Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.155
Peer-review started: August 19, 2021
First decision: September 29, 2021
Revised: October 9, 2021
Accepted: November 22, 2021
Article in press: November 22, 2021
Published online: January 7, 2022
Processing time: 133 Days and 4.4 Hours
Incidentally found thyroid tumor (thyroid incidentaloma, TI) on F-18 fluorode
To distinguish malignant hypermetabolic TIs from benign disease by analyzing F-18 FDG PET-CT parameters and to identify a cut-off value.
Totally, 12761 images of patients who underwent F-18 FDG PET-CT for non-thyroid purposes at our hospital between January 2016 and December 2020 were retrospectively reviewed, and 339 patients [185 men (mean age: 68 ± 11.2) and 154 women (mean age: 63 ± 15.0)] were found to have abnormal, either focal or diffuse, thyroid FDG uptake. After a thorough review of their medical records, US, and cytological/histological reports, 46 eligible patients with focal hyper
Each of the 46 patients [12 men (26.1%; mean age: 62 ± 13.1 years) and 34 women (73.9%; mean age: 60 ± 12.0 years)] with focal hypermetabolic TIs had one focal hypermetabolic TI. Among them, 26 (56.5%) were malignant and 20 (43.5%) were benign. SUVmax, SUVpeak, SUVmean, and TLG were all higher in malignant lesions than benign ones, but the difference was statistically significant (P = 0.012) only for SUVmax. There was a positive linear correlation (r = 0.339) between SUVmax and the diagnosis of malignancy. ROC curve analysis for SUVmax revealed an area under the curve of 0.702 (P < 0.05, 95% confidence interval: 0.550-0.855) and SUVmax cut-off of 8.5 with a sensitivity of 0.615 and a specificity of 0.789.
More than half of focal hypermetabolic TIs on F-18 FDG PET-CT were revealed as malignant lesions, and SUVmax was the best parameter for discriminating between malignant and benign disease. Unexpected focal hypermetabolic TIs with the SUVmax above the cut-off value of 8.5 may have a greater than 70% chance of malignancy; therefore, further active assessment is required.
Core Tip: An unexpected focal thyroid incidentaloma (TI) is detected on various medical imaging studies. The lesion may harbor a risk of malignancy and the differentiation between malignant and benign disease is important. Standardized uptake value (SUV) is often measured for metabolism on F-18 fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT). Parameters of FDG PET-CT, including SUV, have been studied for many years in the fields of nuclear medicine and oncology. We conducted the present study to distinguish malignant TI from benign disease with an analysis of FDG PET-CT parameters.
- Citation: Lee H, Chung YS, Lee JH, Lee KY, Hwang KH. Characterization of focal hypermetabolic thyroid incidentaloma: An analysis with F-18 fluorodeoxyglucose positron emission tomography/computed tomography parameters. World J Clin Cases 2022; 10(1): 155-165
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/155.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.155
The incidence of thyroid cancer has been increasing worldwide since the last few decades[1-3], although its mortality rate is relatively stable[4,5]. According to a recent report from a national institute of South Korea, the disease was ranked as the second most frequent cancer in women after breast cancer in 2018, and it was three times more common in women than in men[6]. Cancer predominantly occurs in older individuals; however, thyroid cancer and breast cancer have their highest frequencies at relatively young ages[7]. In both sexes, thyroid cancer is most frequently found between the ages of 15 and 34 years[6,7]. Moreover, the age-standardized incidence of thyroid cancer is reported to be 48.9 for both sexes, and it is 75.5 in women, which is higher than 65.6 for breast cancer[6]. Thyroid cancer is becoming more common among younger women.
2-Deoxy-2-[18F] fluoroglucose or F-18 fluorodeoxyglucose (F-18 FDG) positron emission tomography-computed tomography (PET-CT) is used widely in the diagnosis, treatment evaluation, and follow-up of cancer. However, its role in thyroid cancer is not as definite as in other cancers. This imaging modality is rather limited and might be used for thyroid cancer in cases of elevated blood thyroglobulin without obvious abnormal iodine uptake on a whole-body scan after total thyroidectomy and/or radioactive iodine therapy[8-10].
In this situation, an unexpectedly detected thyroid lesion (thyroid incidentaloma, TI) with high F-18 FDG uptake (hypermetabolism) may have important implications. This retrospective study was conducted to distinguish malignant hypermetabolic TIs from benign disease by analysing FDG PET-CT parameters of hypermetabolic TIs on PET-CT performed at our hospital for non-thyroid purposes and to identify an optimal cut-off value.
