Published online Mar 20, 2026. doi: 10.5662/wjm.v16.i1.112458
Revised: September 1, 2025
Accepted: November 14, 2025
Published online: March 20, 2026
Processing time: 197 Days and 20.9 Hours
Small cell lung cancer (SCLC) constitutes about 15% of lung cancers and is an aggressive disease with uveal metastasis. An isolated iris lesion in such patients may or may not be a manifestation of disseminated disease. We report two cases – one patient with SCLC and another with non-small cell lung cancer, each with varied presentation.
The first case was a 49-year-old female who presented with redness in the left eye. She was a known case of SCLC with multi-organ involvement. The best corrected visual acuity (BCVA) was 20/100. Slit-lamp bio-microscopy showed an iris mass with neovascularization. Cytology from anterior chamber paracentesis was su
Iris lesions in lung carcinoma patients may not necessarily be metastases. Sometimes they can be reactive nodules mimicking secondaries.
Core Tip: Though iris metastasis is rare in cases of systemic malignancies, a relatively rich vascular supply of uveal tissue makes it vulnerable compared to other ocular structures. Iris masses are often unilateral, may be painful and are vision-threatening. The lesion can be single or multiple, usually well-defined, inducing anterior chamber reaction leading to secondary glaucoma. With treatment advances like platinum doublet chemotherapy and targeted therapy for specific mutations, the disease progression can be arrested with alleviation of symptoms. However, some cases may require surgical intervention to manage the complications.
- Citation: Nayak B, Mohapatra PR, Chakraborty K, Nanda J, Haripriya S, Mantha SP, Sethy M, Panda BB. Are iris masses in lung carcinoma always a metastasis: Two case reports. World J Methodol 2026; 16(1): 112458
- URL: https://www.wjgnet.com/2222-0682/full/v16/i1/112458.htm
- DOI: https://dx.doi.org/10.5662/wjm.v16.i1.112458
Lung cancer is histologically classified broadly into two primary types: Small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). These types differ significantly in their clinical behaviour, with SCLC being markedly more aggressive. SCLC and NSCLC account for approximately 15% and 85% of lung cancer cases, respectively[1]. While uveal metastases are the most common intraocular malignancy in adults, iris involvement is rare, representing less than 10% of such metastases[2]. The most frequent primary origins are breast (37%) and lung (27%) carcinomas followed by kidney (4%) and gastrointestinal tract (4%)[2-5]. Patients with iris mass usually present with blurring of vision, defect in visual field, pain and photophobia[2,5]. Clinical signs often include a unilateral, yellow-white, solitary or multifocal mass, which may be associated with posterior synechiae, localized neovascularization, hyphaema, anterior chamber cells, and se
In this manuscript we report two different types of primary lung malignancies and their varied presentations. We are emphasizing that iris masses may not always the secondaries of the primary cancer, rather these may be due to inflammation. Our patient with SCLC was found to be having iris metastasis in the left eye, confirmed by cytopathological examination. But the other patient with NSCLC had multiple iris nodules in the right eye, and both cytology and tissue biopsy, were inconclusive for malignant cells.
Case 1: A 49-year-old female presented with chief complaints of redness and decreased distant vision in the left eye for the last month.
Case 2: A 54-year-old woman presented with complaints of decreased vision in oculus dexter (OD) for the last month, along with pain, redness, and watering.
Case 1: The symptoms were sudden in onset and progressive in nature. There was associated chest pain and cough for the last two months.
Case 2: She had a sudden onset of decreased vision in OD with dull aching pain and redness one month prior to presentation. She consulted a physician in her locality and was started on antiglaucoma medications.
Case 1: There were no similar episodes of ocular symptoms in the past.
Case 2: There were no similar episodes of ocular symptoms in the past. The patient was diagnosed with stage IV ade
Case 1: She had a history of biomass fuel exposure for over 30 years and was a non-smoker. There was no family history of any malignancy.
Case 2: There was no family history of any malignancy, and she was a non-smoker.
