INTRODUCTION
Acute hyponatremia can cause death if cerebral edema and seizures are not treated promptly[1]. Conversely, osmotic demyelination syndrome (ODS) will occur with rapid correction of severe chronic hyponatremia (serum sodium concentration 120 mmol/L or less) that has been present for more than 2 or 3 d, the time required for the cerebral adaptation to occur[2]. Excessive correction of chronic hyponatremia triggers a cascade of injury in the brain beginning with breakdown of the blood-brain barrier and culminating in the programmed death of oligodendrocytes, the cells that form myelin sheaths in the central nervous system[3]. Known risk factors for ODS are hyponatremia (both in duration and severity), rapid correction of hyponatremia with more than 12 mmol/L in less than 24 h, hypokalemia on presentation[4-6], low BUN with malnourished state, alcoholism, liver disease and seizures on presentation[7,8].
CASE REPORT
A 50-year-old African American male with history of hypertension and chronic intractable hiccups was brought to the emergency department (ED) by family members after three episodes of seizures at home, with the last episode few hours prior to admission and lasting for 10 min. The patient has been having hiccups for several years, which worsened acutely over 3 wk prior to admission. In order to control his hiccups, he has been drinking large amount of water and a proprietary carbonated beverage (Sprite®), as well. He reported nausea and daily vomiting and avoided solid food for the last two weeks prior to admission. He observed that his hiccups have improved temporarily with vomiting and the persisting and crescendo hiccups frustrated him enough to induce voluntary vomiting repeatedly.
On admission, his vital signs included a temperature of 36.4 °C, blood pressure the 168/73 mmHg with a heart rate of 81 beats/min, respiratory rate 18/min and body weight of 72.7 kg. General physical examination revealed an alert malnourished African-American male, oriented to person, place, and time with no focal neurological findings. Physical exam was unremarkable with no detectable peripheral edema. In the ED, the patient’s serum electrolyte concentrations were: sodium of 107 mmol/L, potassium less than 1.5 mmol/L, chloride less than 60 mmol/L, bicarbonate of 38 mmol/L with a pH of 7.60. Blood urea nitrogen (BUN) measured 4 mg/dL, creatinine 0.7 mg/dL and serum osmolality 217 mOsm/kg. Liver function tests were within normal limits. EKG noted prolonged QT intervals (616 ms) and non-specific ST-T abnormalities. No arrhythmia was observed. Emergent treatment included 150 mL 3% saline IV bolus to control seizures, intravenous chlorpromazine (Thorazine®) and baclofen to control hiccups, nausea and vomiting and 1 L of normal saline with 40 mmol/L of potassium-chloride over 5 h. Six hours after the ED presentation, he was admitted to the medical intensive care unit (ICU) for close monitoring.
Shortly after admission to the ICU, the patient developed large volume free water diuresis with 6 L of dilute urine over 8 h (initial Uosm 60 mOsm/kg; repeat 4 h later 40 mOsm/kg). The patient’s serum sodium rapidly rose to 126 mmol/L within 12 h and he became drowsy. At that point of time, the decision was made to start desmopressin at 1 mcg iv twice a day to minimize dilute urine output, increased to 2 mcg iv twice daily the next day. We also administered 5% dextrose in water (D5W) to replace free water over 10 h, calculated to decrease serum sodium to 120 mmol/L. The patient’s serum sodium concentration dropped to 118 mmol/L in 12 h after starting desmopressin and his urine output decreased to ≤ 2 L/d for the next several days. Thereafter, serum sodium was corrected gradually in 2-3 mmol/L daily increments until 130 mmol/L was reached with continued water restriction (Figure 1). After that point, serum potassium was slowly corrected with per os potassium supplements, with the resultant and expected auto-correction of metabolic alkalosis, once serum potassium normalized. The patient was released from the medical ICU on day 5 to the medical ward and discharged home on day 11th day with a weight of 71.4 kg. The patient fully recovered without any neurological sequelae and was discharged home with appropriate instructions, including limiting his fluid intake and avoiding self-induced nausea and vomiting.
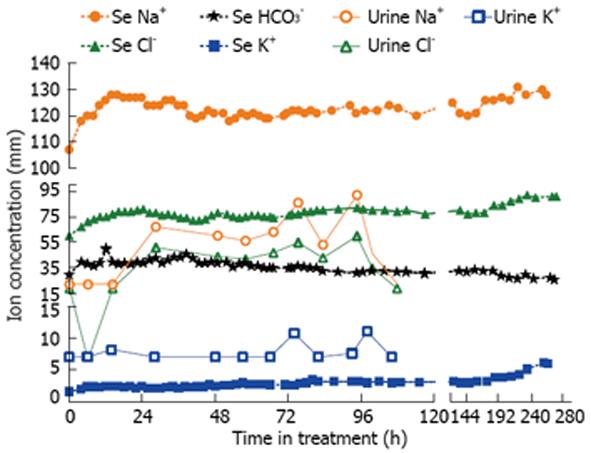
Figure 1 Serum and urine electrolyte concentrations over time.
The patient spent 0-120 h in the intensive care unit.
DISCUSSION
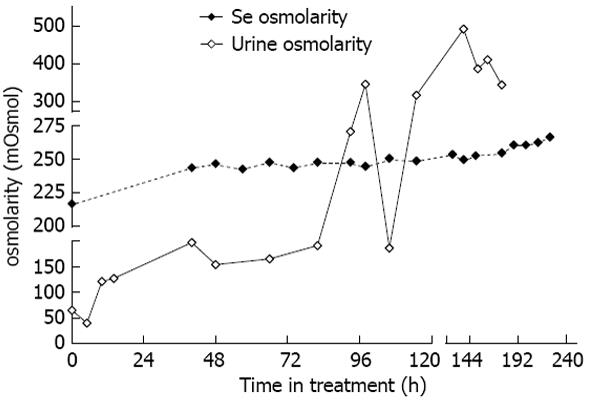
Based on the co-morbid features during admission (hypokalemia, seizures, low BUN, rapid rise of sodium), our patient was at a very high risk of developing ODS[9,10]. In our patient, nausea and vomiting contributed to the development of both hyponatremia[11] and metabolic alkalosis[12]. The presenting clinical picture for our patient on arrival was similar to the syndrome of inappropriate anti-diuretic hormone secretion (SIAD)[13,14]. In his case, however, the causes for excessive release of vasopressin (or anti-diuretic hormone) were reversible ones: nausea and hiccups. Nonetheless, once his nausea was resolved, alternative stimuli to maintain the vasopressin level (e.g., hypovolemia) were absent. The patient excreted the free water accumulated prior to admission in the form of dilute urine, leading to the observed large rise of serum sodium. We could not correct metabolic alkalosis with iv acid infusions, as neither ammonium chloride nor hydrochloric acid[15] were available either in our institution or from any of the surrounding healthcare facilities. We specifically monitored serum osmolality to reflect on all osmotically active substances (sodium, potassium and BUN; Figure 2) and avoided sudden correction of hypokalemia, which could have resulted in increased global osmolality[3,10]. Immediate correction of hypokalemia is associated with ODS[6,16,17], perhaps due to rise in intracellular potassium.
Figure 2 Serum and urine osmolarity over time.
The patient spent 0-120 h in the intensive care unit.
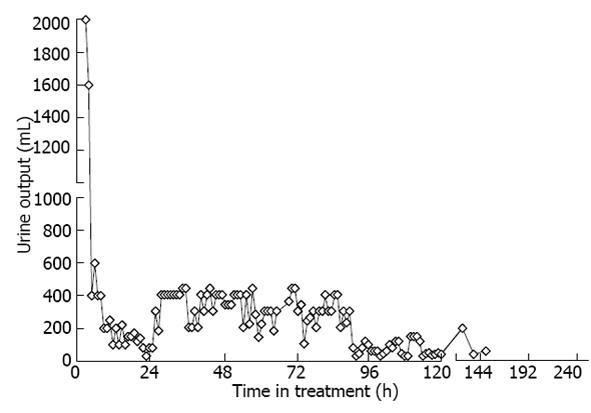
This case illustrates that administration of desmopressin can be a successful strategy both to ameliorate sodium overcorrection and to avoid inadvertent overcorrection of hyponatremia, if significant free water diuresis emerges during recovery from hyponatremia. Inadvertent overcorrection of hyponatremia is, in fact, common[18] and should be viewed as a medical emergency[19]. The administration of hypertonic saline sometimes increases serum sodium more than expected because of unanticipated water diuresis that may develop during the course of therapy. This was the case in our patient, who had additional risk factors for concentration impairment in the kidneys. This prompted us, along with free water administration, to use desmopressin to minimize urine output and alleviate overcorrection of hyponatremia (Figure 3). Avoiding overcorrection with oral intake is difficult since hyposmolality suppresses thirst and patients may reject water that is offered to them. Oral intake is not an option in patients with altered mental status. Finally, attempting to match urinary water losses with intravenous or orally administered electrolyte-free water requires intensive monitoring of fluid balance that is often impractical. Such a strategy has been reported in the medical literature[20], including in a series of 20 patients, where pre-emptive administration of desmopressin prevented excessive water diuresis and fewer patients required 5% dextrose in water (D5W) administration for therapeutic re-lowering of the sodium[21]. For these reasons, administration of vasopressin or synthetic vasopressin analog, such as desmopressin may be a more attractive strategy. Our paper strengthens and confirms the limited published experience to date with the use of desmopressin to prevent or reverse overcorrection of hyponatremia, in face of co-existing complex electrolyte disturbances[22].
Figure 3 Urine output over time.
The patient spent 0-120 h in the intensive care unit.
In conclusion, controlling nausea or any other reversible causes of excessive vasopressin release may lead to unpredictable free water diuresis in euvolemic hyponatremic patients. Polyuria after symptomatic hyponatremia on presentation is a serious warning sign. Early addition of an antidiuretic hormone analog, such as desmopressin, can limit urine output and prevent unpredictable free water losses with sudden rise in serum sodium, simplifying the managements of these complex and high-risk scenarios.
P- Reviewer Pasquale E S- Editor Zhai HH L- Editor A E- Editor Ma S