Published online Mar 20, 2026. doi: 10.5662/wjm.v16.i1.109580
Revised: June 7, 2025
Accepted: September 10, 2025
Published online: March 20, 2026
Processing time: 271 Days and 14 Hours
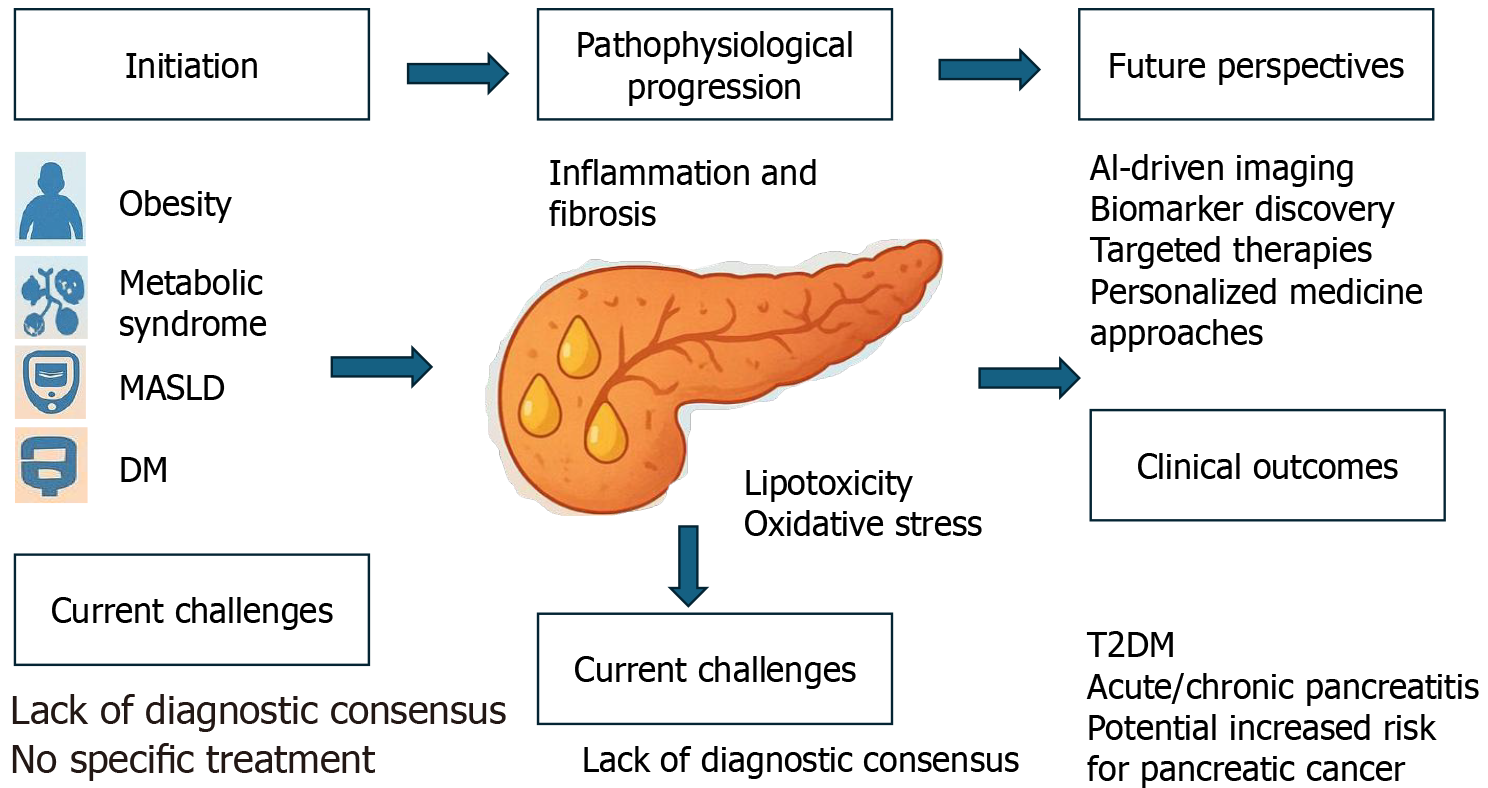
With the global increase in obesity rates, a newly emerging disease, non-alcoholic fatty pancreatic disease (NAFPD), has garnered increasing attention. The most important characteristic of NAFPD is fat accumulation in the pancreas in indivi
Core Tip: With the global increase in obesity cases, non-alcoholic fatty pancreatic disease (NAFPD), characterized by fat accumulation in the pancreas with minimal alcohol consumption, has emerged as a condition of growing interest. Despite its parallels with metabolic dysfunction-associated steatotic liver disease, the pathogenesis, diagnosis, and clinical implications of NAFPD remain poorly understood; therefore, further research is necessary to advance early detection and treatment.