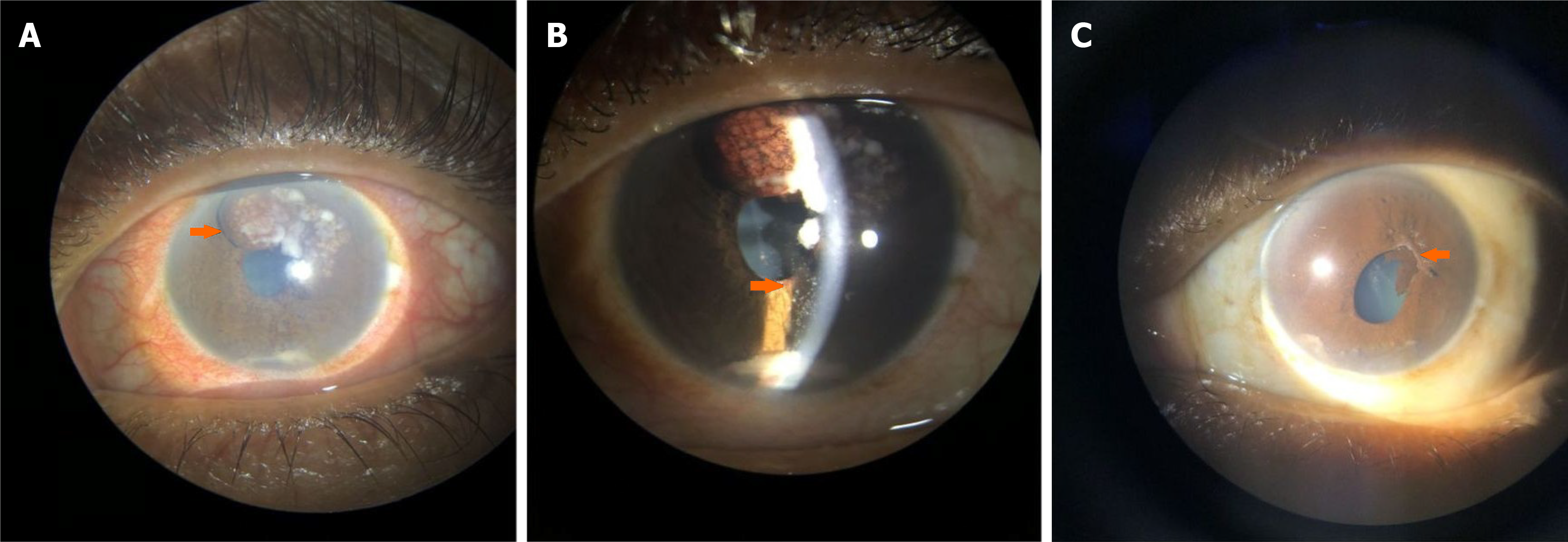
Case 1: On ophthalmic examination, OD had best-corrected visual acuity (BCVA) of 20/30 and intraocular pressure (IOP) of 14 mm without any congestion. The BCVA was 20/100 in oculus sinister (OS) with an IOP of 20 mmHg. On slit-lamp examination of OS, there was ciliary congestion, presence of an iris mass from 11 o’clock to 1 o’clock along with neovascularization of the iris (NVI) (Figure 1A), associated with whitish flocculent material deposits on the iris and in the anterior chamber (Figure 1B). The pupil was sluggishly reacting to torchlight and poorly dilating with mydriatics. The fundus view of OS was not clear. The fundus of OD was normal.
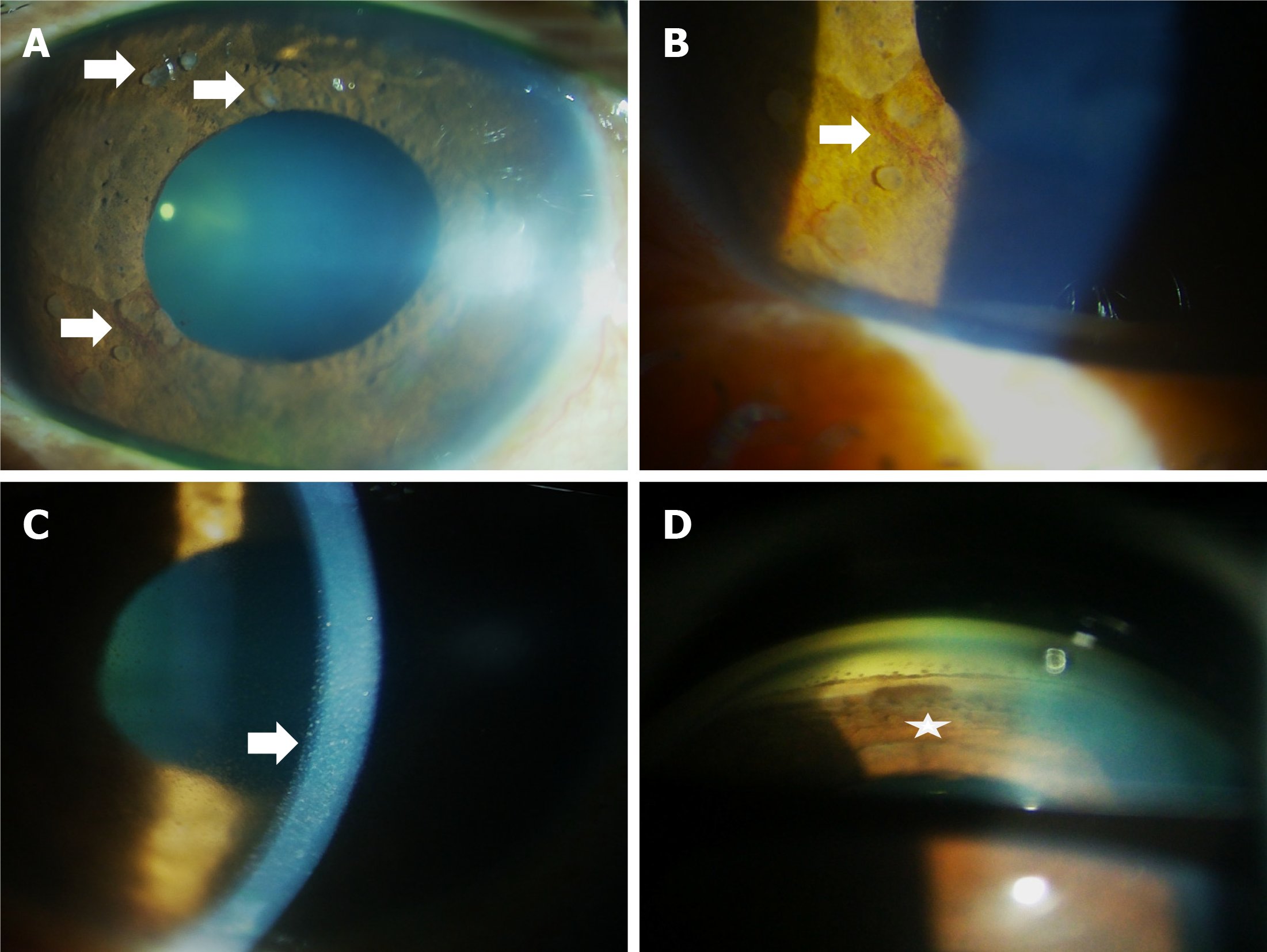
Case 2: Her BCVA was 20/200 in OD and 20/30 in OS. The IOP in OD and OS was 36 and 12 mmHg, respectively. On slit lamp examination of OD, ciliary congestion was noted, and multiple semi-transparent iris nodules of varying sizes and shapes were found in different quadrants (Figure 2A). There was NVI between 7 o'clock and 9 o'clock (Figure 2B) in the affected eye. There were pigments on the endothelium (Figure 2C), and flare 2+ was noted in the anterior chamber. Gonioscopy revealed open angles in oculus uterque (OU) with localized peripheral anterior synechiae in OD (Figure 2D). Dilated fundus examination showed glaucomatous optic disc changes having vertical cup-disc ratio (VCDR) of 0.9 in OD; Fundus findings of OS were normal with VCDR of 0.4.
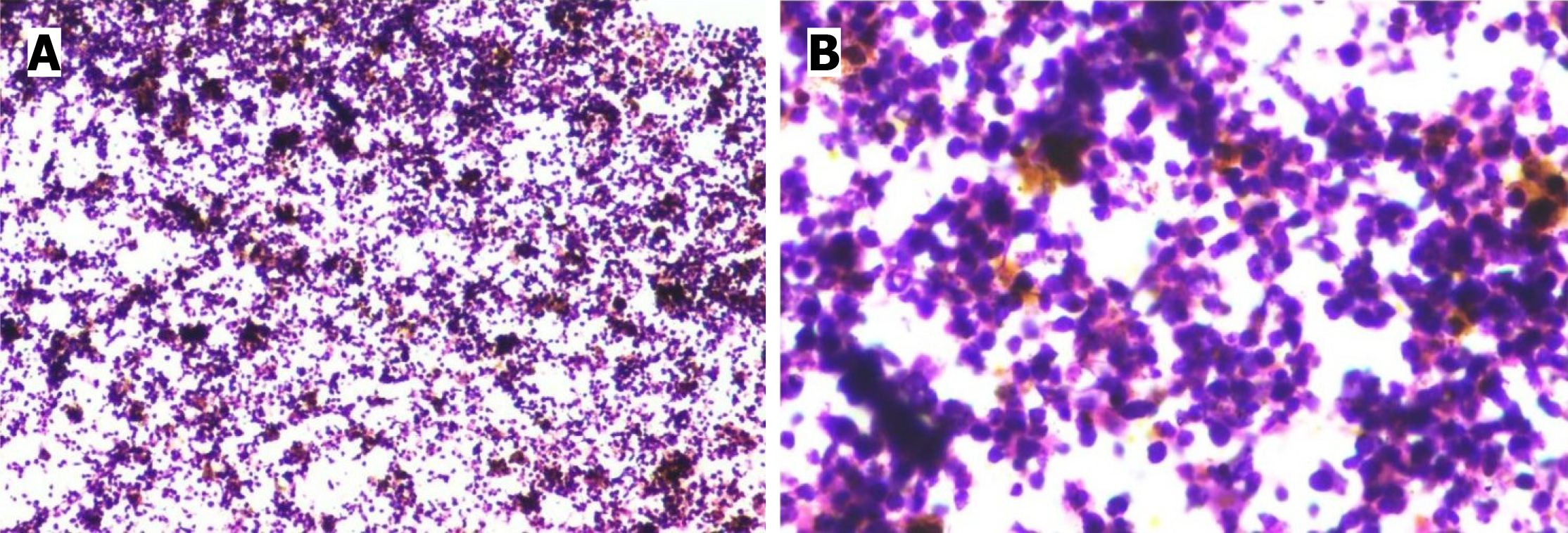
Case 1: Aspiration from the anterior chamber and of the flocculent material on the iris mass was done using a 26 G needle with a tuberculin syringe, and the cytology report was positive for malignancy (Figure 3). Endobronchial biopsy con
Case 2: On genetic analysis, EGFR exon 19 deletion and T790M (Exon 20) mutation were detected. Aqueous sample aspiration was done using 26G needle with tuberculin syringe and the sample was sent for cytopathological examination, and the report was negative for malignant cells. During trabeculectomy, repeat aqueous aspiration done and sent for cytological examination. The iris tissue with a nodule was excised and sent for histopathological examination in a formalin-containing container. Neither report showed any evidence of malignancy. Cytopathology showed only inflammatory changes. On immunohistochemistry tissue was negative for TTF-1 and Napsin A.
Case 1: Ultrasonography of the posterior segment and optical coherence tomography (OCT) didn’t reveal any mass in the choroid and posterior segment. UBM didn’t reveal any ciliary body mass. Contrast-enhanced computed tomography (CECT) of the thorax and abdomen revealed a mass in the right lung with endobronchial growth in the right intermediate bronchus, along with metastasis to the right adrenal gland and spine. Magnetic resonance imaging (MRI) of orbits showed an enhancing lesion in the anterior chamber abutting the lens and iris in OS. MRI of the brain showed pineal gland metastasis. The bone scan was suggestive of D4 vertebra metastasis.
Case 2: Posterior segment ultrasound and OCT didn’t reveal any mass in the choroid or posterior segment. UBM didn’t reveal any ciliary body mass. CECT of the thorax and abdomen revealed multiple lesions in the bilateral lungs, liver, and bones suggestive of metastasis. CEMRI of the brain showed bilateral cerebral and right cerebellar hemisphere lesions suggestive of disseminated disease. Positron emission tomography CT suggested focal FDG avidity in the above-said organs, and no pathological hypermetabolic lesion was found elsewhere in the body.
A diagnosis of left eye iris metastasis secondary to SCLC was made.
Considering the above history and clinical findings, she was diagnosed to have right eye secondary open-angle glaucoma with reactive iris nodules with stage IV adenocarcinoma of the lung.
The patient received whole-brain radiotherapy (WBRT) for brain metastasis, and subsequently, six cycles of platinum doublet chemotherapy were given.
She was managed medically at first, but as the target IOP was not achieved with maximum antiglaucoma medications, trabeculectomy was done. Pulmonary consultation opined to continue Osimertinib as the disease was stable.
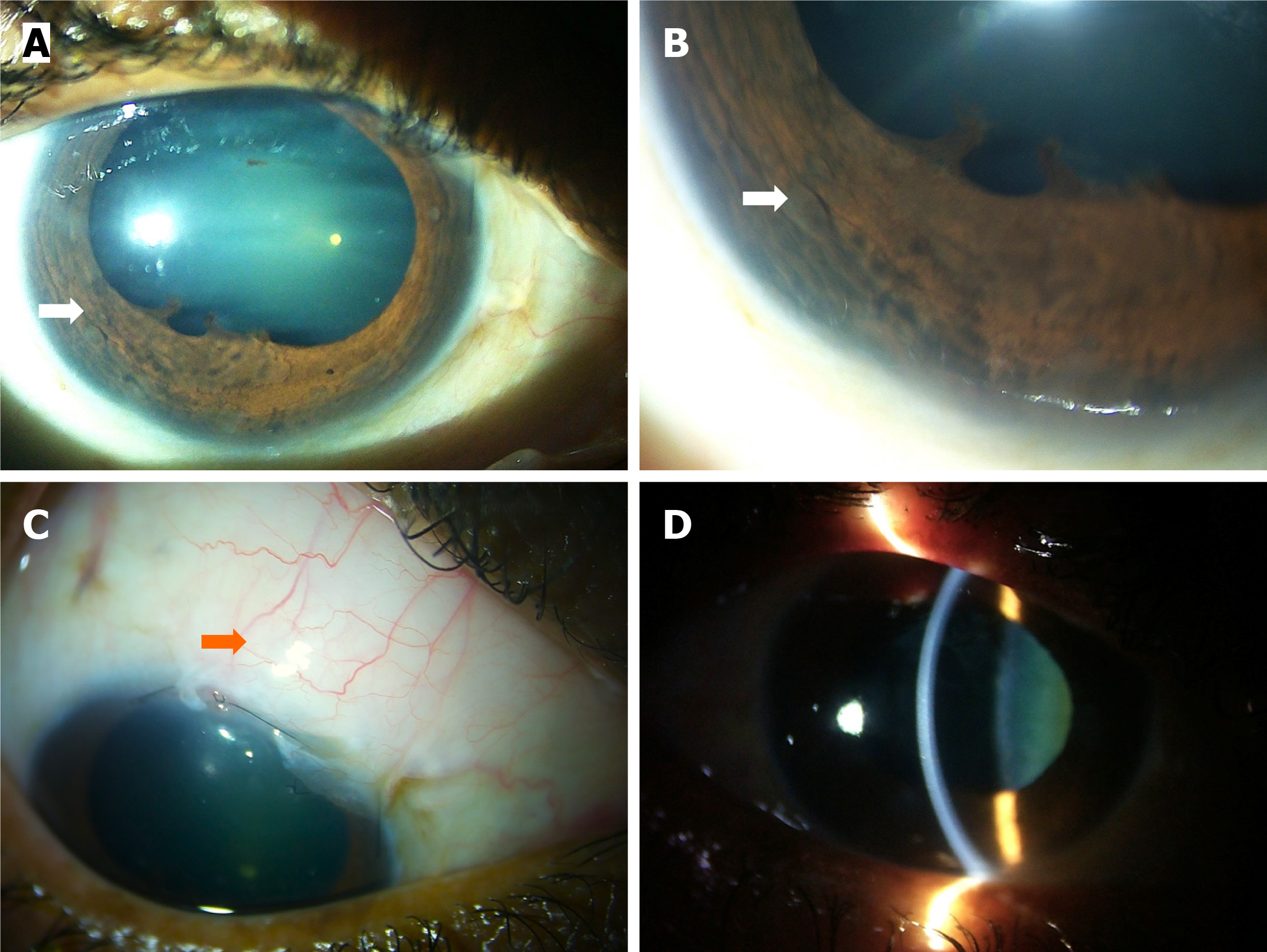
Post chemotherapy, the OS was quiet with a normal IOP of 14 mmHg and a vision of 20/50. The iris mass, the NVI, and also the white flocculent material disappeared. Instead, there was ectropion uvea and post-inflammatory posterior synechiae from 12 o’clock to 3 o’clock (Figure 1C). The lens showed early cataractous changes. Fundus examination was normal with a VCDR of 0.4 in OU.
Postoperatively, the OD was quiet with a vision of 20/70, iris nodules, and NVI showed regression (Figure 4A and B). IOP was 12 mmHg with a well-functioning bleb, and without any antiglaucoma medication (Figure 4C), and the cornea was clear (Figure 4D).
According to previously reported literature, different theories postulate uveal metastasis. The basis behind all these theories is seedlings from primary cancer and a rich vascular supply of uveal tissue, making it prone to secondaries. Once the tumour cells settle in one tissue, these cells drive metabolic activity and clearance of metabolic byproducts initiates, which drives neovascularization in and around the secondaries[6]. Among the uveal tissue, the choroid is the most common site, whereas the iris metastasis has an incidence of less than 10% of all uveal metastases[2]. Among iris tu
The definitive diagnosis of metastasis needs to be confirmed by cytopathology or biopsy. Aqueous and the flocculent material on the iris mass was also aspirated and sent for cytopathological examination, which confirmed the diagnosis (Figure 2A and B) in one of our patients. In our second patient we have sent aqueous aspiration sample on presentation, which came out to be negative for malignancy. So, keeping in mind that aqueous aspiration using a 26G needle might have yielded a low cell count due to its smaller bore size, we went for repeat aspiration of aqueous and tissue biopsy during trabeculectomy. Both cytology and the histopathology of the sample containing iris tissue were found to be inconclusive for malignant cells, as reported by Shields et al[7] and showed only inflammatory cells. The iris tissue was also negative for TTF-1 and Napsin A.
Due to inherent disseminated conditions, chemotherapy with WBRT has been the treatment available for over three decades. Because of the systemic disease with distant metastasis, chemotherapy is the first choice in the treatment of iris metastasis. Our first case received WBRT and platinum doublet chemotherapy, following which the iris mass and NVI resolved. In our second case, the patient denied WBRT and was on osimertinib, a third-generation EGFR tyrosine kinase inhibitor (EGFR-TKI). According to literatures, regression of iris mass was noted with erlotinib, a first-generation EGFR-TKI monotherapy as described by Ye et al[12], and Kim et al[13] reported regression of iris mass when treated with erlotinib and three intravitreal bevacizumab injections; Maturu et al[14] described gefitinib, a first-generation EGFR-TKI with four intravitreal bevacizumab injections helped in regression of mass; Chen et al[15] demonstrated complete resolution of iris mass with osimertinib monotherapy; whereas, Wong et al[16] reported osimertinib along with three intravitreal bevacizumab injections caused regression of the mass.
Due to the paucity of reported literature, it is unclear whether EGFR-TKI monotherapy or combination with intravitreal anti-VEGF is more effective in the regression of iris masses. A randomized controlled trial should be conducted to consider the different modalities of treatment for a better understanding.
In the present study follow-up period was 6 months for both of our patients. None of our patients had received anti-VEGF injections during treatment. Our patients had received topical antibiotics and non-steroidal anti-inflammatory drugs after the aqueous aspiration in both cases; after tissue biopsy and trabeculectomy in second case. As our second patient had been on osimertinib for the last two years, there were multiple nodules, and cytology showed only inflammatory cells; we thought those to be reactive iris nodules due to the drug as reported by Deitch-Harel et al[17].
These contrasting cases highlight the diagnostic and therapeutic complexities of iris lesions in patients with advanced lung carcinoma. We demonstrate that while iris masses can represent metastatic disease, as confirmed by cytology in our SCLC case, they may also manifest as reactive, inflammatory nodules secondary to targeted therapies like osimertinib, as evidenced by negative histopathology in our NSCLC patient.
A high index of clinical suspicion is paramount. Diagnosis hinges on a multimodal approach, combining advanced ocular imaging with cytopathological and histopathological analysis, though results can be inconclusive. Treatment must be highly individualized; systemic chemotherapy and radiotherapy effectively addressed the metastatic lesion in SCLC, while targeted EGFR-TKI therapy alone led to the regression of inflammatory nodules in NSCLC, with surgical in
These outcomes underscore that iris abnormalities in oncology patients are not always malignant and may reflect a spectrum of disease, from direct metastasis to treatment-related inflammation. A collaborative, multidisciplinary strategy is essential to optimize both oncologic and ophthalmic outcomes, preserving vision and quality of life. Further research is needed to establish definitive diagnostic criteria and treatment protocols for EGFR-TKI-associated inflammatory iris nodules.
| 1. | Wang Y, Zou S, Zhao Z, Liu P, Ke C, Xu S. New insights into small-cell lung cancer development and therapy. Cell Biol Int. 2020;44:1564-1576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Shields CL, Kalafatis NE, Gad M, Sen M, Laiton A, Silva AMV, Agrawal K, Lally SE, Shields JA. Metastatic tumours to the eye. Review of metastasis to the iris, ciliary body, choroid, retina, optic disc, vitreous, and/or lens capsule. Eye (Lond). 2023;37:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 625] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 4. | Almanassra M, Sucharita S, Padhy SK, Tripathy D. Iris metastasis: clues to the primary through immunohistochemistry. BMJ Case Rep. 2025;18:e264917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Liu T, Bai F, Yang L, Liu L, Xiao J, Liu X. Primary Tumour Type, Clinical Features, Treatment and Outcome of Patients with Iris Metastasis. Ocul Immunol Inflamm. 2022;30:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Mathis T, Jardel P, Loria O, Delaunay B, Nguyen AM, Lanza F, Mosci C, Caujolle JP, Kodjikian L, Thariat J. New concepts in the diagnosis and management of choroidal metastases. Prog Retin Eye Res. 2019;68:144-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Shields JA, Shields CL, Kiratli H, de Potter P. Metastatic tumors to the iris in 40 patients. Am J Ophthalmol. 1995;119:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 116] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Shields CL, Manquez ME, Ehya H, Mashayekhi A, Danzig CJ, Shields JA. Fine-needle aspiration biopsy of iris tumors in 100 consecutive cases: technique and complications. Ophthalmology. 2006;113:2080-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Lu P, Huang J. Bilateral Iris Metastasis From Pulmonary Adenocarcinoma. JAMA Ophthalmol. 2018;136:e182381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Sobol EK, Pasquale LR. Iris Mass and Secondary Glaucoma: The Presenting Sign of Lung Adenocarcinoma. Ophthalmol Glaucoma. 2020;3:217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Konno S, Yuzawa S, Kinouchi R. A case of masquerade syndrome caused by metastatic iris tumor diagnosed by a high CEA level in the aqueous humor and iris biopsy. Diagn Pathol. 2024;19:128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Ye X, Kaliki S, Shields CL. Rapid regression of choroidal metastasis from lung cancer using erlotinib (Tarceva). Oman J Ophthalmol. 2014;7:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to non-small-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223:411-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Maturu VN, Singh N, Bansal P, Rai Mittal B, Gupta N, Behera D, Gupta A. Combination of intravitreal bevacizumab and systemic therapy for choroidal metastases from lung cancer: report of two cases and a systematic review of literature. Med Oncol. 2014;31:901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Chen KJ, Chao AN, Wang CL. Iris Metastasis Regression Following Osimertinib Treatment. JAMA Ophthalmol. 2020;138:e202095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Wong M, Frank JH, Shields CL. Non-small cell lung cancer with iris metastasis controlled with osimertinib and monthly intravitreal bevacizumab. Am J Ophthalmol Case Rep. 2022;25:101269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Deitch-Harel I MD, Raskin E MD, Habot-Wilner Z MD, Friling R MD, Amer R MD, Kramer M MD. Uveitis Induced by Biological Agents Used in Cancer Therapy. Ocul Immunol Inflamm. 2021;29:1370-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/