We retrospectively reviewed the imaging data of 12761 patients who underwent F-18 FDG PET-CT to evaluate or follow-up their newly or previously diagnosed malignant disease, except for thyroid cancer, at our hospital between January 2016 and December 2020. We identified 339 patients (185 men and 154 women with mean age 68 ± 11.2 years and 63 ± 15.0 years, respectively) whose images presented incidentally abnormal hypermetabolism in their thyroid. From those, we selected patients with focal thyroid hypermetabolism after exclusion of the cases with known thyroid lesions and diffuse FDG uptake in or around the thyroid. The reports of ultrasonography (US) and, as a gold standard, cytological/histological examinations from fine-needle aspiration cytology or thyroidectomy were collected for the selected patients. Those with the reports of all three examinations were eligible for inclusion in this study.
All patients were required to fast for 4-6 h and had their blood glucose level checked before acquiring F-18 FDG PET-CT to ensure optimal image quality. When the blood glucose level was greater than or equal to 11 mmol/L (200 mg/dL), the scan was rescheduled. Scanning was performed 60 min after intravenously injecting 185 MBq F-18 FDG. Images from the skull base to the upper thigh were acquired using a dedicated PET-CT scanner, Biograph mCT 128 (Siemens Healthcare GmbH, Erlangen, Germany). Individually optimized images with lower patient radiation exposure were obtained with the emission scan performed for 3 min per bed by the step and shoot method and the CT scan performed in the continuous spiral mode with functions such as CareDose4D and CARE kV based on the default values of 60 mAs and 120 kVp, respectively. No contrast material was used for the CT scan. Both PET and CT images were reconstructed by the iterative reconstruction method, and fusion PET-CT images were generated on the dedicated image acquisition workstation provided with the PET-CT equipment.
Two nuclear medicine physicians examined the F-18 FDG PET-CT images. Once they identified an abnormal FDG uptake by the thyroid, they looked up the patient’s medical record to obtain the US and cytological/histological reports, then the lesion was categorized as malignant or benign according to the cytological/histological report when available. The maximum, peak, and mean of the semi-quantitative standardized uptake value (SUV) of focal TI were measured. SUVs of the contralateral thyroid were also measured. Additionally, the metabolic tumor volume (MTV) of TI was measured. The volume of interest (VOI) for measuring MTV can be drawn differently using different SUV thresholds. In this study, multiple SUV thresholds from 2 to 5 with an increment of 0.5 were used to obtain multiple MTVs. Finally, total lesion glycolysis (TLG) was calculated by multiplying MTV by the mean SUV. All imaging analyses were performed on a dedicated PET-CT workstation equipped with SyngoMMWP (Siemens Healthcare GmbH, Erlangen, Germany). These five para
Both parametric and non-parametric methods were used to compare SUVmax, SUVpeak, SUVmean, MTV, and TLG between the malignant and benign lesions. Point biserial correlation was performed for the parameter(s) and malignancy. ROC curves were plotted, and the area under the curve (AUC) was calculated to determine an optimal cut-off value. Statistical analysis was performed using SPSS 16 (IBM, Armonk, New York, United States). A P value of less than 0.05 was considered statistically sig
This retrospective study was approved by the institutional review board of our hospital (IRB no. GAIRB2020-297), and the requirement to obtain informed consent was waived. The study was conducted in accordance with the 1964 Declaration of Helsinki and later amendments.