- Citation: Okasha HH, Alyouzbaki AZ, Tehami N, Abdellatef A. Fatty pancreas: Current insights and future perspectives. World J Methodol 2026; 16(1): 109580
- URL: https://www.wjgnet.com/2222-0682/full/v16/i1/109580.htm
- DOI: https://dx.doi.org/10.5662/wjm.v16.i1.109580
Excess fat accumulation in the body leads to significant health problems, such as fatty liver; however, fat accumulation is not restricted to the liver but may involve other organs, including the pancreas. Despite the well-established patho
Fat infiltration in the pancreas, which is a characteristic of FP, can disrupt both endocrine and exocrine functions, contributing to a broad spectrum of clinical complications (Figure 1). On the endocrine front, excess pancreatic fat is associated with β-cell dysfunction, leading to impaired insulin secretion and compromised glycemic control, which are the dominant factors in the development and progression of type 2 diabetes mellitus (T2DM)[11].
This dysfunction arises partly from lipotoxicity and inflammation, which damage islet cells. Simultaneously, exocrine function may be affected by the reduced secretion of digestive enzymes, such as lipase, amylase, and proteases, resulting in malabsorption, steatorrhea, and other gastrointestinal symptoms[12].
These dual impairments underscore the systemic nature of FP and highlight the need for its recognition as a clinically significant condition that warrants comprehensive metabolic and digestive evaluations.
This review aims to move beyond descriptive synthesis by offering a structured analysis of the current understanding and emerging trends in pancreatic disease research. Specifically, the goal was to consolidate existing knowledge while critically evaluating diagnostic frameworks and highlighting gaps in the literature. By examining the clinical manifestations, mechanistic insights, and growing roles of biomarkers and imaging, this review seeks to inform both clinical practice and future research. In doing so, it positions itself not only as a resource for current knowledge but also as a foundation for developing more precise diagnostic and therapeutic strategies.
Recent reports estimate the prevalence of FP at 16%-35% when assessed by abdominal ultrasonography[13,14]. In recent years, a new era has emerged in understanding FP and its clinical implications, including its association with other non-cancerous conditions, such as diabetes mellitus, metabolic syndrome (MetS), and cardiovascular diseases, as well as with metabolic dysfunction-associated steatotic liver disease (MASLD) and cancerous conditions, such as pancreatic cancer[15,16]. A meta-analysis of 11 studies encompassing 12675 individuals reported a prevalence of FIP at 33% (95%CI: 24%-41%)[17]. Notably, several studies have shown that FP is a risk factor for acute pancreatitis[18] associated with precancerous pancreatic mucinous neoplasms[19]. Many risk factors are associated with FP, including age, obesity, diabetes mellitus, and MetS. Several studies have reported that fat accumulation in the pancreas increases with age until approximately 60 years of age, reaching a steady state. The volume of the pancreatic parenchyma increases with age until approximately 30 years of age, after which it gradually decreases. These changes result in an increased fat-to-parenchyma ratio in middle-aged and older individuals[20-23].
In a cohort study of adult Hong Kong Chinese volunteers, it was reported that the prevalence of FP progressively increased with age, with a low prevalence in children. However, among children who are overweight or obese, the prevalence was higher in those with fatty liver than in those without, at 2.28% vs 1.77%, respectively[24]. In a study by Aleshina et al[25], 70% of the overweight children had pancreatic steatosis, whereas only 46.6% had hepatic steatosis. Owing to the increasing rates of overnutrition and obesity worldwide, the number of individuals with MetS has risen rapidly, with an estimated increase from 10.1% to 12.1% over 5 years among the 35-59-year-old Chinese population[26], along with an increase in mean body mass index (BMI) by 0.4-0.5 kg/m2 per decade globally[27]. FP is associated with abdominal obesity, insulin resistance, T2DM, dyslipidemia, arterial hypertension, and MetS[2,17]. Moreover, a fatty liver has been reported as a risk factor for a FP[28]. Obesity and insulin resistance are the key contributors to pancreatic adipocyte infiltration, ultimately leading to FIP[29]. A significant association has been reported between pancreatic steatosis and atherosclerosis in non-obese patients with T2DM[30]. In a study by Tomita et al[31], the fatty degeneration rate in the pancreas with pancreatic ductal adenocarcinoma (PDAC) was higher than in those without PDAC, at 72% and 44%, respectively. Accurate estimates of NAFPD and MASLD rates remain undetermined[32]. Lee et al[2] reported that only 2.2% of the patients with fatty liver had a normal pancreas, whereas 29.9% of the patients with FP had a normal liver. The prevalence of FP varies according to ethnicity, with higher rates among Hispanic and Caucasian populations than among African Americans[33,34]. Environmental risk factors, particularly cigarette smoking and alcohol consumption, have been associated with the development of FP[35,36].
However, its exact pathogenesis remains unknown[37]. Alcohol and steroid hormones have been implicated in FP development[22]; additionally, certain medications and chemical agents, such as antiretroviral therapy[38], rosiglitazone[39], and chronic ethanol feeding[40] have been associated. However, there is no evidence to support a genetic predisposition to FP[41]. Most published data have reported a strong association among diabetes, MASLD, hypertension, cardiovascular disease, and FP[42-44]. The lack of a clear distinction between triglyceride (TG) accumulation in acinar cells and beta cells and intrapancreatic adipose tissue infiltration has contributed to the interchangeable use of various terms, including pancreatic steatosis, pancreatic lipomatosis, and FP[5].
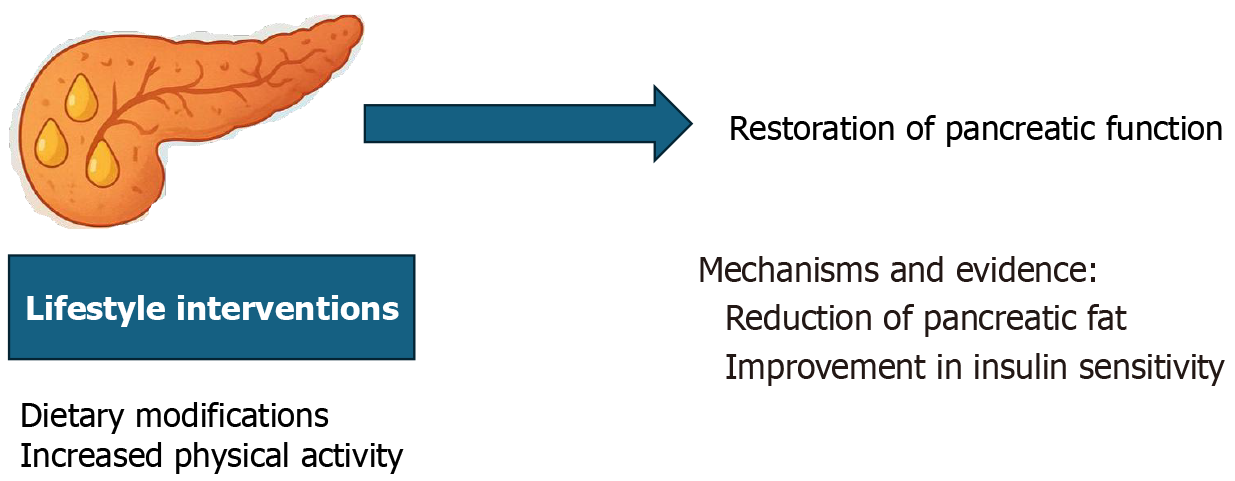
An important and promising aspect of pancreatic health is the potential reversibility of early pancreatic dysfunction, particularly in cases of fat infiltration or metabolic stress. Emerging research shows that targeted lifestyle changes, primarily dietary modifications and increased physical activity, can significantly improve or even restore pancreatic function to a more normal state[45].
For instance, reducing caloric intake and engaging in regular exercise can decrease intra-pancreatic fat and improve insulin sensitivity, thereby enhancing both endocrine and exocrine functions[46].
These changes are especially relevant in the early stages of T2DM or NAFPD, where interventions may prevent progression to irreversible damage. The mechanisms underlying this recovery were linked to improved metabolic flexibility, reduced lipotoxicity, and enhanced cellular repair processes in the pancreas. Understanding and commu
Many patients with FP remain asymptomatic, with detection typically dependent on diagnostic testing[5]. Due to the deep anatomical location of the pancreas, tissue sampling is not practical for the routine evaluation of fatty infiltration. Instead, imaging modalities offer a non-invasive means of detecting and quantifying pancreatic fat accumulation in clinical practice[47]. Several diagnostic techniques have been established to diagnose FP, including transabdominal ultrasonography, endoscopic ultrasonography (EUS), magnetic resonance imaging (MRI) and computed tomography (CT)[48]. However, transabdominal ultrasonography remains the preferred diagnostic tool because it is widely available, non-invasive, and cost-effective[2]. The added difficulty in correctly diagnosing FP is that the echogenicity on abdominal ultrasonography is influenced by peripancreatic fat deposition rather than pancreatic parenchymal deposition. Unfortunately, this correlates with age and subcutaneous fat, which limits the utility of sonography as a valuable tool for dia
Histopathology remains the gold standard for diagnosing many diseases, such as FP. It is characterized by adipocyte infiltration and intracellular fat deposition in acinar and islet cells. However, pancreatic biopsy after surgery has limited indications and cannot be widely adopted, given the low incidence of pancreatic surgery in individuals at risk for FP[56].
The presence of FP can be assessed by comparing pancreatic echogenicity to that of the spleen using EUS. A grading system for EUS was proposed based on the echogenic characteristics of the pancreatic parenchyma, including the presence of distinctive fine "salt and pepper" speckling and the clarity of the pancreatic duct margin[14,57].
EUS-guided fine-needle aspiration biopsy can be performed. However, it carries the risk of complications and is generally considered ethical only, especially in situations, such as differentiating between malignant and benign localized pancreatic tumors[9]. While EUS provides high-resolution imaging owing to the proximity of the probe to the pancreas, it shares certain limitations with transabdominal US, notably operator dependency. Additionally, the requirement for an endoscopic procedure renders EUS relatively more invasive than other non-invasive imaging modalities[45].
CT is a widely used imaging technique to quantify pancreatic fat. Because the radiodensity of tissues varies on CT images, pancreatic fat measurement is often compared to that of an internal reference tissue, such as the spleen, which contains minimal lipid content. In cases of FP, non-enhanced CT images typically show reduced pancreatic attenuation. Moreover, the difference in attenuation between the pancreas and spleen provides an objective means of assessing the severity of fatty infiltration. The advantages of CT include its broad availability and relatively short imaging time. However, the potential risk of radiation exposure remains a significant limitation[58-60]. Table 1 contains a summary of the diagnostic modalities for FP.
| Modality | Advantages | Limitations | Clinical use |
| US | Widely available, non-invasive, low cost | Operator-dependent; limited sensitivity in obese patients | Initial screening tool |
| Computed tomography scan | High-resolution imaging; detects fat attenuation | Radiation exposure; limited functional assessment | Incidental detection during imaging |
| Magnetic resonance imaging-proton density fat fraction | Quantifies fat content accurately; no radiation | Expensive; limited availability | Research and advanced diagnostic use |
| Endoscopic US | High-resolution, closer proximity to pancreas | Invasive; requires sedation and trained operator | Detailed assessment, especially in ambiguous cases |
Currently, no reliable biomarker exists for FP diagnosis, and histopathology and imaging continue to serve as primary diagnostic tools. Nonetheless, a meta-analysis reported a modest positive association between FP and TG levels as well as a modest negative association with high-density lipoprotein cholesterol among lipid metabolism indicators. Furthermore, several markers of glucose metabolism, including glycated hemoglobin, fasting insulin, homeostasis model assessment of insulin resistance, and fasting glucose, have demonstrated significant correlations with FP[5].
Inflammatory markers, such as high-sensitivity C-reactive protein (CRP) and plasminogen activator inhibitor-1 (PAI-1), have minor associations with pancreatic fat content. CRP is an acute-phase protein produced in the liver in response to the pro-inflammatory cytokine interleukin-6 and is a well-established inflammatory marker. However, it is not consi
There is no universally accepted grading system for assessing FP severity. However, a cross-sectional study involving 367 patients who underwent pancreatoduodenectomy for PDAC proposed a histological classification based on fat infiltration as mild (< 10% of pancreatic tissue), moderate (10%-20%), and severe (> 20%). These findings highlight the need for standardized diagnostic methods and clinically relevant thresholds to accurately evaluate the presence and severity of FP in routine clinical practice[61].
Despite the increasing interest in the clinical implications of FP, several key questions remain unanswered. One critical area of uncertainty is the lack of standardized thresholds for defining clinically relevant FP using imaging techniques, such as MRI-PDFF. While MRI-PDFF has shown promise in non-invasively quantifying pancreatic fat, there is no con
In countries where alcohol consumption is uncommon, the clinical implications of FP remain debatable[32].
Diseases that affect the pancreas, such as pancreatitis, pancreatic cancer, and diabetes mellitus, have significant clinical consequences that extend beyond localized symptoms. Patients may experience severe abdominal pain, digestive issues, and weight loss due to malabsorption, particularly in chronic pancreatitis[62].
Malnutrition is a common complication due to reduced pancreatic enzyme output, and chronic pain can severely affect the quality of life. Diabetes resulting from impaired insulin production leads to long-term complications, including cardiovascular diseases, kidney dysfunction, and neuropathy[63].
Pancreatic cancer often presents late with vague symptoms and is associated with a poor prognosis due to rapid metastasis. These conditions may trigger systemic inflammation, organ dysfunction, and psychological distress. Early diagnosis and effective management are critical to improving patient outcomes and reducing the overall burden on healthcare systems[64].
Pancreatic cancer ranks among the leading causes of cancer-related mortality worldwide. Unfortunately, it is one of the cancers that harbors delayed diagnoses and, in most cases, is discovered in the late stages. However, there are currently no screening programs for pancreatic cancer. FP has been strongly suggested to be involved in pancreatic carcinogenesis[41,65,66].
Recent studies have reported an association between FP and an increased risk of PDAC, beyond the effect of obesity alone[65]. Furthermore, recent studies have demonstrated a direct correlation among FP, pancreatic intraepithelial neoplasia, and ductal adenocarcinoma development[65,67]. Moreover, FP has been associated with a poor prognosis in pancreatic cancer by facilitating cancer dissemination. The pathogenesis of this relationship involves two mechanisms[68].
The first mechanism is linked to obesity, which contributes to oxidative stress and an imbalance in adipokines, thereby creating a chronic low-grade systemic inflammatory state. Persistent inflammation increases the risk of recurrent pancreatitis, a well-established risk factor for pancreatic cancer[69].
The second mechanism involves intrapancreatic fat infiltration, which induces steatopancreatitis, leading to pancreatic cell injury, fibrosis, and ultimately malignant transformation[70,71].
Additionally, emerging evidence has highlighted the role of gut microbiota in the development and progression of pancreatic cancer. Studies have shown that patients with pancreatic cancer exhibit reduced gut microbial diversity, characterized by an increase in potentially pathogenic bacteria, such as Enterobacteriaceae and a decrease in beneficial microbes, such as Bifidobacterium and butyrate-producing bacteria. These findings suggest that restoring a healthy gut microbiota may positively influence disease outcomes in patients with pancreatic cancer[72].
