Published online Sep 16, 2013. doi: 10.12998/wjcc.v1.i6.197
Revised: August 8, 2013
Accepted: August 20, 2013
Published online: September 16, 2013
Processing time: 88 Days and 13.3 Hours
The development of chronic pain after amputations is not an uncommon event. In some cases the most disabling problem is represented by the symptom called dynamic mechanical allodynia, characterized by the painful sensation evoked by gently stroking the skin. Despite the growing interest in understanding pain mechanisms, little is known about the mechanism sustaining this peculiar type of pain. We present here the case of a 53-year-old female patient who complained of severe tactile allodynia in the hand after amputation of her left second finger, resistant to several medical and surgical treatments. In order to gain information about the pain mechanism, two neurodiagnostic skin biopsies were obtained from the area of tactile allodynia and from the contralateral, normal skin area. Skin biopsies showed an unexpected increased innervation of the allodynic skin compared to the contralateral, normal skin area (+ 80.1%). Hyperinnervation has been proposed as a mechanism of pain following nerve lesions, but the increased innervation described here could be also attributed to neuronal plasticity occurring in chronic inflammatory conditions. Independently from the uncertain cause of the epidermal hyperinnervation, in this patient we tried to reduce the elevated number of epidermal nerve fibres by treating the skin with topical capsaicin (0.075%) three times a day, and obtained a persistent pain relief. In conclusion, neurodiagnostic skin biopsy might represent an useful tool for detecting derangements of epidermal innervation in patients with dynamic mechanical allodynia and can help to select an individually tailored therapeutic strategy in such difficult clinical conditions. Further studies are needed to clarify this issue and try to gain better understanding of chronic pain mechanisms in patients who underwent finger amputation.
Core tip: In some patients with post-amputation chronic pain dynamic mechanical allodynia (a painful sensation evoked by gentle stroking the skin) represents the most disabling problem. So far, little is known about the mechanism of this peculiar type of pain. We present here a patient who complained of severe dynamic mechanical allodynia in the hand after amputation of the left second finger. The neurodiagnostic skin biopsy showed an increased innervation of the allodynic skin compared to the contralateral, normal skin area (+ 80.1%), suggesting hyperinnervation as a possible pain mechanism. Interestingly, topical capsaicin (0.075%) relieved allodynia for a long period.
- Citation: Buonocore M, Gagliano MC, Bonezzi C. Dynamic mechanical allodynia following finger amputation: Unexpected skin hyperinnervation. World J Clin Cases 2013; 1(6): 197-201
- URL: https://www.wjgnet.com/2307-8960/full/v1/i6/197.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v1.i6.197
The development of chronic pain after finger amputation is not an uncommon event[1-4]. In this context, one of the most disabling symptoms is dynamic mechanical allodynia (or brush evoked allodynia), which has been defined as the pain induced by a light moving mechanical stimuli on the skin[5-7]. Although it has been described some decades ago, its mechanisms are far to be completely understood[5,8].
We describe here the case of a 53-year-old female patient who complained of severe tactile allodynia in her hand after amputation of the left index finger at the metacarpal-phalangeal joint and discuss the possibility that the painful sensation was sustained by an hyperinnervation of the skin.
In 2001, following a minor trauma, the patient experienced acute pain in the tip of her left index finger. Surprisingly, the pain persisted, despite any efforts to relieve it by several analgesic drugs including paracetamol, nonsteroidal antiinflammatory drugs and tramadol. In order to relieve the persistent pain, in the period 2002-2004, three operations for removal of neuromas of the digital nerves to the index finger were performed, but they provided only transient pain relief.
Due to the persistence of pain, on January 2006 the patient underwent amputation of her left second finger at the metacarpal-phalangeal joint. In particular, the palmar collateral nerves of the index were exposed and the radial one was found adherent to the underneath structures. It was isolated, resected and sutured end to end to the ulnar collateral nerve according to the principle of centro-central loop coaptation as described by Fourrier et al[9]. Skin suture was performed and a volar slab was applied and kept for 3 wk.
Following amputation the patient experienced a significant pain relief, but 4 mo later the pain reappeared in the hand with the characteristics of deep allodynia during flexion or extension of left middle finger. In the following weeks dynamic mechanical allodynia spread to the skin of both the palm and dorsum of the hand.
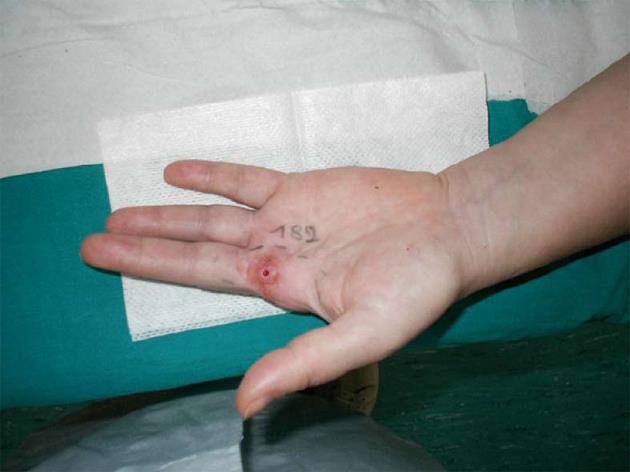
Since the pain relief was only transient and incomplete and because of the inefficacy of several pharmacological agents including gabapentin, pregabalin and amitriptyline, on October 2006 the patient presented at our hospital for the first time. In order to get more information on the possible mechanism of her pain, the patient underwent several diagnostic tests and a neurodiagnostic skin biopsy was proposed. The patient consented to skin biopsy and signed an informed consent approved by the Ethics Committee of the “Salvatore Maugeri Foundation”. On March 2007, after an intradermal injection of 2% lidocaine (0.5 mL), a 3 mm punch biopsy was obtained from the skin area more severely affected by dynamic mechanical allodynia (Figure 1). A second skin biopsy was obtained from the contralateral, normal skin area.
Epidermal nerve fibres (ENFs) were labelled with indirect immunofluorescence and the images of optical sections were collected with a fluorescence microscope system[10].
According to recent guidelines of the European Federation of Neurological Societies[11], the density of ENFs (ENFD) was calculated by counting only the ENFs crossing the basement membrane and expressed as the number of ENFs per millimetre of epidermis (ENFs/mm)[11,12].
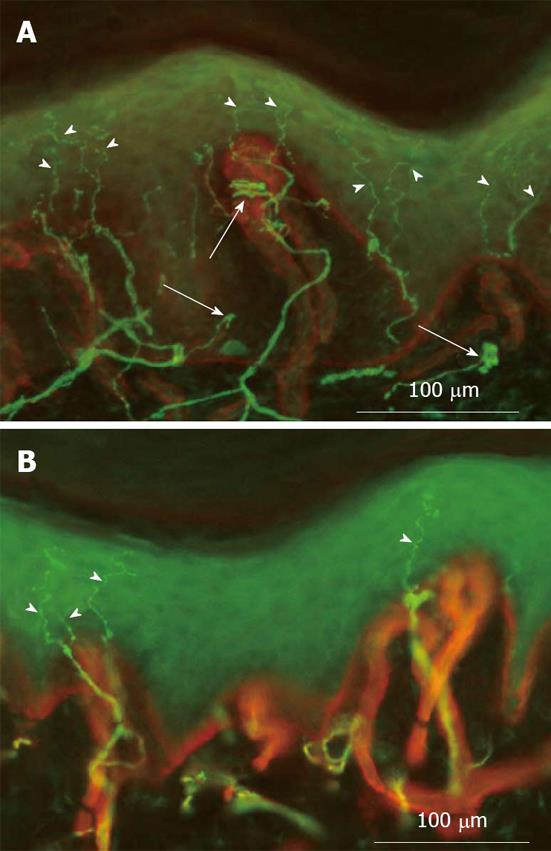
In the patient presented here, skin biopsy showed a marked asymmetry of ENFD with a significant higher value (+ 80.1%) in the allodynic skin (Figure 2). In particular ENFD was 23.6 fibres/mm in the allodynic skin and 13.1 fibres/mm in the contralateral, normal skin area, with an epidermal innervation symmetry ratio of 0.55 (normal value > 0.6)[13].
In the case described here, the neurodiagnostic skin biopsy provided evidence for an increased innervation of the allodynic skin, suggesting it as a possible pain mechanism.
Hyperinnervation has already been proposed as a possible mechanism of pain[14,15] and previous papers have documented an increased innervation in patients with painful conditions. For example, an increase in the innervation of the lateral retinaculum was correlated with anterior knee pain in a group of patients with symptomatic patellofemoral malalignment[16,17]. Hyperinnervation has been also described in patients with inflamed appendix[18], painful lumbar discopathy[19] and vulvodynia[20]. However, the relationship between hyperinnervation and allodynia has been rarely investigated. The presence of both hyperinnervation and allodinia has been described in an animal study where the injection of doxorubicin (an anti-cancer agent) into eyelids of adult rabbits produced inflammation, increased sensitivity to touch and hyperinnervation[21].
Since the development of mechanical allodynia during inflammation has been widely described in literature both in animal[22-24] and human studies[25-27], a possible explanation of the increased innervation described in our case report is the neuronal plasticity occurring in inflammatory conditions. In fact, it has been reported that acute skin inflammation tends to induce neurodegeneration, while chronic inflammation leads to increased innervation[28]. This seems to be confirmed by the increased number of nerve fibres observed in skin chronic inflammatory diseases such as prurigo nodularis[29,30], psoriasis[31,32] and atopic dermatitis[33].
Another possible explanation of the increased skin innervation is the excessive regeneration of amputated nerve axons. Although in humans neuropathic pain has been associated with poor nerve regeneration following traumatic nerve lesions[34,35], mechanical allodynia is indeed strictly related to the well known development of mechanosensitivity in regenerating primary afferents[35-38]. When some regenerating axons directly reach the peripheral target tissues, non-neuronal cells synthesise growth factors which are able to induce the sprouting of the lesioned fibres towards the target tissues[39,40].
All that considered, increased number of skin afferents with a reduced threshold for mechanical stimuli could explain the dynamic mechanical allodynia observed in this case.
According to this hypothesis, a targeted therapeutic strategy was carried out in this patient. Considering the ENFs degeneration after topical application of capsaicin[41] we topically treated the patient’s skin with capsaicin cream (0.075%) in the tactile allodynia area three times a day. In order to prevent the unbearable burning sensation, sometimes associated with capsaicin based therapies, we instructed the patient to a gradual dose titration. After 4 wk of treatment, the tactile allodynia was still present, but the patient reported a significant reduction in both its intensity and extension. Capsaicin treatment was then discontinued, but the antalgic effect was still present on a mid-term follow-up (3 mo).
In conclusion, the case described here seems to confirm, at least in some patients, the possible association between epidermal hyperinnervation and the clinical development of dynamic mechanical allodynia. Moreover, skin biopsy might represent an useful tool to better define the underlying pathology of allodynic conditions and can help to select an individually tailored therapeutic strategy in such difficult clinical conditions. Further studies are warranted in order to improve the knowledge about the mechanisms underlying the onset of dynamic mechanical allodynia after amputation.
The authors would like to thank the neuropathophysiology technicians Michela Canti and Rosa Bagnasco for their essential role in the paper draft and Dr. Anna Maria Gatti for helping in the preparation of figures.
P- Reviewers Ge HY, Wang FZ S- Editor Gou SX L- Editor A E- Editor Liu XM
| 1. | Fisher GT, Boswick JA. Neuroma formation following digital amputations. J Trauma. 1983;23:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Kumar N, Stevenson JH. Intractable digital neuroma pain; the ultimate solution? Br J Plast Surg. 1990;43:122-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Belcher HJ, Pandya AN. Centro-central union for the prevention of neuroma formation after finger amputation. J Hand Surg Br. 2000;25:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Hattori Y, Doi K, Ikeda K, Estrella EP. A retrospective study of functional outcomes after successful replantation versus amputation closure for single fingertip amputations. J Hand Surg Am. 2006;31:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Baron R, Maier C. Painful neuropathy: C-nociceptor activity may not be necessary to maintain central mechanisms accounting for dynamic mechanical allodynia. Clin J Pain. 1995;11:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Attal N, Brasseur L, Chauvin M, Bouhassira D. A case of ‘pure’ dynamic mechano-allodynia due to a lesion of the spinal cord: pathophysiological considerations. Pain. 1998;75:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000;16:S12-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Bowsher D. Dynamic mechanical allodynia in neuropathic pain. Pain. 2005;116:164-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Fourrier P, Papot G, Cayrol M. [Value of Samii’s method in the treatment of digital neuromas]. Ann Chir Main. 1986;5:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Kennedy WR. Opportunities afforded by the study of unmyelinated nerves in skin and other organs. Muscle Nerve. 2004;29:756-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17:903-912, e44-e49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 601] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 12. | Smith AG, Howard JR, Kroll R, Ramachandran P, Hauer P, Singleton JR, McArthur J. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci. 2005;228:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Buonocore M, Gatti AM, Demartini L. Epidermal innervation symmetry ratio (EISR) for the diagnosis of unilateral neuropathic pain. In: Abstract Book of the ¡°4th International Congress on Neuropathic Pain¡±; 2013 May 23-26. Toronto: CDN 2013; USB key: 740. |
| 14. | Woolf CJ. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, Lipton R, Loeser JD, Payne R, Torebjork E. Towards a mechanism-based classification of pain? Pain. 1998;77:227-229. [PubMed] |
| 16. | Sanchis-Alfonso V, Roselló-Sastre E, Monteagudo-Castro C, Esquerdo J. Quantitative analysis of nerve changes in the lateral retinaculum in patients with isolated symptomatic patellofemoral malalignment. A preliminary study. Am J Sports Med. 1998;26:703-709. [PubMed] |
| 17. | Sanchis-Alfonso V, Roselló-Sastre E. Anterior knee pain in the young patient--what causes the pain? “Neural model”. Acta Orthop Scand. 2003;74:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Di Sebastiano P, Fink T, Weihe E, Friess H, Beger HG, Büchler M. Changes of protein gene product 9.5 (PGP 9.5) immunoreactive nerves in inflamed appendix. Dig Dis Sci. 1995;40:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Coppes MH, Marani E, Thomeer RT, Groen GJ. Innervation of “painful” lumbar discs. Spine (Phila Pa 1976). 1997;22:2342-239; discussion 2349-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 285] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Harrison AR, McLoon LK. Reduction in touch sensitivity and hyperinnervation in vesicant-injured rabbit eyelid by direct injection of corticotropin releasing factor. Neurosci Lett. 2006;400:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Mansikka H, Pertovaara A. Supraspinal influence on hindlimb withdrawal thresholds and mustard oil-induced secondary allodynia in rats. Brain Res Bull. 1997;42:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Ambalavanar R, Moutanni A, Dessem D. Inflammation of craniofacial muscle induces widespread mechanical allodynia. Neurosci Lett. 2006;399:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Hughes A, Macleod A, Growcott J, Thomas I. Assessment of the reproducibility of intradermal administration of capsaicin as a model for inducing human pain. Pain. 2002;99:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Hendiani JA, Westlund KN, Lawand N, Goel N, Lisse J, McNearney T. Mechanical sensation and pain thresholds in patients with chronic arthropathies. J Pain. 2003;4:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Owatz CB, Khan AA, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. The incidence of mechanical allodynia in patients with irreversible pulpitis. J Endod. 2007;33:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Hendrix S, Peters EM. Neuronal plasticity and neuroregeneration in the skin--the role of inflammation. J Neuroimmunol. 2007;184:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Liang Y, Marcusson JA, Johansson O. Light and electron microscopic immunohistochemical observations of p75 nerve growth factor receptor-immunoreactive dermal nerves in prurigo nodularis. Arch Dermatol Res. 1999;291:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Liang Y, Jacobi HH, Reimert CM, Haak-Frendscho M, Marcusson JA, Johansson O. CGRP-immunoreactive nerves in prurigo nodularis--an exploration of neurogenic inflammation. J Cutan Pathol. 2000;27:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Jiang WY, Raychaudhuri SP, Farber EM. Double-labeled immunofluorescence study of cutaneous nerves in psoriasis. Int J Dermatol. 1998;37:572-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Raychaudhuri SP, Jiang WY, Smoller BR, Farber EM. Nerve growth factor and its receptor system in psoriasis. Br J Dermatol. 2000;143:198-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Urashima R, Mihara M. Cutaneous nerves in atopic dermatitis. A histological, immunohistochemical and electron microscopic study. Virchows Arch. 1998;432:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Ochs G, Schenk M, Struppler A. Painful dysaesthesias following peripheral nerve injury: a clinical and electrophysiological study. Brain Res. 1989;496:228-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Wall PD, Gutnick M. Properties of afferent nerve impulses originating from a neuroma. Nature. 1974;248:740-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 284] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Johnson RD, Munson JB. Regenerating sprouts of axotomized cat muscle afferents express characteristic firing patterns to mechanical stimulation. J Neurophysiol. 1991;66:2155-2158. [PubMed] |
| 37. | Devor M, Govrin-Lippmann R, Angelides K. Na+ channel immunolocalization in peripheral mammalian axons and changes following nerve injury and neuroma formation. J Neurosci. 1993;13:1976-1992. [PubMed] |
| 38. | England JD, Happel LT, Kline DG, Gamboni F, Thouron CL, Liu ZP, Levinson SR. Sodium channel accumulation in humans with painful neuromas. Neurology. 1996;47:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 125] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987;104:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 792] [Cited by in RCA: 792] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 40. | Taherzadeh O, Otto WR, Anand U, Nanchahal J, Anand P. Influence of human skin injury on regeneration of sensory neurons. Cell Tissue Res. 2003;312:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 324] [Article Influence: 12.0] [Reference Citation Analysis (0)] |