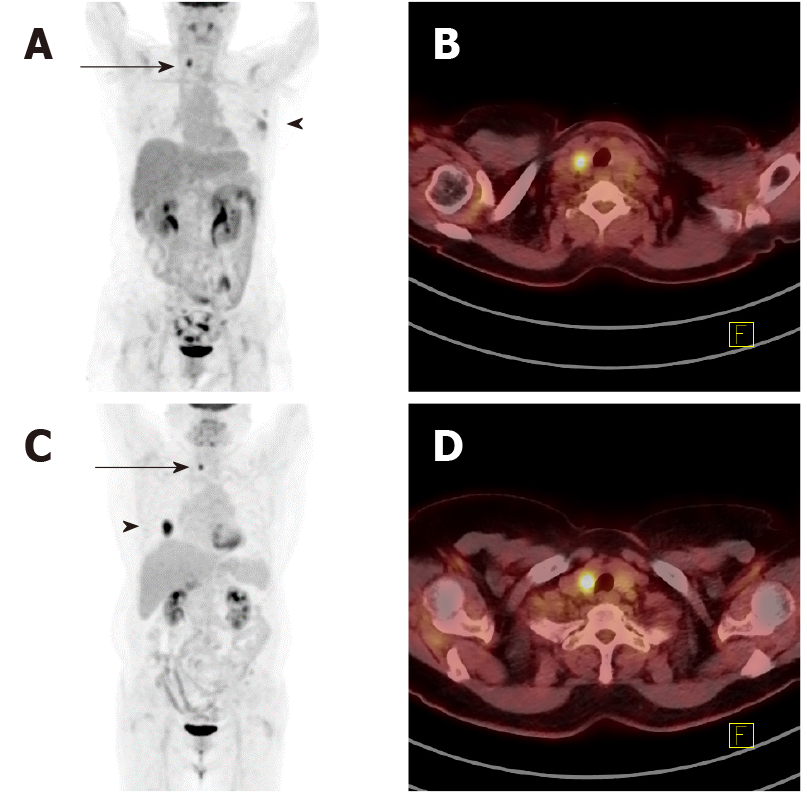
Approximately 2.7% (339/12761) of all FDG PET-CT images reviewed initially showed abnormal thyroid hypermetabolism. The demographic and clinical characteristics of these 339 patients are shown in Table 1. Amongst the 339 non-thyroid disease patients [185 men (mean age: 68 ± 11.2) and 154 women (mean age: 63 ± 15.0 years)] with incidental suspicious hypermetabolism of the thyroid gland, 46 patients [13.6%, 12 men (mean age: 62 ± 13.1 years) and 34 women (mean age: 60 ± 12.0 years)] had focal hypermetabolism on PET-CT, and the hypermetabolic location was identified as a nodule on US and confirmed by cytological/histological analysis. Figure 1 shows some representative PET-CT images of such cases. Overall, 56.5% (26/46) of the cases were malignant, and the rest 43.5% (20/46) were benign. Amongst malignancy cases, 84.6% (22/26) were papillary, 3.8% (1/26) follicular, 3.8% (1/26) poorly differentiated, and 7.7% (2/26) Hurthle cell malignancies. Their primary cancers and cytolo
| Characteristics | Male | Female | Total |
| Subjects (n) | 185 | 154 | 339 |
| Age (yr, mean ± SD) | 68 ± 11.2 | 63 ± 15.0 | 66 ± 13.3 |
| Primary malignancy (n) | |||
| Lung | 64 | 33 | 97 (28.6%) |
| Colorectal | 31 | 11 | 42 (12.4%) |
| Breast | 0 | 50 | 50 (14.7%) |
| Lymphoma | 15 | 15 | 30 (8.8%) |
| Gastrointestinal | 20 | 8 | 28 (8.3%) |
| Hepatobiliary | 20 | 8 | 28 (8.3%) |
| Head and neck | 13 | 2 | 15 (4.4%) |
| Other | 22 | 27 | 49 (14.5%) |
| Primary cancer | Malignant | Benign | Total |
| Breast | 5 | 6 | 11 |
| Kidney | 1 | 0 | 1 |
| Colorectal | 2 | 3 | 5 |
| GIST | 0 | 1 | 1 |
| Lung | 8 | 8 | 16 |
| Stomach | 2 | 0 | 2 |
| Uterine cervix | 3 | 0 | 3 |
| Sarcoma | 1 | 0 | 1 |
| Urinary bladder | 1 | 0 | 1 |
| Salivary gland | 1 | 0 | 1 |
| Lymphoma | 1 | 2 | 3 |
| Bone | 1 | 0 | 1 |
| Total | 26 | 20 | 46 |
Five representative parameters of PET-CT (SUVmax, SUVpeak, SUVmean, MTV, and TLG) were compared to evaluate the differences between malignant and benign lesions. Table 3 shows an example of these parameters. The average SUVmax of 26 malignant lesions and their contralateral isometabolic thyroid areas without US-identified lesions was 10.8 ± 7.5 and 2.5 ± 1.2, respectively, with a statistically significant difference (P < 0.05). Similarly, the average SUVmax of benign lesions and their contralateral thyroid areas was 6.5 ± 3.0 and 2.1 ± 0.7, respectively, also with statistical significance. There was a significant difference between the SUVmax of malignant and benign focal thyroid lesions (P = 0.012). The SUVmax of contralateral thyroid areas of both malignant and benign lesions presented no significant difference. Point biserial correlation resulted in a statistically significant positive linear correlation (r = 0.339) between SUVmax and the malignant cytological/histological report (P < 0.05).
| Parameters | Malignant (n = 26) | Benign (n = 20) | P value |
| SUVmax | 10.8 ± 7.5 | 6.5 ± 3.0 | 0.012 |
| SUVpeak | 6.8 ± 5.7 | 4.4 ± 2.0 | 0.058 |
| MTV2 | 5.06 ± 5.2 | 6.7 ± 6.6 | 0.354 |
| SUVmean2 | 3.8 ± 1.5 | 3.0 ± 0.5 | 0.011 |
| TLG2 | 25.5 ± 48.9 | 21.3 ± 24.0 | 0.735 |
| MTV2.5 | 3.5 ± 4.5 | 3.7 ± 4.2 | 0.872 |
| SUVmean2.5 | 4.5 ± 1.6 | 3.6 ± 0.7 | 0.014 |
| TLG2.5 | 22.0 ± 47.6 | 14.8 ± 19.0 | 0.532 |
SUVpeak presented no statistical significance with a P-value of 0.058, which was close to significance. The SUVmean showed statistical significance with a threshold of 2 (P = 0.011) and 2.5 (P = 0.014). The SUVmean with other thresholds, MTV, and TLG failed to show any statistical significance.
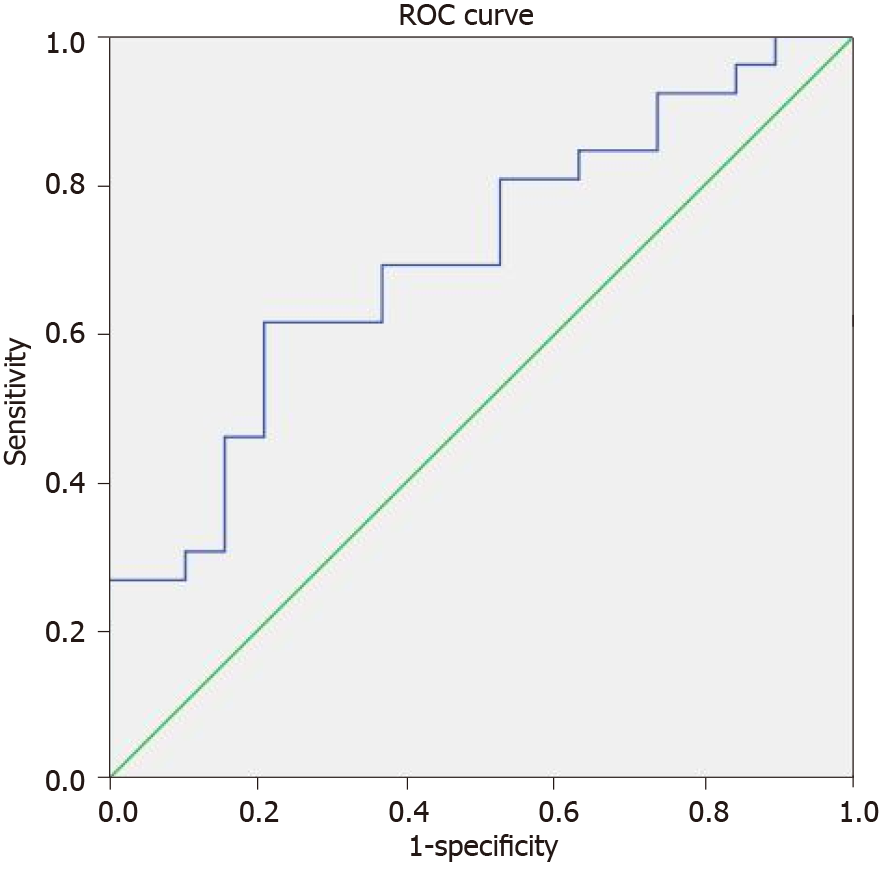
An ROC curve was plotted for SUVmax (Figure 2), and the AUC was 0.702 (P < 0.05, 95% confidence interval: 0.550-0.855). The SUVmax cut-off value was 8.5 with a sen
The number of diagnoses of thyroid cancer has been increasing for several decades, and this includes TIs identified by PET-CT, CT, magnetic resonance imaging, and US conducted for non-thyroid purposes. Well-differentiated thyroid cancers such as papillary and follicular cancers, which develop from thyroid follicular cells, comprise more than 85% of all thyroid cancers[11,12]. Well-differentiated thyroid cancers are known to be less aggressive and have a better prognosis than other thyroid cancers such as poorly differentiated thyroid cancer, anaplastic thyroid cancer, or Hurthle cell cancer; however, up to 5% of well-differentiated thyroid cancers could become dedifferentiated and aggressive[13-15]. Dedifferentiated thyroid cancer is generally not very responsive to radioactive iodine therapy, while well-differentiated cancer shows a good response. FDG is easily taken up by aggressive cancers with less/non-iodine-avidity or by tumors with increased malignancy due to the elevated expression of glucose transporter 1. As the majority of thyroid cancers are slow-growing well-differentiated types, they are generally less FDG avid, and F-18 FDG PET-CT has a limited role in the initial evaluation. It is usually only used for the evaluation of recurrences after resection and/or iodine therapy when the thyroglobulin level in the serum is suspicious without definite abnormal findings on US or an iodine whole-body scan. Therefore, the focus of this study is not on the initial evaluation of thyroid cancer but on unexpectedly identified FDG uptake by the thyroid on PET-CT per
Diffuse thyroid FDG uptake has a greater chance of being benign thyroid diseases such as thyroiditis or hypothyroidism than cancer[16,17]. However, about 25%-50% of focal hypermetabolic TIs, with a prevalence of 2.5%-5%, have malignant cytolo
Of the 23 well-differentiated malignant lesions of this study, 19 were available for the BRAF mutation test, and 100% (19/19) lesions were proved to have the mutation. This (dedifferentiation) could be associated with a change in FDG avidity from low to high. As this study was conducted on any hypermetabolic lesions discovered with the naked eye, lesions not yet advanced, which is why they had low FDG uptake and therefore had less chance to be observed on images, were likely excluded from the study. This unrecognized selection bias probably resulted in a high FDG uptake even in lesions of well-differentiated thyroid cancer. Conversely, if thyroid cancer was diagnosed pathologically first and then FDG PET-CT was performed, there would be more lesions with low FDG uptake.
All of the patients involved in this study already had one type of cancer but not thyroid cancer, and we excluded PET-CT images acquired for benign diseases or health check-ups. This patient selection might influence the malignancy rate, especially since there is a report describing the prevalence of TI being higher in patients with cancer than in healthy subjects[19].
The higher the semi-quantitative SUV on F-18 FDG PET-CT, the higher is the possibility of cancer with various reported cut-off values, and this is related to the prognosis and overall survival[23-26]. Among the five PET parameters associated with SUV and the metabolic volume of the tumor, SUVmax showed good performance in discriminating malignant lesions from benign ones. The mean value of SUVmean was higher in the malignant group and presented a statistical significance difference comparable to SUVmax in some conditions (SUV threshold of 2.0 and 2.5). This could be associated with a larger volume of benign lesions with the same SUV thresholds. There was no statistical significance for the SUVmean with other SUV thresholds where the volumes were all larger in the malignant group. In a situation with a low SUV threshold, the VOI might include areas outside the tumor, and consequently, the final measured volume could be larger than the real volume. SUVmean might be influenced and reduced as the volume of benign lesions is unintentionally larger, and this could lead to a significant statistical difference from that of malignant lesions. The mean values of SUVpeak and TLG were higher in the malignant group but without statistical significance, although SUVpeak caught our attention with a P-value of 0.058, which was close to significance.
There are studies on TIs reporting that MTV, TLG, or both are useful parameters in distinguishing malignant lesions from benign ones[27-31], while other reported different conclusions[32]. The roles of MTV and TLG in other cancers are still open to debate[33-36]. In this study, both MTV and TLG were not useful in the discrimination. TLG was expected to be a good discriminator initially like SUVmax, but it was not. This might have something to do with MTV. There are reports that a specific range of thyroid nodule sizes had a greater prevalence of malignancy, while others found no increased risk of malignancy over a specific nodule size[37-41]. These findings imply that a larger size does not necessarily mean a higher possibility of malignancy. MTV might be thought of in a similar way, and thus a larger MTV does not always mean malignancy. In this way, there is a possibility that TLG, which is the product of SUVmean and MTV, might not reflect the risk of malignancy well. Finally, SUVmax was the only reliable discriminator, while SUVpeak might be a candidate. In contrast, the other parameters had no discernible statistical impact.
SUVmax was chosen for the ROC curve. Based on the AUC[42,43], SUVmax has a power of fair discrimination with approximately 70% probability for malignancy in an unexpectedly identified focal hypermetabolic thyroid lesion. The lesions with SUVmax higher than 8.5 have a greater chance to be malignant with a sensitivity of 61.5% and a specificity of 78.9%. Some cases of Hurthle cell adenoma, which might have high FDG uptake[44-46], were included in the benign group and these could reduce the sensitivity and AUC, making the discrimination difficult. The reading of PET-CT images relies mainly on the naked eye qualitatively and it is not simple to distinguish malignant lesions from benign ones with a high FDG uptake. Relatively rare metastatic lesions from other cancers could also have a high FDG uptake[47-49]. Therefore, SUVmax with a reference of suggested cut-off value should be measured in cases of hypermetabolic TI, and further active examination is recommended to characterize lesions above the threshold.
More than half of the focal hypermetabolic TIs on F-18 FDG PET-CT were revealed as malignant. SUVmax was the best parameter for discriminating malignant and benign lesions. The unexpected focal hypermetabolic TIs with an SUVmax above the cut-off value of 8.5 may have a greater than 70% chance of malignancy; therefore, further active assessment is required to characterize these lesions.
Thyroid incidentaloma (TI) is detected on imaging studies for non-thyroid purposes and the lesion may harbor a risk of malignancy. It is critical to distinguish malignant TI from benign disease.
The higher the metabolism on F-18 fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) image, the higher the possibility of malignancy. TI might be characterized depending on the FDG metabolism.
To distinguish malignant hypermetabolic TIs from benign disease by analyzing F-18 FDG PET-CT parameters and to identify a cut-off value.
The values of parameters from FDG PET-CT of 46 focal hypermetabolic thyroid lesions were measured, calculated, and compared. Receiver operating characteristic (ROC) curve was plotted to determine a cut-off value.
Standardized uptake value (SUV)max was the only statistically significant discriminator in differentiation. From the ROC curve, the AUC was 0.702 and the SUVmax cut-off value was 8.5.
TIs with SUVmax above the cut-off value 8.5 may have a greater than 70% chance of malignancy. A further active assessment is required.
Other studies and controversies on the parameters included in this study are ongoing. Further studies with a large number of subjects are guaranteed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ankrah AO, Silano F S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Rossi ED, Pantanowitz L, Hornick JL. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol. 2021;9:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Olson E, Wintheiser G, Wolfe KM, Droessler J, Silberstein PT. Epidemiology of Thyroid Cancer: A Review of the National Cancer Database, 2000-2013. Cureus. 2019;11:e4127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide Thyroid-Cancer Epidemic? N Engl J Med. 2016;375:614-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 805] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 4. | Ahn HS, Kim HJ, Kim KH, Lee YS, Han SJ, Kim Y, Ko MJ, Brito JP. Thyroid Cancer Screening in South Korea Increases Detection of Papillary Cancers with No Impact on Other Subtypes or Thyroid Cancer Mortality. Thyroid. 2016;26:1535-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 5. | Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1097] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 6. | Korea Central Cancer Registry. Annual report of cancer statistics in Korea in 2018. [cited from 15 August 2021]. Available from: https://ncc.re.kr/cancerStatsView.ncc?bbsnum=558&searchKey=total&searchValue=&pageNum=1. |
| 7. | Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES; Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2017. Cancer Res Treat. 2020;52:335-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 327] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 8. | Bannas P, Derlin T, Groth M, Apostolova I, Adam G, Mester J, Klutmann S. Can (18)F-FDG-PET/CT be generally recommended in patients with differentiated thyroid carcinoma and elevated thyroglobulin levels but negative I-131 whole body scan? Ann Nucl Med. 2012;26:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Bertagna F, Bosio G, Biasiotto G, Rodella C, Puta E, Gabanelli S, Lucchini S, Merli G, Savelli G, Giubbini R, Rosenbaum J, Alavi A. F-18 FDG-PET/CT evaluation of patients with differentiated thyroid cancer with negative I-131 total body scan and high thyroglobulin level. Clin Nucl Med. 2009;34:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Okuyucu K, Ince S, Alagoz E, Emer O, San H, Balkan E, Ayan A, Meric C, Haymana C, Acıkel C, Gunalp B, Karacalioglu AO, Arslan N. Risk factors and stratification for recurrence of patients with differentiated thyroid cancer, elevated thyroglobulin and negative I-131 whole-body scan, by restaging 18F-FDG PET/CT. Hell J Nucl Med. 2016;19:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Shah JP. Thyroid carcinoma: epidemiology, histology, and diagnosis. Clin Adv Hematol Oncol. 2015;13:3-6. [PubMed] |
| 12. | Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, Dickson P, Duh QY, Ehya H, Goldner W, Haymart M, Hoh C, Hunt JP, Iagaru A, Kandeel F, Kopp P, Lamonica DM, McIver B, Raeburn CD, Ridge JA, Ringel MD, Scheri RP, Shah JP, Sippel R, Smallridge RC, Sturgeon C, Wang TN, Wirth LJ, Wong RJ, Johnson-Chilla A, Hoffmann KG, Gurski LA. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J Natl Compr Canc Netw. 2018;16:1429-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 13. | Antonelli A, Ferri C, Ferrari SM, Sebastiani M, Colaci M, Ruffilli I, Fallahi P. New targeted molecular therapies for dedifferentiated thyroid cancer. J Oncol. 2010;2010:921682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Sturgeon C, Angelos P. Identification and treatment of aggressive thyroid cancers. Part 1: subtypes. Oncology (Williston Park). 2006;20:253-260. [PubMed] |
| 15. | Braga-Basaria M, Ringel MD. Clinical review 158: Beyond radioiodine: a review of potential new therapeutic approaches for thyroid cancer. J Clin Endocrinol Metab. 2003;88:1947-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Liu Y. Clinical significance of thyroid uptake on F18-fluorodeoxyglucose positron emission tomography. Ann Nucl Med. 2009;23:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Karantanis D, Bogsrud TV, Wiseman GA, Mullan BP, Subramaniam RM, Nathan MA, Peller PJ, Bahn RS, Lowe VJ. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. J Nucl Med. 2007;48:896-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Burguera B, Gharib H. Thyroid incidentalomas. Prevalence, diagnosis, significance, and management. Endocrinol Metab Clin North Am. 2000;29:187-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Choi JY, Lee KS, Kim HJ, Shim YM, Kwon OJ, Park K, Baek CH, Chung JH, Lee KH, Kim BT. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47:609-615. [PubMed] |
| 20. | Kwak JY, Kim EK, Yun M, Cho A, Kim MJ, Son EJ, Oh KK. Thyroid incidentalomas identified by 18F-FDG PET: sonographic correlation. AJR Am J Roentgenol. 2008;191:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Bertagna F, Treglia G, Piccardo A, Giubbini R. Diagnostic and clinical significance of F-18-FDG-PET/CT thyroid incidentalomas. J Clin Endocrinol Metab. 2012;97:3866-3875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Gavriel H, Tang A, Eviatar E, Chan SW. Unfolding the role of PET FDG scan in the management of thyroid incidentaloma in cancer patients. Eur Arch Otorhinolaryngol. 2015;272:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Kim SH, Song BI, Kim HW, Won KS, Son YG, Ryu SW. Prognostic Value of Restaging F-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography to Predict 3-Year Post-Recurrence Survival in Patients with Recurrent Gastric Cancer after Curative Resection. Korean J Radiol. 2020;21:829-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Purandare NC, Puranik A, Shah S, Agrawal A, Puri A, Gulia A, Nayak P, Rekhi B, Rangarajan V. Can 18F-FDG PET/CT diagnose malignant change in benign chondroid tumors? Nucl Med Commun. 2019;40:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Kohutek ZA, Wu AJ, Zhang Z, Foster A, Din SU, Yorke ED, Downey R, Rosenzweig KE, Weber WA, Rimner A. FDG-PET maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non-small cell lung cancer. Lung Cancer. 2015;89:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Nguyen NC, Kaushik A, Wolverson MK, Osman MM. Is there a common SUV threshold in oncological FDG PET/CT, at least for some common indications? Acta Oncol. 2011;50:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Erdoğan M, Korkmaz H, Torus B, Avcı M, Boylubay ŞM, Çiriş M, Yıldız M, Şengül SS. The Role of Metabolic Volumetric Parameters in Predicting Malignancy in Incidental Thyroid Nodules Detected in 18F-FDG PET/CT Scans. Mol Imaging Radionucl Ther. 2021;30:86-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Sollini M, Cozzi L, Pepe G, Antunovic L, Lania A, Di Tommaso L, Magnoni P, Erba PA, Kirienko M. [18F]FDG-PET/CT texture analysis in thyroid incidentalomas: preliminary results. Eur J Hybrid Imaging. 2017;1:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Shi H, Yuan Z, Yang C, Zhang J, Shou Y, Zhang W, Ping Z, Gao X, Liu S. Diagnostic Value of Volume-Based Fluorine-18-Fluorodeoxyglucose PET/CT Parameters for Characterizing Thyroid Incidentaloma. Korean J Radiol. 2018;19:342-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Ceriani L, Milan L, Virili C, Cascione L, Paone G, Trimboli P, Giovanella L. Radiomics Analysis of [18F]-Fluorodeoxyglucose-Avid Thyroid Incidentalomas Improves Risk Stratification and Selection for Clinical Assessment. Thyroid. 2021;31:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Kim BH, Kim SJ, Kim H, Jeon YK, Kim SS, Kim IJ, Kim YK. Diagnostic value of metabolic tumor volume assessed by 18F-FDG PET/CT added to SUVmax for characterization of thyroid 18F-FDG incidentaloma. Nucl Med Commun. 2013;34:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Thuillier P, Bourhis D, Roudaut N, Crouzeix G, Alavi Z, Schick U, Robin P, Kerlan V, Salaun PY, Abgral R. Diagnostic Value of FDG PET-CT Quantitative Parameters and Deauville-Like 5 Point-Scale in Predicting Malignancy of Focal Thyroid Incidentaloma. Front Med (Lausanne). 2019;6:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Chen B, Feng H, Xie J, Li C, Zhang Y, Wang S. Differentiation of soft tissue and bone sarcomas from benign lesions utilizing 18F-FDG PET/CT-derived parameters. BMC Med Imaging. 2020;20:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Hu Y, Zhou W, Sun S, Guan Y, Ma J, Xie Y. 18F-fluorodeoxyglucose positron emission tomography-based prediction for splenectomy in patients with suspected splenic lymphoma. Ann Transl Med. 2021;9:1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Morita T, Tatsumi M, Ishibashi M, Isohashi K, Kato H, Honda O, Shimosegawa E, Tomiyama N, Hatazawa J. Assessment of Mediastinal Tumors Using SUVmax and Volumetric Parameters on FDG-PET/CT. Asia Ocean J Nucl Med Biol. 2017;5:22-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 36. | Shen CT, Qiu ZL, Sun ZK, Wei WJ, Song HJ, Zhang XY, Luo QY. Dual time-point 18F-FDG PET/CT imaging with multiple metabolic parameters in the differential diagnosis of malignancy-suspected bone/joint lesions. Oncotarget. 2017;8:71188-71196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Al-Hakami HA, Alqahtani R, Alahmadi A, Almutairi D, Algarni M, Alandejani T. Thyroid Nodule Size and Prediction of Cancer: A Study at Tertiary Care Hospital in Saudi Arabia. Cureus. 2020;12:e7478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Kamran SC, Marqusee E, Kim MI, Frates MC, Ritner J, Peters H, Benson CB, Doubilet PM, Cibas ES, Barletta J, Cho N, Gawande A, Ruan D, Moore FD Jr, Pou K, Larsen PR, Alexander EK. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Chung SR, Baek JH, Choi YJ, Sung TY, Song DE, Kim TY, Lee JH. The relationship of thyroid nodule size on malignancy risk according to histological type of thyroid cancer. Acta Radiol. 2020;61:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Cavallo A, Johnson DN, White MG, Siddiqui S, Antic T, Mathew M, Grogan RH, Angelos P, Kaplan EL, Cipriani NA. Thyroid Nodule Size at Ultrasound as a Predictor of Malignancy and Final Pathologic Size. Thyroid. 2017;27:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 41. | Aydoğan Bİ, Şahin M, Ceyhan K, Deniz O, Demir Ö, Emral R, Tonyukuk Gedik V, Uysal AR, Çorapçıoğlu D. The influence of thyroid nodule size on the diagnostic efficacy and accuracy of ultrasound guided fine-needle aspiration cytology. Diagn Cytopathol. 2019;47:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Muller MP, Tomlinson G, Marrie TJ, Tang P, McGeer A, Low DE, Detsky AS, Gold WL. Can routine laboratory tests discriminate between severe acute respiratory syndrome and other causes of community-acquired pneumonia? Clin Infect Dis. 2005;40:1079-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Kang YS, Kang JH, Kim MC, Yu BY, Sung EJ, Lee SY, Lee YJ. Cutoff of Percent Body Fat to Predict Obesity and Metabolic Risk in Children and Adolescents: 2007 Children and Adolescent Physical Growth Standard. Korean J Fam Med. 2009;30:887-894. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Pathak KA, Klonisch T, Nason RW, Leslie WD. FDG-PET characteristics of Hürthle cell and follicular adenomas. Ann Nucl Med. 2016;30:506-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Hassan A, Riaz S, Asif A. Hypermetabolic Hurthle Cell Adenoma on 18F-FDG PET/CT. Mol Imaging Radionucl Ther. 2018;27:96-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Yu R, Auerbach MS. FDG-Avid Hürthle Cell Thyroid Adenoma. Clin Nucl Med. 2019;44:752-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Nagarajah J, Ho AL, Tuttle RM, Weber WA, Grewal RK. Correlation of BRAFV600E Mutation and Glucose Metabolism in Thyroid Cancer Patients: An ¹⁸F-FDG PET Study. J Nucl Med. 2015;56:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Yoon S, An YS, Lee SJ, So EY, Kim JH, Chung YS, Yoon JK. Relation Between F-18 FDG Uptake of PET/CT and BRAFV600E Mutation in Papillary Thyroid Cancer. Medicine (Baltimore). 2015;94:e2063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 49. | Kim H, Na KJ, Choi JH, Ahn BC, Ahn D, Sohn JH. Feasibility of FDG-PET/CT for the initial diagnosis of papillary thyroid cancer. Eur Arch Otorhinolaryngol. 2016;273:1569-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |