
Rheumatoid arthritis (RA) is a systemic autoimmune disease with the main clinical manifestations of invasive arthritis[1]. The prevalence of RA is estimated to be 0.5%–1.0% globally[2]. The clinical features of blood system damage in patients with RA usually include anemia, neutropenia, thrombocytopenia, and hematological malignancies[3]. RA can also lead to acquired coagulation dysfunction, such as acquired hemophilia. Previous studies indicated that 4%–8% of acquired hemophilia cases were related to RA and that rituximab was effective for acquired FVIII inhibitors in RA patients[4,5]. Compared with healthy people, Dimitroulas et al[6] found that RA patients had higher levels of coagulation factors, such as tissue plasminogen activator, plasminogen activator inhibitor, fibrinogen (FBG), prothrombin fragments 1 and 2, and thrombomodulin, indicating that an imbalance of the coagulation and fibrinolysis systems was common in RA, although the underlying mechanism is not fully understood. Nevertheless, cases of acquired coagulation dysfunction caused by RA combined with vitamin K-dependent coagulation factors deficiency are rare. To further explore the possible etiology of coagulopathy in RA patients, we report a case of acquired coagulation dysfunction resulting from vitamin K-dependent coagulation factor deficiency associated with RA.
A 64-year-old female patient was admitted to the hospital on November 27, 2019, with a chief complaint of “painless gross hematuria for 2 wk”.
Two weeks previously, the patient had developed painless gross hematuria with no obvious cause. She presented with a whole course of hematuria, including blood clots, infrequent urination, urgent urination, and urodynia, without pain in the waist or lower abdomen, nausea, or vomiting. At the beginning, the patient was treated at The Fifth People’s Hospital of Jingzhou City, Hubei Province, China. A computed tomography scan of the urinary system displayed a soft tissue density in her bladder. A cystoscopy was performed and intravesical blood clots were subsequently removed on November 19, 2019. However, the gross hematuria symptoms recurred after the treatment. Hence, the patient attended our hospital for medical treatment. She was admitted for hematuria of unknown etiology.
The patient had a history of RA for 40 years, with regular treatment of oral indomethacin and prednisone tablets. At the time of admission, the patient had suffered joint pain and stiffness symptoms for several months.
The patient had suffered joint pain and stiffness symptoms.
After admission, laboratory tests revealed the following results: Prothrombin time (PT), 38.6 s; international normalized ratio (INR), 3.97; FBG, 4.85 g/L; activated partial thromboplastin time (APTT), 109.8 s; thrombin time, 16.9 s; and D-dimer, 0.89 µg/mL FEU. The results for blood coagulation factor (F) activity were as follows: FII, 7.0%; FV, 122.0%; and FX, 6.0%. Thus, vitamin K-dependent coagulation factor deficiency was considered. Thromboelastography produced the following findings: R, 10.2 min; K, 3.6 min; angle, 57.4; MA, 76.1 mm; CI, −2.1; and LY30, 0.0%. Routine urine examination showed red blood cells (occult blood), 3+; white blood cells (granular), 3+; nitrite-positive urinary protein, 3+; specific gravity > 1.030; urine glucose, ±; ketonuria, 2+; and urinary bilirubin, 3+. An APTT mixed correction experiment was performed on December 4. The patient’s APTT was 55.7 s. Her blood was then mixed with blood from a normal patient in a 1:1 ratio, and the APTT was determined immediately after mixing. The results showed that the mixed blood APTT was 41.4 s (normal control APTT, 37.3 s), and the Rosner index was 7.4 KUA/I, indicating a lack of coagulation factors. The level of rheumatoid factor (RF) was 35.4 IU/mL, RF IgG type was 25.87 RU/mL, RF IgM type was 103.81 RU/mL, and anti-cyclic citrulline polypeptide antibody was 48.4 U/mL. Antinuclear antibodies showed nuclear homogeneous type (1:100), suggesting the presence of trace amounts of antinuclear antibodies. Anti-neutrophil cytoplasmic antibody was positive for perinuclear type. C-reactive protein was 34.2 mg/L, indicating the possibility of infection or inflammation. The disease activity score in 28 joints was 3.7, indicating moderate disease activity.
Routine blood tests showed the following results: Red blood cells, 3.35 × 1012/L; hemoglobin, 86.0 g/L; white blood cells, 5.6 × 109/L; neutrophils, 3.42 × 109/L; lymphocytes 1.49 × 109/L; and platelets, 343.0 × 109/L. Liver function tests were: Alanine aminotransferase, < 5 U/L; glutamic oxaloacetic transaminase, 19 U/L; total protein, 62.1 g/L ↓; albumin, 28.8 g/L ↓; globulin, 33.3 g/L; total bilirubin, 5.5 µmol/L; direct bilirubin, 2.9 µmol/L; indirect bilirubin, 2.6 µmol/L; alkaline phosphatase, 96 U/L; γ-glutamyl transpeptidase, 13 U/L ; total cholesterol, 3.69 mmol/L; and lactic dehydrogenase, 244 U/L ↓. Other examinations did not show any obviously abnormal values, suggesting that no other disease condition was present.
The final diagnosis was hematuria, acquired coagulation dysfunction, RA, and vitamin K-dependent coagulation factor deficiency.
The patient was given intramuscular injection of vitamin K1 (10 mg) on day 1 after admission. From November 29 to December 8, she received daily intravenous administration of 20 mg of vitamin K1, 3 g of etamsylate, and 3000 mg of vitamin C. Oral leflunomide tablets and prednisone were administered for treatment of RA.
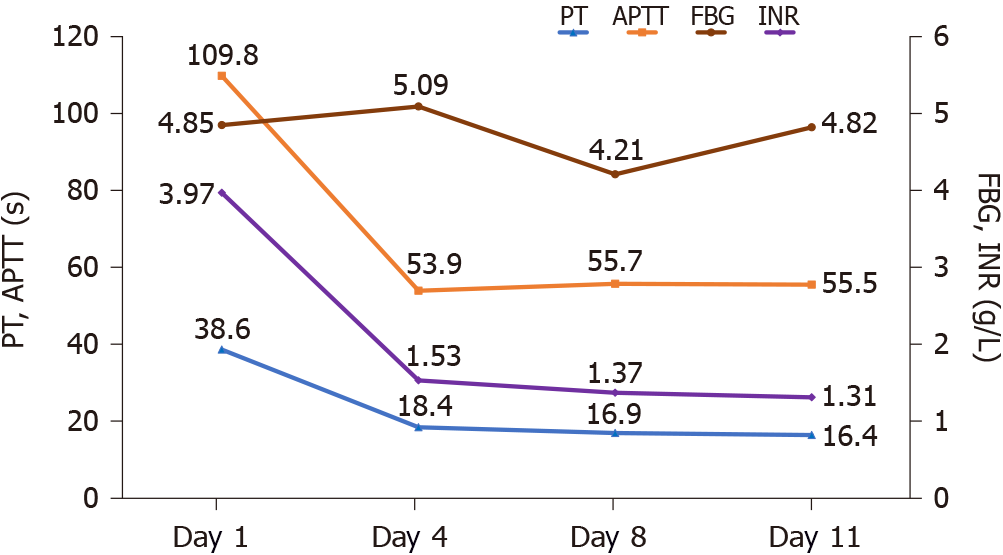
During the treatment period, the results of blood coagulation function tests (Table 1), blood coagulation factor activity test (Table 2) and routine urine tests (Table 3) were reviewed. PT, INR, FBG, and particularly APTT were significantly decreased (Table 1), and a trend toward gradual recovery of coagulation function was observed (Figure 1). The results for blood coagulation factor activity are shown in Table 2; FV activity returned to normal, while FII and FX activities did not return to their normal ranges, but did show significant increases compared with the previous results. A routine urine test on December 5 showed: Red blood cells (occult blood), 3+; white blood cells (granular), 1+; urinary protein, 1+; and specific gravity, 1.009. Other indicators were all normal, and the patient’s hematuria symptoms had improved considerably. On December 7, the patient’s urine color returned to normal. The marked improvement in the patient’s symptoms continued, and she was discharged from hospital. Besides anti-rheumatoid therapy, she continued receiving vitamin K1 orally for 3 mo after discharge. The patient was followed for 18 mo, without any hemorrhagic events.
| Hospitalization days | PT | INR | FBG | APTT | TT | D-D | FDPs | AT | PTA |
| 2 | 38.6 | 3.97 | 4.85 | 109.8 | 16.9 | 0.89 | - | - | 19 |
| 4 | 18.4 | 1.53 | 5.09 | 53.9 | 16.4 | 0.94 | < 4.0 | 80 | 54 |
| 8 | 16.9 | 1.37 | 4.21 | 55.7 | 17.3 | 0.81 | < 4.0 | 67 | 62 |
| 11 | 16.4 | 1.31 | 4.82 | 55.5 | 16.7 | 1.28 | 4.2 | 75 | 65 |
| Hospitalization days | II | V | VII | VIII | IX | X | XI | XII |
| 2 | 7 | 122 | - | - | - | 6 | - | - |
| 9 | 36 | 96 | 95 | 168 | 71 | 25 | 86 | 40 |
| Hospitalization days | RBCs | WBCs | Urinary protein | Nitrite | Specific gravity | Ketonuria | Urinary bilirubin | Urine glucose |
| 3 | 3+ | 3+ | 3+ | Positive | > 1.030 | 2+ | 3+ | ± |
| 9 | 3+ | 1+ | 1+ | Negative | < 1.009 | Negative | Negative | Negative |
Acquired coagulation dysfunction has a complicated etiology, and can arise secondary to liver diseases, vitamin K-dependent coagulation factor deficiency, pregnancy, neoplastic diseases, autoimmune diseases, and use of certain drugs. The present patient had no family history of hemophilia or severe bleeding tendency during the previous 60 years, or of serious bleeding during pregnancy and delivery. In the previous 2 years, in addition to oral indomethacin and prednisone tablets for treatment of RA, she was prescribed nifedipine sustained-release tablets and insulin to control blood pressure and blood glucose, respectively, with no other suspicious drug usages or toxic exposures. After combining the medical history and other laboratory examinations, the possibility of liver disease or neoplastic disease was ruled out.
The diagnosis was made on the basis of the following clinical features. First, hematuria was the main clinical symptom. Second, increased PT, APTT, and INR indicated coagulation dysfunction. Third, prolongation of the R parameter on thromboelastography suggested that coagulation factor activity was decreased. Analyses revealed that FII was 7.0% and FX was 6.0%, indicating possible vitamin K-dependent coagulation factor deficiency. The Rosner index of 7.4 (< 11) in the APTT mixed correction experiment also suggested a lack of coagulation factors. Finally, after treatment with vitamin K1, the results of routine urine analysis, coagulation function, and coagulation factor activity were significantly improved, and the urine color returned to normal. Therefore, the coagulation dysfunction in this patient may have been due to vitamin K-dependent coagulation factor deficiency, which is usually caused by a lack of vitamin K.
Vitamin K is a coenzyme for many γ-glutamyl carboxylase enzymes[7,8]. When vitamin K is lacking, the γ-glutamyl carboxylases lose their biological activity and ability to synthesize vitamin K-dependent coagulation factors, leading to the disorder of the coagulation function. A previous study found that the serum levels of vitamin K1, menaquinone-4, and menaquinone-7 in patients with RA were significantly lower than those in healthy people[9]. Therefore, RA may be the direct cause of the vitamin K-dependent coagulation factor deficiency, which in turn caused the coagulation dysfunction in the present patient.
Vitamin K supplementation is the main therapeutic measure for treatment of vitamin K-dependent coagulation factor deficiency[10]. Under such circumstances, patients showed improved coagulation factor activity, PT, APTT, and bleeding symptoms after treatment with vitamin K1[7]. In the present case, the patient’s symptoms improved markedly after intravenous administration of vitamin K1, etamsylate, and vitamin C.
The relationship between RA and vitamin K-dependent coagulation factor deficiency has rarely been reported. The present case report provides some references for diagnosis and treatment of RA patients with coagulation dysfunction symptoms. Clinicians should consider investigating vitamin K deficiency in such RA cases. It remains unclear whether the vitamin K-dependent coagulation factor deficiency caused by RA was accidental, or whether there was an internal relationship with autoimmunity. The mechanism for how RA can cause vitamin K deficiency requires further elucidation.
RA can result in vitamin K-dependent coagulation factor deficiency, which leads to acquired coagulation dysfunction. Vitamin K1 supplementation has an obvious effect on coagulation dysfunction under these circumstances.
| 1. | Chinese Rheumatology Association. [2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis]. Zhonghua Nei Ke Za Zhi. 2018;57:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 2. | Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1693] [Article Influence: 211.6] [Reference Citation Analysis (0)] |
| 3. | Bowman SJ. Hematological manifestations of rheumatoid arthritis. Scand J Rheumatol. 2002;31:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Ghozlani I, Mounach A, Ghazi M, Kherrab A, Niamane R. Targeting Acquired Hemophilia A with Rheumatoid Arthritis by a Rituximab Shot: A Case Report and Review of the Literature. Am J Case Rep. 2018;19:582-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Oliveira B, Arkfeld DG, Weitz IC, Shinada S, Ehresmann G. Successful rituximab therapy of acquired factor VIII inhibitor in a patient with rheumatoid arthritis. J Clin Rheumatol. 2007;13:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Dimitroulas T, Douglas KM, Panoulas VF, Toms T, Smith JP, Treharne GJ, Nightingale P, Hodson J, Kitas GD. Derangement of hemostasis in rheumatoid arthritis: association with demographic, inflammatory and metabolic factors. Clin Rheumatol. 2013;32:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Brenner B, Kuperman AA, Watzka M, Oldenburg J. Vitamin K-dependent coagulation factors deficiency. Semin Thromb Hemost. 2009;35:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3:1873-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 269] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Khojah HM, Ahmed S, Abdel-Rahman MS, Alkhalil KM, Hamza AB. Vitamin K homologs as potential biomarkers for disease activity in patients with rheumatoid arthritis. J Bone Miner Metab. 2017;35:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Napolitano M, Mariani G, Lapecorella M. Hereditary combined deficiency of the vitamin K-dependent clotting factors. Orphanet J Rare Dis. 2010;5:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdel Ghafar MT S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang H