Published online Apr 16, 2013. doi: 10.12998/wjcc.v1.i1.4
Revised: March 26, 2013
Accepted: March 28, 2013
Published online: April 16, 2013
Processing time: 136 Days and 13.6 Hours
Back pain is a common chronic disorder that represents a large burden for the health care system. There is a broad spectrum of available treatment options for patients suffering from chronic lower back pain in the setting of degenerative disorders of the lumbar spine, including both conservative and operative approaches. Lumbar arthrodesis techniques can be divided into sub-categories based on the part of the vertebral column that is addressed (anterior vs posterior). Furthermore, one has to differentiate between approaches aiming at a solid fusion in contrast to motion-sparing techniques with the proposed advantage of a reduced risk of developing adjacent disc disease. However, the field of application and long-term outcomes of these novel motion-preserving surgical techniques, including facet arthroplasty, nucleus replacement, and lumbar disc arthroplasty, need to be more precisely evaluated in long-term prospective studies. Innovative surgical treatment strategies involving minimally invasive techniques, such as lateral lumbar interbody fusion or transforaminal lumbar interbody fusion, as well as percutaneous implantation of transpedicular or transfacet screws, have been established with the reported advantages of reduced tissue invasiveness, decreased collateral damage, reduced blood loss, and decreased risk of infection. The aim of this study was to review well-established procedures for lumbar spinal fusion with the main focus on current concepts on spinal arthrodesis and motion-sparing techniques in degenerative disorders of the lumbar spine.
Core tip: There is a broad spectrum of surgical techniques that can be performed in order to fuse lumbar motions segments. The aim of this study was to review well-established procedures for lumbar spinal fusion with the main focus on current concepts on spinal arthrodesis and motion-sparing techniques in degenerative disorders of the lumbar spine, including minimally invasive interbody fusion, total disc arthroplasty, nucleus replacement systems, percutaneous implantation of pedicle and facet screws, facet arthroplasty, and interspinous implants.
- Citation: Lykissas MG, Aichmair A. Current concepts on spinal arthrodesis in degenerative disorders of the lumbar spine. World J Clin Cases 2013; 1(1): 4-12
- URL: https://www.wjgnet.com/2307-8960/full/v1/i1/4.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v1.i1.4
Back pain is a common chronic disorder that has been reported to affect more than a quarter of the adult population in the United States, representing a large burden for the health care system[1]. Within the last decades health care costs of back and neck pain have increased tremendously. In a recent report the increase in expenditures was estimated at 65% between 1997 (52.1 billions of US dollars) and 2005 (85.9 billions of US dollars)[2].
There is a broad spectrum of available treatment options for patients with lower back pain due to degenerative disorders of the lumbar spine, including both conservative and operative approaches. Furthermore, novel and innovative surgical treatment strategies involving minimally invasive and motion-sparing techniques have emerged within the last decade. The aim of this study was to review well-established procedures for lumbar spinal fusion with the main focus on current concepts on spinal arthrodesis and motion-sparing techniques in degenerative disorders of the lumbar spine.
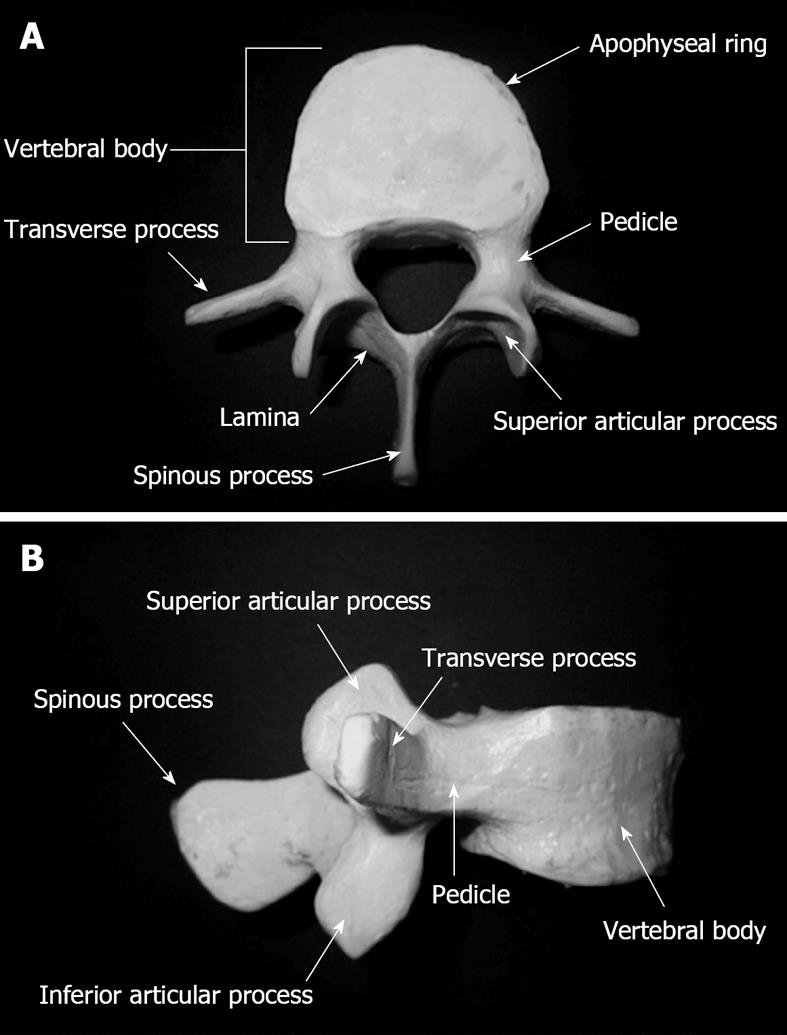
The lumbar vertebral column is usually made up of five vertebral bodies (L1-L5), each providing a dense apophyseal ring at the top and bottom surfaces. The pedicles are bony processes projecting dorsally to merge into the two laminae, which fuse to one spinous process at the posterior midline. At the pediculo-laminar junction, the transverse process projects laterally on each side. Vertebral bodies articulation is executed via the intervertebral spinal disc as well as the superior and inferior articular processes extending from the superior and inferior laminar margins bilaterally. The superior articular process of the inferior lumbar vertebra articulates with the inferior articular process of the superior vertebral body to form the facet joint, also referred to as the zygapophyseal joint (Figure 1). The superior articular process forms the anterior part of the facet joint with a concavely shaped articular surface, compared to the convex shape of the inferior facet. The bony joint surfaces are covered by hyaline cartilage and lined by a synovial membrane. The lumbar spine can be further divided into three parts: the thoraco-lumbar junction (Th12-L1), the mid-lumbar spine (L1-L5), and the lumbo-sacral junction (L5-S1). Within the thoraco-lumbar junction, there is a transition from the rigid kyphotic thoracic to the more flexible lordotic lumbar spine, representing a zone of increased shear stress to the intervertebral motion segment. After exiting through the foramen magnum at the base of the skull, the spinal cord travels within the spinal canal, made up of the dorsal vertebral body surfaces, the pedicles, as well as the laminae. The abdominal aorta and the inferior vena cava travel anterior to the vertebral column and bifurcate to supply the pelvis and the lower extremities. The lumbar spinal roots exit the intervertebral foramen beneath the pedicle of the corresponding vertebral body into the pelvis to form the lumbar plexus, which travels through the posterior third of the psoas muscle with branches exiting at its anterior or lateral surfaces[3-6].
While the lumbar plexus travels within the posterior third of the psoas in the majority of cases, recent studies have underlined its possible anatomical location posterior to the psoas muscle[7].
Lumbar spinal fusion is increasingly utilized to treat a broad spectrum of degenerative spine disorders, including scoliosis, spondylolisthesis, and spinal stenosis. Traditionally, fusion of a motion segment can be achieved by mechanical roughening and decortication of articular surfaces and packing the joint space with bone graft material, including iliac crest autograft, allograft material, or biologic adjuncts such as bone morphogenetic proteins (BMPs)[4,8-16].
There is a wide spectrum of lumbar spinal arthrodesis techniques addressing different parts of the vertebral column. With regard to the well-established three-column theory of the spine[17], plates, cages, and disc arthroplasty devices can be implanted with the aim to correctly align and stabilize the anterior two columns, while wiring systems, hook-based systems, pedicle screws, translaminar screws, facet replacement systems, and interspinous implants address the posterior column.
The selection of the appropriate surgical arthrodesis technique for lumbar spinal fusion is influenced by factors, such as the number of diseased motions segments, the affected number of columns per level, and the degree of instability, among others. Furthermore, to achieve adequate bony fusion, factors such as local and systemic bone quality, diabetes, smoking, and corticosteroid use, among others, have to be considered[4]. The main advantages and disadvantages of current procedures for the treatment of degenerative disorders of the lumbar spine are summarized in Table 1.
| Surgical technique | Advantages | Disadvantages |
| ALIF | Direct visualization of disc space | Intra-abdominal vascular and visceral injury[31,32] |
| Small incisions and reduced tissue invasiveness if minimally invasive approach performed[27-30] | ||
| PLIF | Avoiding intra-abdominal complications associated with anterior approach | Increased risk of surgical damage to neural structures, dural layer, and epidural veins[33-35] |
| LLIF | Avoiding surgical complications associated with anterior and posterior approaches | Limited surgical accessibility of L5-S1 level due to iliac crest |
| Sparing of anterior longitudinal ligament (ligamentotaxis)[36] | Injury to lumbar plexus during transpsoas approach with post-operative approach-related neurological deficits[37-40] | |
| Stable implantation of device due to bilateral utilization of the dense apophyseal ring[36] | ||
| TLIF | Minimally-invasive | Less reduction in ROM compared to LLIF, if performed as stand-alone procedures[41] |
| Reduced nerve root retraction[41] | ||
| Circumferential fusion[27,41-43] | ||
| Total disc arthroplasty | Reduced risk of adjacent segment disease due to preservation of motion[47] | Narrow spectrum of indications[48,49] |
| No fusion required[47] | ||
| Nucleus replacement | Preservation of motion | Risk of device migration, extrusion, and subsidence[53,54] |
| Multiple approach and revision options[53] | ||
| Sublaminar wiring | Can be implanted as an adjunct to other devices (hybrid)[4] | Risk of neurological injury[58,59] |
| Pedicle screws | Involvement of all three columns[4] | High costs[4] |
| Rigid segmental fixation[4] | Damage to neural and vascular structures[4] | |
| High fusion rates[4] | Adjacent segment disease[62] | |
| Adequate deformity correction[4] | ||
| Percutaneous approach possible[65-67] | ||
| Translaminar screws | Minimally-invasive percutaneous approach available[4,68,69] | Not indicated for multilevel arthrodesis[4,68,69] |
| Facet arthroplasty | Preservation of motion | Complex anatomy of facet joints |
| Reduce the risk of adjacent segment disease[71] | Narrow spectrum of indications | |
| Interspinous implants | Preservation of motion | Narrow spectrum of indications |
In a recent systematic review on 26 articles including a total number of 3060 patients, Phillips et al[18] concluded that lumbar arthrodesis is a viable treatment strategy for patients with degenerative disc disease related low back pain who are refractory to non-surgical treatment, both with regard to pain reduction and functional improvement.
The implantation of anterior instrumentation systems has previously been shown to successfully restore immediate post-operative stability[19], and to correct post-traumatic deformities such as progressive kyphosis[20]. The spectrum of relative contraindications for the anterior approach includes severe osteoporosis and scaring due to previous abdominal surgery[4]. Gurwitz et al[21] compared three different approaches for short-segment instrumentation in a lumbar spine burst fracture model: posterior instrumentation alone (VSP plates: Acromed, Cleveland, OH) or with an anterior strut graft, and anterior instrumentation (Kaneda system: Acromed, Cleveland, OH) with an anterior strut graft. Posterior instrumentation alone indicated 76% less axial stiffness compared to the intact spine. Posterior instrumentation supplemented by anterior strut grafting revealed axial stiffness results that were not significantly different from the intact lumbar spine. Finally, anterior instrumentation with anterior strut grafting indicated 15% more axial stiffness than the intact spine. However, the three approaches showed 30%, 26%, and 24% decreased rigidity when compared to the intact spine. In their biomechanical study on anterior and posterior lumbar stabilization procedures, Flamme et al[22] compared three systems: anterior instrumentation alone [modular anterior construct system (MACS), Aesculap AG and Co. KG, Tuttlingen, Germany], MACS anterior instrumentation with intercorporal Pyramesh cage augmentation (Medtronic Sofamor Danek, Memphis, TN), and posterior screw-rod instrumentation alone (SOCON: SOlid CONnection, Aesculap AG and Co. KG, Tuttlingen, Germany). When compared to the physiologic lumbar motion segments all three systems demonstrated increased stability and reduced mobility. The authors concluded that anterior instrumentation with intercorporal cage augmentation results in comparable or even greater stability than posterior stabilization, with the exception of flexion/extension.
Since augmentation of the intervertebral space with bone graft alone has resulted in insufficient support of the anterior vertebral column of the lumbar spine with a resulting high rate of pseudarthrosis, intercorporal implantation of cage devices has emerged in recent years[23]. The principle of achieving intervertebral fusion with cage implantation is to expose the intervertebral disc space, to perform complete diskectomy as well as end plate preparation (i.e., removal of cartilage), and to implant a synthetic device. Commercially available cages can be loaded with bone graft supplements, including vertebral body and iliac crest aspirate, iliac crest bone graft (ICBG) material, β tricalcium phosphate, stem cell allografts, demineralized bone matrix, and biologic adjuncts, such as BMPs[4].
Various techniques for intervertebral cage implantation have been described. The diverse spectrum of common interbody fusion techniques comprises anterior lumbar interbody fusion (ALIF), lateral lumbar interbody fusion (LLIF), transforaminal lumbar interbody fusion (TLIF), and posterior lumbar interbody fusion (PLIF), each characterized by a different approach to access the lumbar spine.
ALIF: Since the early 1930s, when the anterior approach for lumbar arthrodesis via bone grafting was first described as a surgical alternative for the management of spondylolisthesis[24,25], the spectrum of conditions being addressed by the ALIF technique has widened. Currently, ALIF can be used to treat spondylolisthesis, lumbar instability, degenerative disc disease, and pseudarthrosis[26,27]. Via either a retroperitoneal or a transperitoneal approach, the spine surgeon gains access to the lumbar intervertebral motions segments to excise the disk and insert a cage at the anterior part of the intervertebral space. A suggested advantage of ALIF over other interbody fusion techniques is direct visualization of the intervertebral space, potentially associated with improved post-operative outcome. In contrast to traditional invasive ALIF approaches, a minimally invasive surgical approach has been emerged recently, with the advantage of small incisions and reduced tissue invasiveness[28-31]. ALIF has been reported to be associated with an increased risk of surgical collateral damage, such as intra-abdominal vascular and visceral injury[32,33].
PLIF: The PLIF procedure, as originally described by Cloward[34] in 1953, is characterized by sparing the facet joints, and by gaining access to the lumbar motion segment via laminotomy/laminectomy, followed by diskectomy, decortication of vertebral body end plates, and the implantation of an interbody fusion device/graft. A suggested advantage of the posterior approach in PLIF is the avoidance of intra-abdominal vascular and visceral injury as seen in anterior approaches to the lumbar spine (e.g., ALIF). However, PLIF is associated with an increased risk of damage to neural structures, epidural vein injury potentially resulting in increased peri-operative blood loss, and dural laceration, among others[34-36].
LLIF: Due to reduced risk of surgical collateral damage associated with the ALIF or PLIF approach[32,33,35,36], the minimally-invasive LLIF procedure has recently been established to address lumbar motion segments L1-L5, as described by Ozgur et al[37] in detail. The implantation of LLIF cages at the L5-S1 level can be difficult due to the presence of the iliac crest potentially blocking the surgical access. The LLIF approach requires blunt dissection of the psoas muscle in order to insert minimally invasive tubular retractors. Following diskectomy the procedure utilizes the dense apophyseal ring for device implantation, allowing a more stable fixation of the device and preventing subsidence. Furthermore, when compared to ALIF, the surgical approach in LLIF spares the anterior longitudinal ligament, leading to increased post-operative vertebral column stability and improved alignment via ligamentotaxis[37]. However, due to the proximity of the lumbar plexus, which usually travels within the posterior third of the psoas muscle[6,7], concerns regarding approach-related neurological adverse sequelae have arisen[38-41]. Analysis of our unpublished data on 919 treated levels revealed that neurological deficits following LLIF, although high in the immediate post-operative setting, steadily decrease over time, which underlines the transiency of the majority of these deficits.
TLIF: TLIF is another minimally invasive approach to achieve lumbar arthrodesis, which has been reported to reduce the extent of nerve root retraction associated with the PLIF procedure[42]. Unilateral facetectomy and/or laminectomy/laminotomy are followed by implantation of pedicle screws, diskectomy at the appropriate level, gradual distraction of the intervertebral disk space, and surgical preparation of vertebral bony endplates. By careful retraction of the thecal sac and protection of the traversing nerve root, interbody fusion cages can be implanted through the intervertebral foramen, and the pedicle screws can be connected via a rod. Postero-lateral fusion can further be achieved by decortication of the transverse processes and augmentation with ICBG. Due to surgeon’s ability to address both the anterior as well as the posterior columns of the spine, TLIF has become a favorable procedure to achieve circumferential fusion[28,42-44]. In their study on comparative effectiveness and cost-utility analysis comparing minimally invasive TLIF (MIS TLIF) vs the open TLIF procedure for degenerative spondylolisthesis, Parker et al[45] reported a similar post-operative patient-reported outcome for both techniques, but significantly less lengths of both hospital stay and return to the work force for MIS TLIF, resulting in a reduction in both societal and hospital costs.
Biomechanics of interbody cages: According to Oxland et al[46], the implantation of anterior lumbar interbody cages alone provides stability of the vertebral column in flexion, axial rotation, and lateral bending, when compared to the intact spine, but no stabilization in extension. Posterior implantation of a titanium interbody cage has been reported to achieve higher stiffness, when compared to both the intact spine and the augmentation with bone graft alone, and to result in similar stiffness, when compared to posterior instrumentation supplemented by bone graft[47]. In their biomechanical study, Cappuccino et al[48] evaluated the range of motion (ROM) after the implantation of LLIF cages. The authors compared their results with current literature and concluded that the implantation of LLIF devices without supplemental instrumentation (i.e., stand-alone LLIF) results in greater segmental reduction in ROM, when compared to stand-alone ALIF or TLIF procedures. Furthermore, the authors demonstrated that supplemental bilateral posterior instrumentation with pedicle screws results in the largest decrease of ROM.
The principle of total disc arthroplasty (TDA) is to replace the degenerated disc by an intervertebral prosthesis with the theoretical advantage of preservation of ROM. Due to reduced shear stresses at the adjacent level based on preserved motion, a decreased risk for adjacent segment disease has been suggested. Furthermore, since no fusion is required, arthrodesis-associated adverse sequelae such as pseudarthrosis and donor site morbidity due to bone graft harvesting can be avoided[49]. However, the surgical indications for performing TDA at the lumbar spine remain narrow. Previous studies have suggested young patients, with disc disease involving one motion segment, normal bone quality, intact facet joints, and absence of scoliosis and spinal instability (e.g., spondylolisthesis, spinal fracture) as the ideal candidates to undergo lumbar TDA[50,51]. In their prospective, randomized, multicenter study, Blumenthal et al[52] revealed that the implantation of the CHARITé artificial disc (DePuy Spine, Raynham, MA) results in at least equivalent clinical outcomes, when compared to ALIF. Zigler et al[53] compared ProDisc-L (Synthes Spine, West Chester, PA) lumbar TDA with circumferential fusion for the treatment of single-level lumbar degenerative disc disease. The authors concluded that ProDisc-L, with careful patient selection, achieves superior clinical results compared to circumferential fusion. The efficacy of ProDisc-L implantation was supported by a recent study on the long-term post-operative outcome. Although the results support both ProDisc-L and circumferential arthrodesis as adequate approaches to treat single-level degenerative disc disease, patients who had undergone TDA demonstrated more rapid improvement, with regard to post-operative pain, disability, and neurological function[54].
Nucleus replacement (nucleoplasty) devices can be functionally divided into two major groups: elastomeric and mechanical nucleus devices, with elastomeric devices further being divided into hydrogel and non-hydrogel devices that are either injectable or preformed. Mechanical nucleus devices can further be sub-classified as one- or two-piece systems. The proposed advantages of nucleoplasty are the variety of minimally invasive surgical approaches that can be performed, and the multiple revision options after failed nucleoplasty, including lumbar disc arthroplasty and spinal fusion[55]. Furthermore, as seen in other motion sparing techniques[49], the risk of adjacent segment disease may also be reduced due to preservation of mobility of the addressed motion segment. However, the risk of device migration or extrusion, as well as subsidence remain a source of concern[55,56]. The evaluation of post-operative outcome following NUBAC™ implantation, a novel nucleus disc device made of polyetheretherketone and two articulating pieces, revealed absence of major intra- and post-operative complications as well as significant post-operative decrease in visual analogue scale and oswestry disability index parameters in addition to symptomatic improvement in all patients, underlining both the efficacy and safety of the approach, likely attributable to the reduced invasiveness of the procedure[57]. However, further prospective studies on long-term outcomes and the influence on the adjacent motion segments are warranted.
One of the most common wiring procedures is the Luque technique, utilizing sublaminar wires for segmental spinal stabilization[4,58-60]. This procedure has been associated with an increased risk of neurological injury, especially in the thoracic spine[60,61]. Wires can also be used to attach the implanted rod to the spinous process (“Wisconsin method”[62]), thereby avoiding the risk of injury to the spinal cord associated with the Luque technique[60,61,63]. Currently, wiring systems are more commonly implanted supplemental to other fusion or stabilization devices such as pedicle screws, instead of being utilized alone (hybrid systems)[4].
Transpedicular screw fixation, a common procedure aiming at the stabilization of the vertebral column, is the only available surgical technique that addresses all three columns of the spine. It has been reported to achieve rigid segmental fixation, high fusion rates, and deformity correction. Disadvantages include the high cost and the risk of damage to the thecal sac, the nerve roots, and major vascular structures[4]. Furthermore, pedicular screw insertion has been shown to be associated with a higher risk of developing adjacent segment disease (12.2%-18.5%) compared to patients with a different instrumentation technique (posterior midline and interbody arthrodesis) or non-instrumented fusion (5.2%-5.6%)[64]. When a recent retrospective series evaluated adverse sequelae related to the implantation of transpedicular screws in 648 patients screw misplacement was evident in three cases, nerve root impingement in one case, leakage of cerebrospinal fluid in two cases, pedicular fracture in two cases, deep wound infection in four cases, screw loosening in two cases, and rod-screw disconnection in one case[65]. In a recent meta-analysis, comparing different constructs in terms of mid- to long-term outcomes following instrumented posterior spinal fusion for adolescent idiopathic scoliosis, Cotrel-Dubousset instrumentation achieved higher degree of correction in the coronal plane, as well as better restoration of thoracic kyphosis and lumbar lordosis when compared to all-pedicle screw constructs. All-pedicle screw fixation was associated with the lower risk of pseudarthrosis, infection, neurologic deficit, and revision surgery[66]. A novel technique for pedicle screw implantation is the percutaneous approach supplemental to an ALIF procedure for the treatment of spondylolisthesis. Advantages include reduced surgical time, blood loss and collateral tissue damage, high fusion rates, and low incidence of adjacent disc degeneration[67-69].
Compared to transpedicular screw fixation, the translaminar approach has been shown to be associated with reduced soft tissue damage when screws are implanted via a minimally invasive percutaneous approach. However, translaminar screw technique is not indicated for multilevel arthrodesis since it does not provide enough strength of fixation[4,70,71].
Replacement systems of the facet joint, such as the total facet arthroplasty system (TFAS) (Archus Orthopedics, Redmond, WA), can be implanted following posterior decompression in the setting of degenerative facet complex disease, and degenerative lumbar spinal stenosis[72], with the aim to avoid the need for lumbar spinal fusion[4]. By restoring the ROM at the operated motion segment to intact values and to almost physiologic kinematics at the adjacent levels, TFAS may reduce the risk of adjacent segment disease[73]. TFAS is characterized by the transpedicular implantation of two straight metal stems into the inferior vertebral bodies and two bent metal stems into the superior ones. The two superior L-shaped metal stems are connected to a cross-arm with spherically-shaped ends that articulate with the bearing surfaces at the tops of the two inferior straight metal stems during flexion and extension[72-74]. Due to financial problems the company had to discontinue distribution of the TFAS system, with a resulting lack of data regarding long-term outcomes. Other facet replacement systems, being characterized by individually sizing all articulating bony components in order to satisfactorily emulate the individual’s anatomy of the facet joint, are currently under investigation. Clinical studies focusing on the long-term outcomes of these devices are warranted[4].
Interspinous spacers, including the X-STOP device (Medtronic, Minneapolis, MN), have recently been introduced as motion-preserving implants for the treatment of lumbar degenerative conditions such as spinal stenosis, due to increase of flexion and prevention of extension at the motion segment level, in addition to distraction of the spinous processes[4]. This concurs with the results of a randomized, controlled, prospective multicenter trial underlining the efficacy of the X-STOP interspinous spacer in the treatment of spinal stenosis. After 2 years of follow-up, patients treated with X-STOP showed an improvement of 45.5% in terms of disease symptom severity, compared to an improvement of only 7.4% recorded in conservatively treated patients[75]. The Wallis System (Zimmer, Warsaw, IN) is implanted into the lumbar spine without permanent bony fixation (“floating” system) with the aim to decrease the risk of device loosening. It is recommended in the setting of diskectomy for massive and/or recurrent disc herniation, adjacent segment disc degeneration, and chronic low-back pain due to mild degenerative disc disease (Modic I)[76].
There is a broad spectrum of surgical techniques that can be performed in order to fuse lumbar motions segments. Advantages and disadvantages of each arthrodesis technique have to be taken into consideration during pre-operative surgical planning. The transition from open to minimally invasive surgical procedures, potentially supplemented by biologic adjuncts such as BMPs, is promising. The field of application and long-term outcomes of novel motion-sparing surgical techniques, such as facet arthroplasty, nucleus replacement, and lumbar disc arthroplasty, need to be more precisely evaluated in further, ideally prospective, studies.
| 1. | Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976). 2006;31:2724-2727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 663] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 2. | Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1069] [Cited by in RCA: 1115] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 3. | Bogduk N. Clinical and Radiological Anatomy of the Lumbar Spine. 5th ed. Edinburgh; New York: Elsevier/Churchill Livingstone 2012; . |
| 4. | Herkowitz HN, Garfin SR, Eismont FJ, Bell GR, Balderston RA. Rothman Simeone The Spine. 6th ed. Philadelphia: Saunders 2011; . |
| 5. | Sarazin L, Chevrot A, Pessis E, Minoui A, Drape JL, Chemla N, Godefroy D. Lumbar facet joint arthrography with the posterior approach. Radiographics. 1999;19:93-104. [PubMed] |
| 6. | Lu S, Chang S, Zhang YZ, Ding ZH, Xu XM, Xu YQ. Clinical anatomy and 3D virtual reconstruction of the lumbar plexus with respect to lumbar surgery. BMC Musculoskelet Disord. 2011;12:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kirchmair L, Lirk P, Colvin J, Mitterschiffthaler G, Moriggl B. Lumbar plexus and psoas major muscle: not always as expected. Reg Anesth Pain Med. 2008;33:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976). 2002;27:2662-2673. [PubMed] |
| 9. | Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 476] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery - the case for restraint. N Engl J Med. 2004;350:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 377] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997;22:2807-2812. [PubMed] |
| 12. | Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802-808. [PubMed] |
| 13. | Kerr EJ, Jawahar A, Wooten T, Kay S, Cavanaugh DA, Nunley PD. The use of osteo-conductive stem-cells allograft in lumbar interbody fusion procedures: an alternative to recombinant human bone morphogenetic protein. J Surg Orthop Adv. 2011;20:193-197. [PubMed] |
| 14. | Möller H, Hedlund R. Surgery versus conservative management in adult isthmic spondylolisthesis--a prospective randomized study: part 1. Spine (Phila Pa 1976). 2000;25:1711-1715. [PubMed] |
| 15. | Sandhu HS, Boden SD, An H, Kang J, Weinstein J. BMPs and gene therapy for spinal fusion: summary statement. Spine (Phila Pa 1976). 2003;28:S85. [PubMed] |
| 16. | Boden SD, Martin GJ, Horton WC, Truss TL, Sandhu HS. Laparoscopic anterior spinal arthrodesis with rhBMP-2 in a titanium interbody threaded cage. J Spinal Disord. 1998;11:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 132] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983;8:817-831. [PubMed] |
| 18. | Phillips FM, Slosar PJ, Youssef JA, Andersson G, Papatheofanis F. Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine (Phila Pa 1976). 2013;38:E409-E422. [PubMed] |
| 19. | Gurr KR, McAfee PC, Shih CM. Biomechanical analysis of anterior and posterior instrumentation systems after corpectomy. A calf-spine model. J Bone Joint Surg Am. 1988;70:1182-1191. [PubMed] |
| 20. | Ghanayem AJ, Zdeblick TA. Anterior instrumentation in the management of thoracolumbar burst fractures. Clin Orthop Relat Res. 1997;89-100. [PubMed] |
| 21. | Gurwitz GS, Dawson JM, McNamara MJ, Federspiel CF, Spengler DM. Biomechanical analysis of three surgical approaches for lumbar burst fractures using short-segment instrumentation. Spine (Phila Pa 1976). 1993;18:977-982. [PubMed] |
| 22. | Flamme CH, Hurschler C, Heymann C, von der Heide N. Comparative biomechanical testing of anterior and posterior stabilization procedures. Spine (Phila Pa 1976). 2005;30:E352-E362. [PubMed] |
| 23. | Zdeblick TA, Phillips FM. Interbody cage devices. Spine (Phila Pa 1976). 2003;28:S2-S7. [PubMed] |
| 24. | Burns BH. Two Cases of Spondylolisthesis. Proc R Soc Med. 1932;25:571-573. [PubMed] |
| 25. | Capener N. Spondylolisthesis. Br J Surg. 1932;19:374-386. [RCA] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Saraph V, Lerch C, Walochnik N, Bach CM, Krismer M, Wimmer C. Comparison of conventional versus minimally invasive extraperitoneal approach for anterior lumbar interbody fusion. Eur Spine J. 2004;13:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Zdeblick TA, David SM. A prospective comparison of surgical approach for anterior L4-L5 fusion: laparoscopic versus mini anterior lumbar interbody fusion. Spine (Phila Pa 1976). 2000;25:2682-2687. [PubMed] |
| 28. | Shen FH, Samartzis D, Khanna AJ, Anderson DG. Minimally invasive techniques for lumbar interbody fusions. Orthop Clin North Am. 2007;38:373-386; abstract vi. [PubMed] |
| 29. | McAfee PC, Regan JJ, Geis WP, Fedder IL. Minimally invasive anterior retroperitoneal approach to the lumbar spine. Emphasis on the lateral BAK. Spine (Phila Pa 1976). 1998;23:1476-1484. [PubMed] |
| 30. | Mummaneni PV, Haid RW, Rodts GE. Lumbar interbody fusion: state-of-the-art technical advances. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Regan JJ, McAfee PC, Guyer RD, Aronoff RJ. Laparoscopic fusion of the lumbar spine in a multicenter series of the first 34 consecutive patients. Surg Laparosc Endosc. 1996;6:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Rajaraman V, Vingan R, Roth P, Heary RF, Conklin L, Jacobs GB. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999;91:60-64. [PubMed] |
| 33. | Fantini GA, Pappou IP, Girardi FP, Sandhu HS, Cammisa FP. Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine (Phila Pa 1976). 2007;32:2751-2758. [PubMed] |
| 34. | Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10:154-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 374] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Lin PM. Posterior lumbar interbody fusion technique: complications and pitfalls. Clin Orthop Relat Res. 1985;90-102. [PubMed] |
| 36. | Chrastil J, Patel AA. Complications associated with posterior and transforaminal lumbar interbody fusion. J Am Acad Orthop Surg. 2012;20:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 980] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 38. | Pumberger M, Hughes AP, Huang RR, Sama AA, Cammisa FP, Girardi FP. Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J. 2012;21:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976). 2011;36:26-32. [PubMed] |
| 40. | Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech. 2011;24:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 41. | Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976). 2010;35:S322-S330. [PubMed] |
| 42. | DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. J Am Acad Orthop Surg. 2008;16:130-139. [PubMed] |
| 43. | Hee HT, Castro FP, Majd ME, Holt RT, Myers L. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord. 2001;14:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Rosenberg WS, Mummaneni PV. Transforaminal lumbar interbody fusion: technique, complications, and early results. Neurosurgery. 2001;48:569-574; discussion 574-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 185] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 45. | Parker SL, Mendenhall SK, Shau DN, Zuckerman SL, Godil SS, Cheng JS, McGirt MJ. Minimally Invasive Versus Open Transforaminal Lumbar Interbody Fusion (TLIF) for Degenerative Spondylolisthesis: Comparative Effectiveness and Cost-Utility Analysis. World Neurosurg. 2013;Epub ahead of print. [PubMed] |
| 46. | Oxland TR, Hoffer Z, Nydegger T, Rathonyi GC, Nolte LP. A comparative biomechanical investigation of anterior lumbar interbody cages: central and bilateral approaches. J Bone Joint Surg Am. 2000;82:383-393. [PubMed] |
| 47. | Brodke DS, Dick JC, Kunz DN, McCabe R, Zdeblick TA. Posterior lumbar interbody fusion. A biomechanical comparison, including a new threaded cage. Spine (Phila Pa 1976). 1997;22:26-31. [PubMed] |
| 48. | Cappuccino A, Cornwall GB, Turner AW, Fogel GR, Duong HT, Kim KD, Brodke DS. Biomechanical analysis and review of lateral lumbar fusion constructs. Spine (Phila Pa 1976). 2010;35:S361-S367. [PubMed] |
| 49. | Lin EL, Wang JC. Total disk arthroplasty. J Am Acad Orthop Surg. 2006;14:705-714. [PubMed] |
| 50. | Gamradt SC, Wang JC. Lumbar disc arthroplasty. Spine J. 2005;5:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Hochschuler SH, Ohnmeiss DD, Guyer RD, Blumenthal SL. Artificial disc: preliminary results of a prospective study in the United States. Eur Spine J. 2002;11 Suppl 2:S106-S110. [PubMed] |
| 52. | Blumenthal S, McAfee PC, Guyer RD, Hochschuler SH, Geisler FH, Holt RT, Garcia R, Regan JJ, Ohnmeiss DD. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine (Phila Pa 1976). 2005;30:1565-175; discussion 1565-175;. [PubMed] |
| 53. | Zigler J, Delamarter R, Spivak JM, Linovitz RJ, Danielson GO, Haider TT, Cammisa F, Zuchermann J, Balderston R, Kitchel S. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976). 2007;32:1155-162; discussion 1163. [PubMed] |
| 54. | Zigler JE, Delamarter RB. Five-year results of the prospective, randomized, multicenter, Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential arthrodesis for the treatment of single-level degenerative disc disease. J Neurosurg Spine. 2012;17:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Coric D, Mummaneni PV. Nucleus replacement technologies. J Neurosurg Spine. 2008;8:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Bertagnoli R, Karg A, Voigt S. Lumbar partial disc replacement. Orthop Clin North Am. 2005;36:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Balsano M, Zachos A, Ruggiu A, Barca F, Tranquilli-Leali P, Doria C. Nucleus disc arthroplasty with the NUBAC™ device: 2-year clinical experience. Eur Spine J. 2011;20 Suppl 1:S36-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Luque ER, Cordosa A. Segmental correction of scoliosis with rigid internal fixation: preliminary report. Orthop Trans. 1977;1:136-137. |
| 59. | Mohan AL, Das K. History of surgery for the correction of spinal deformity. Neurosurg Focus. 2003;14:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Winter RB, Anderson MB. Spinal arthrodesis for spinal deformity using posterior instrumentation and sublaminar wiring. A preliminary report of 100 consecutive cases. Int Orthop. 1985;9:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Zdeblick TA, Becker PS, McAfee PC, Sutterlin CE, Coe JD, Gurr KR. Neuropathologic changes with experimental spinal instrumentation: transpedicular versus sublaminar fixation. J Spinal Disord. 1991;4:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 62. | Drummond D, Guadagni J, Keene JS, Breed A, Narechania R. Interspinous process segmental spinal instrumentation. J Pediatr Orthop. 1984;4:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Jeng CL, Sponseller PD, Tolo VT. Outcome of Wisconsin instrumentation in idiopathic scoliosis. Minimum 5-year follow-up. Spine (Phila Pa 1976). 1993;18:1584-1590. [PubMed] |
| 64. | Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976). 2004;29:1938-1944. [PubMed] |
| 65. | Faraj AA, Webb JK. Early complications of spinal pedicle screw. Eur Spine J. 1997;6:324-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Lykissas MG, Jain VV, Nathan ST, Pawar V, Eismann EA, Sturm PF, Crawford AH. Mid- to long-term outcomes in adolescent idiopathic scoliosis after instrumented posterior spinal fusion: a meta-analysis. Spine (Phila Pa 1976). 2013;38:E113-E119. [PubMed] |
| 67. | Lee SH, Choi WG, Lim SR, Kang HY, Shin SW. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis. Spine J. 2004;4:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976). 2005;30:123-129. [PubMed] |
| 69. | Kim JS, Choi WG, Lee SH. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis: minimum 5-year follow-up. Spine J. 2010;10:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Shim CS, Lee SH, Jung B, Sivasabaapathi P, Park SH, Shin SW. Fluoroscopically assisted percutaneous translaminar facet screw fixation following anterior lumbar interbody fusion: technical report. Spine (Phila Pa 1976). 2005;30:838-843. [PubMed] |
| 71. | Margulies JY, Seimon LP. Clinical efficacy of lumbar and lumbosacral fusion using the Boucher facet screw fixation technique. Bull Hosp Jt Dis. 2000;59:33-39. [PubMed] |
| 72. | Palmer DK, Inceoglu S, Cheng WK. Stem fracture after total facet replacement in the lumbar spine: a report of two cases and review of the literature. Spine J. 2011;11:e15-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Phillips FM, Tzermiadianos MN, Voronov LI, Havey RM, Carandang G, Renner SM, Rosler DM, Ochoa JA, Patwardhan AG. Effect of the Total Facet Arthroplasty System after complete laminectomy-facetectomy on the biomechanics of implanted and adjacent segments. Spine J. 2009;9:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Zhu Q, Larson CR, Sjovold SG, Rosler DM, Keynan O, Wilson DR, Cripton PA, Oxland TR. Biomechanical evaluation of the Total Facet Arthroplasty System: 3-dimensional kinematics. Spine (Phila Pa 1976). 2007;32:55-62. [PubMed] |
| 75. | Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, Johnson DR, Skidmore GA, Vessa PP, Dwyer JW. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine (Phila Pa 1976). 2005;30:1351-1358. [PubMed] |
| 76. | Sénégas J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: the Wallis system. Eur Spine J. 2002;11 Suppl 2:S164-S169. [PubMed] |
P- Reviewers Pamidi N, Sahebari M, Fourtounas C, Vij M S- Editor Huang XZ L- Editor A E- Editor Zheng XM