Many studies have observed more extensive pancreatic necrosis in areas adjacent to necrotic fat tissue. This phenomenon is thought to result from the local release of lipase, which promotes localized and systemic fat breakdown, leading to elevated levels of unsaturated fatty acids. These unsaturated fatty acids exert direct cytotoxic effects on the pancreatic acinar cells, contributing to acinar cell injury and necrosis within the pancreatic parenchyma[73,74].
To date, there is no conclusive evidence linking NAFPD as a direct cause of chronic pancreatitis[75].
Pancreatic diseases, including pancreatitis and pancreatic cancer, and metabolic dysfunctions, such as FP, are increasingly prevalent worldwide, with a growing burden linked to lifestyle-related risk factors, such as obesity, poor diet, and sedentary behavior. The global incidence of pancreatic cancer has increased steadily, with over 495000 new cases and more than 466000 deaths reported worldwide in 2020[76].
Similarly, non-alcoholic FP disease is emerging as a common comorbidity in populations with MetS, particularly in regions with high obesity rates. These conditions place a substantial strain on healthcare systems, both in terms of direct costs, such as hospitalization, diagnostics, and long-term treatment, and indirect costs, such as loss of productivity and long-term disability. In the United States, the annual economic burden of pancreatic cancer is estimated to exceed 3 billion United State dollar[77].
Given the increasing prevalence and associated costs, urgent public health interventions, early screening programs, and investments in research are critical for mitigating the global impact of pancreatic diseases.
Several studies have reported an increased incidence of postoperative pancreatic fistula (PF) in patients with NAFPD. Factors, such as increased fat infiltration within the pancreas, soft gland texture, small pancreatic duct diameter, and compromised local blood supply, are believed to contribute to the high risk of PF in this patient population[78,79].
A recent meta-analysis of 27 studies involving 24740 patients systematically evaluated the risks and protective factors associated with postoperative PF. The most significant risk factors identified were BMI greater than 25 kg/m2, pancreatic duct diameter less than 3 mm, and soft pancreatic texture. Although the exact mechanisms linking FP to the development of PF remain unclear, it has been postulated that increased fat infiltration contributes to softer pancreatic consistency. This soft texture is often associated with narrower pancreatic ducts, increasing the likelihood of structural damage during surgical procedures, such as anastomosis and suturing[80].
At present, there is no established treatment regimen for NAFPD, and clinical management has yet to progress beyond the preliminary stages[81].
Diet may play a pivotal role in FP development. A study involving 11 individuals with T2DM demonstrated that a low-calorie diet significantly reduced pancreatic fat content by inducing a negative energy balance, highlighting its relevance to T2DM-associated pancreatic fat accumulation[46].
Furthermore, a clinical trial comparing a Mediterranean diet, characterized by a high unsaturated fat content, with a low-fat diet in individuals with abdominal obesity or dyslipidemia, including those with T2DM, reported a significantly low prevalence of FP in the Mediterranean diet group. These findings underscore the potential of dietary modifications to influence pancreatic fat accumulation and improve metabolic health, particularly in individuals with T2DM[82].
FP can often be reversed by weight reduction[83]. The influence of lifestyle factors, including tobacco and alcohol consumption, on the development of FP, notably alcohol, even at moderate levels, has been linked to increased fat accumulation within pancreatic tissue[84].
The pharmacological treatment for NAFPD remains in the experimental phase, with some therapeutic agents demonstrating efficacy primarily in preclinical animal studies[85,86].
The pharmacological management of pancreatic diseases continues to evolve as both traditional and novel therapeutic options are explored. Conventional treatments include analgesics for pain relief, anti-inflammatory agents for managing pancreatic inflammation, and pancreatic enzyme replacement therapy for exocrine insufficiency[87].
In the context of diabetes associated with pancreatic dysfunction, newer pharmacological agents, such as glucagon-like peptide 1 (GLP-1) receptor agonists and sodium-glucose co-transporter 2 (SGLT-2) inhibitors, have gained attention. GLP-1 agonists not only improve glycemic control but also exhibit potential protective effects on pancreatic β-cell function and reduce pancreatic fat accumulation[88].
Similarly, SGLT-2 inhibitors enhance glycemic control and may indirectly improve metabolic profiles by reducing insulin resistance and promoting weight loss[89].
Understanding the mechanisms of these medications and integrating them into patient-specific treatment plans are essential for optimizing outcomes and potentially modifying the disease course. Medications, such as metformin, have shown potential in improving NAFPD[46].
In one study, the authors suggested that a combination of telmisartan and sitagliptin might help control the prog
GLP-1 receptor agonists are the only pharmacological agents that reduce pancreatic fat content. While the literature on this topic is limited, several studies have reported that 6 months of treatment with exenatide, liraglutide, and dulaglutide improved liver fat content in patients with T2DM but did not significantly alter pancreatic fat content as measured by MRI. As these drugs typically induce mild weight loss, their limited effectiveness in reducing pancreatic fat content may be attributed to this factor[91,92].
Several studies have examined the effects of bariatric surgery and the subsequent significant weight loss on pancreatic fat content. These studies demonstrated a marked reduction in pancreatic fat following surgery, with the decrease occurring independently of changes in liver fat content[93-97].
Furthermore, these studies have shown improvements in β-cell function in response to reduced pancreatic fat[93,95].
Research exploring the effects of physical exercise on FP (NAFPD) remains limited and requires further investigation to clarify its underlying mechanisms and clinical outcomes. Nonetheless, regular physical activity is strongly recommended for individuals with these conditions as it enhances caloric expenditure and basal metabolic rate, contributing to favorable changes in both clinical parameters and biochemical profiles[98].
The pancreas plays a critical role in maintaining glucose homeostasis and regulating insulin secretion in response to nutritional and metabolic changes. Emerging research has indicated that pancreatic fat accumulation is associated with diminished β-cell function and disrupted insulin release, contributing to abnormal glucose metabolism, T2DM, and MetS. Moreover, a significant correlation has been identified between pancreatic steatosis and both the presence and severity of MASLD[47].
The pancreas and liver originate from the same embryonic endoderm, which explains the relationship between FP and MASLD. Current evidence from experimental models and human studies suggests that a bidirectional relationship between FP and MASLD[4,9,99,100].
NAFPD and MASLD share common risk factors, including obesity, dyslipidemia, and diabetes, which contribute to fat accumulation in both organs. Therefore, the effective control of these risk factors may reduce the incidence of both conditions[101].
Several studies have established a significant association between FP, also known as NAFPD, and MetS, a cluster of interrelated conditions including obesity, hypertension, insulin resistance, hyperglycemia, and dyslipidemia. The accumulation of ectopic fat within the pancreatic tissue is believed to impair β-cell function, reduce insulin secretion, and exacerbate systemic insulin resistance[102].
Additionally, lipid infiltration may promote local and systemic inflammation, thereby contributing to metabolic dysfunction. These mechanisms underscore a bidirectional relationship in which MetS may drive pancreatic steatosis, and FP can amplify the risk and severity of metabolic abnormalities. Understanding this interplay is crucial, as it opens the door for targeted lifestyle or pharmacological interventions aimed at reducing pancreatic fat and improving both endocrine and metabolic health outcomes.
A study using post-mortem material from 80 patients identified total pancreatic fat as a significant determinant of MASLD. Specifically, intralobular pancreatic adipose accumulation has been associated with metabolic dysfunction-associated steatohepatitis (MASH), as previously reported[29].
Furthermore, a study employing transabdominal ultrasonography to assess the association between MASH and FP revealed that more than half of individuals with MASH also exhibited FP. Specifically, FP at varying stages was present in 51.2% of patients with MASH compared to 14% of those with normal liver function[103].
In another prospective study, nearly 80% of patients with MASH developed FP, with hepatic steatosis showing a significant association with the condition[84].
A systematic review and meta-analysis revealed that FIP in an unadjusted analysis of healthy individuals was linked to a more than 2.5-fold higher co-prevalence of MASLD and showed a strong positive correlation between pancreatic fat and liver fat content[17].
Several cross-sectional studies[13,25,32,103,104] have demonstrated that MASLD is an independent risk factor for FP. MASLD is linked to severe fat accumulation in the pancreas[28,105-108].
Conversely, a recent meta-analysis of 49329 individuals found that FP is independently associated with MASLD, with a relative risk of 2.49 (95%CI: 2.06-3.02)[92]. Additionally, an FP has been correlated with subclinical atherosclerosis in MASLD patients[109]. Cumulative evidence has established a significant association between a FP and more severe histological features of MASLD[44]. Moreover, FP may play a pivotal role in MASLD progression[108].
Consequently, the presence of FP in patients with MASLD warrants careful attention[110].
The clinical significance of FP or NAFPD remains a subject of ongoing debate. Traditionally considered an incidental finding in imaging studies, increasing evidence suggests that fat accumulation within the pancreatic parenchyma may represent more than a benign anomaly. Characterized by ectopic fat deposition, this condition is associated with impaired insulin secretion, β-cell dysfunction, and a heightened risk of developing MetS and T2DM. These associations raise essential questions about whether FP should be reclassified as a distinct pathological entity that requires targeted clinical attention. Recognizing its potential as a modifiable risk factor could prompt earlier interventions aimed at metabolic regulation and pancreatic health preservation, thereby influencing both diagnostic and treatment strategies in clinical practice[14].
Despite growing research on pancreatic fat accumulation, several challenges and controversies have limited our understanding and management of FP. One major issue is the difficulty of establishing standardized diagnostic criteria. The absence of universally accepted thresholds and reliable imaging protocols hampers clinical diagnoses and research comparability. Moreover, the anatomical location of the pancreas and the variability in imaging techniques contribute to inconsistent assessments.
Another unresolved challenge is the unclear nature of the relationship between FP and the associated metabolic disorders. Although numerous studies have demonstrated correlations among pancreatic fat, obesity, T2DM, and MASLD, a definitive causal link has yet to be established. This uncertainty complicates the interpretation of clinical findings and the development of targeted interventions.
There is currently no consensus on the optimal therapeutic approach for the management of FP. Pharmacological treatments and lifestyle interventions are largely extrapolated from related metabolic conditions, and their direct effect on pancreatic fat has not yet been clearly demonstrated in clinical trials.
Although pancreatic weight correlates with overall body weight, suggesting a link with obesity, the clinical signi
Genetic and molecular pathways play crucial roles in the development and progression of FP, and recent studies have suggested that inherited factors and specific molecular mechanisms may influence fat accumulation within the pancreas. Identifying these pathways could lead to a better understanding of and potentially offer new avenues for treatment. Identifying the molecular pathways that mediate the metabolic effects of FP may provide clinicians with therapeutic targets for the management of patients with MASLD and FP.
Advances in imaging technology have significantly improved our understanding of the pathophysiological relationship between FP and other obesity-related disorders, including MASLD. However, standardized diagnostic criteria are urgently needed to consistently identify and quantify FP in clinical settings. These criteria would ensure greater accuracy and reproducibility, allowing for more reliable comparisons and improved patient management.
Recent advances in biomarker research have identified promising candidates that may serve as indicators of pancreatic health and disease progression. In particular, fibroblast growth factor-1 (FGF-1) has emerged as a notable molecule owing to its roles in cellular repair, proliferation, and tissue regeneration. Studies have suggested that FGF-1 may contribute to the maintenance of pancreatic homeostasis and could serve as a marker for monitoring therapeutic responses in conditions, such as pancreatitis and diabetes[111].
Similarly, lipocalins, a family of small secreted proteins involved in inflammation and immune modulation, are potential early markers of pancreatic injury and metabolic dysfunction[112].
These biomarkers not only offer diagnostic and prognostic value but also open new avenues for personalized treatment approaches. Continued research on their mechanisms and clinical relevance could significantly enhance the early detection and management of pancreatic disorders.
Further studies are required to investigate the causality and progression of FP. Understanding whether FP directly contributes to the development of MASLD or whether it is a consequence of metabolic disturbances will provide valuable insights into its role in disease progression.
Finally, there is growing interest in identifying novel therapeutic targets for managing FP, particularly in MASLD. Investigation of the molecular drivers of fat accumulation in the pancreas could reveal new pharmacological and interventional strategies to prevent or reverse this condition and improve patient outcomes in both liver and pancreatic diseases[43].
NAFPD is an emerging clinical entity that has garnered increasing attention in recent years owing to its potential metabolic and systemic implications. FP has been identified as a significant risk factor for pancreatic cancer. Given the growing body of evidence supporting this association and the recognition that FP is a potentially reversible condition, early detection of individuals with FP is crucial. Implementing targeted management strategies, particularly in patients with MetS and obesity, may help reduce pancreatic steatosis and subsequently lower the risk of developing pancreatic cancer. Healthcare providers should actively encourage individuals with FP to modify other lifestyle factors known to contribute to the risk of pancreatic cancer, such as smoking and excessive alcohol consumption.
| 1. | Yin W, Liao D, Kusunoki M, Xi S, Tsutsumi K, Wang Z, Lian X, Koike T, Fan J, Yang Y, Tang C. NO-1886 decreases ectopic lipid deposition and protects pancreatic beta cells in diet-induced diabetic swine. J Endocrinol. 2004;180:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Lee JS, Kim SH, Jun DW, Han JH, Jang EC, Park JY, Son BK, Kim SH, Jo YJ, Park YS, Kim YS. Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol. 2009;15:1869-1875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 175] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Ogilvie RF. The islands of langerhans in 19 cases of obesity. J Pathol. 1933;37:473-481. [RCA] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 4. | Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011;8:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (2)] |
| 5. | Mahyoub MA, Elhoumed M, Maqul AH, Almezgagi M, Abbas M, Jiao Y, Wang J, Alnaggar M, Zhao P, He S. Fatty infiltration of the pancreas: a systematic concept analysis. Front Med (Lausanne). 2023;10:1227188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Patel S, Bellon EM, Haaga J, Park CH. Fat replacement of the exocrine pancreas. AJR Am J Roentgenol. 1980;135:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Mortelé KJ, Rocha TC, Streeter JL, Taylor AJ. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics. 2006;26:715-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Mathur A, Marine M, Lu D, Swartz-Basile DA, Saxena R, Zyromski NJ, Pitt HA. Nonalcoholic fatty pancreas disease. HPB (Oxford). 2007;9:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Petrov MS, Taylor R. Intra-pancreatic fat deposition: bringing hidden fat to the fore. Nat Rev Gastroenterol Hepatol. 2022;19:153-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 10. | Chang ML. Fatty Pancreas-Centered Metabolic Basis of Pancreatic Adenocarcinoma: From Obesity, Diabetes and Pancreatitis to Oncogenesis. Biomedicines. 2022;10:692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK, Mari A, Heine RJ, Diamant M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 317] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 12. | Rugivarodom M, Geeratragool T, Pausawasdi N, Charatcharoenwitthaya P. Fatty Pancreas: Linking Pancreas Pathophysiology to Nonalcoholic Fatty Liver Disease. J Clin Transl Hepatol. 2022;10:1229-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Wang CY, Ou HY, Chen MF, Chang TC, Chang CJ. Enigmatic ectopic fat: prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J Am Heart Assoc. 2014;3:e000297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Sepe PS, Ohri A, Sanaka S, Berzin TM, Sekhon S, Bennett G, Mehta G, Chuttani R, Kane R, Pleskow D, Sawhney MS. A prospective evaluation of fatty pancreas by using EUS. Gastrointest Endosc. 2011;73:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Milovanovic T, Dragasevic S, Stojkovic Lalosevic M, Zgradic S, Milicic B, Dumic I, Kmezic S, Saponjski D, Antic A, Markovic V, Popovic D. Ultrasonographic Evaluation of Fatty Pancreas in Serbian Patients with Non Alcoholic Fatty Liver Disease-A Cross Sectional Study. Medicina (Kaunas). 2019;55:697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Khoury T, Mari A, Sbeit W. A Novel Clinical Score Predicting the Presence of Fatty Pancreas. J Clin Med. 2021;10:5843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Singh RG, Yoon HD, Poppitt SD, Plank LD, Petrov MS. Ectopic fat accumulation in the pancreas and its biomarkers: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2017;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Sbeit W, Khoury T. Fatty Pancreas Represents a Risk Factor for Acute Pancreatitis: A Pilot Study. Pancreas. 2021;50:990-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Sbeit W, Greener T, Kadah A, Mari A, Goldin E, Mahamid M, Khoury T. Pancreatic and hepatobiliary manifestations of nonalcoholic fatty pancreatic disease: a referral multi-center experience. Eur J Gastroenterol Hepatol. 2021;33:e297-e301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Paul J, Shihaz AVH. Pancreatic Steatosis: A New Diagnosis and Therapeutic Challenge in Gastroenterology. Arq Gastroenterol. 2020;57:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Ramkissoon R, Gardner TB. Pancreatic Steatosis: An Emerging Clinical Entity. Am J Gastroenterol. 2019;114:1726-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Rosso E, Casnedi S, Pessaux P, Oussoultzoglou E, Panaro F, Mahfud M, Jaeck D, Bachellier P. The role of "fatty pancreas" and of BMI in the occurrence of pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg. 2009;13:1845-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Saisho Y. Pancreas Volume and Fat Deposition in Diabetes and Normal Physiology: Consideration of the Interplay Between Endocrine and Exocrine Pancreas. Rev Diabet Stud. 2016;13:132-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Pacifico L, Di Martino M, Anania C, Andreoli GM, Bezzi M, Catalano C, Chiesa C. Pancreatic fat and β-cell function in overweight/obese children with nonalcoholic fatty liver disease. World J Gastroenterol. 2015;21:4688-4695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Aleshina EI, Novikova VP, Gur'eva VA, Burnysheva IA, Usychenko EA. [Hepatic steatosis and fatty pancreas--2 targets of metabolic syndrom in children]. Eksp Klin Gastroenterol. 2014;16-20. [PubMed] |
| 26. | Zhu Z, Yin P. Overweight and obesity: The serious challenge faced by Chinese children and adolescents. J Glob Health. 2023;13:03036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 27. | Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3177] [Cited by in RCA: 2970] [Article Influence: 198.0] [Reference Citation Analysis (1)] |
| 28. | Wang D, Yu XP, Xiao WM, Jiao XP, Wu J, Teng DL, Wu KY, Zhang M, Zhu QT, Liu XN, Ding YB, Lu GT. Prevalence and clinical characteristics of fatty pancreas in Yangzhou, China: A cross-sectional study. Pancreatology. 2018;18:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas. 2010;39:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Kim MK, Chun HJ, Park JH, Yeo DM, Baek KH, Song KH, Chung DJ, Kwon HS. The association between ectopic fat in the pancreas and subclinical atherosclerosis in type 2 diabetes. Diabetes Res Clin Pract. 2014;106:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Tomita Y, Azuma K, Nonaka Y, Kamada Y, Tomoeda M, Kishida M, Tanemura M, Miyoshi E. Pancreatic fatty degeneration and fibrosis as predisposing factors for the development of pancreatic ductal adenocarcinoma. Pancreas. 2014;43:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Lesmana CR, Pakasi LS, Inggriani S, Aidawati ML, Lesmana LA. Prevalence of Non-Alcoholic Fatty Pancreas Disease (NAFPD) and its risk factors among adult medical check-up patients in a private hospital: a large cross sectional study. BMC Gastroenterol. 2015;15:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Lê KA, Ventura EE, Fisher JQ, Davis JN, Weigensberg MJ, Punyanitya M, Hu HH, Nayak KS, Goran MI. Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care. 2011;34:485-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Szczepaniak LS, Victor RG, Mathur R, Nelson MD, Szczepaniak EW, Tyer N, Chen I, Unger RH, Bergman RN, Lingvay I. Pancreatic steatosis and its relationship to β-cell dysfunction in humans: racial and ethnic variations. Diabetes Care. 2012;35:2377-2383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Jermendy G, Kolossváry M, Drobni Z, Papp S, Jermendy ÁL, Panajotu A, Dudás I, Tárnoki ÁD, Tárnoki DL, Voros S, Merkely B, Maurovich-Horvat P. Environmental Factors Slightly Outweigh Genetic Influences in the Development of Pancreatic Lipid Accumulation: A Classical Twin Study. Metab Syndr Relat Disord. 2020;18:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Stuart CE, Ko J, Modesto AE, Alarcon Ramos GC, Bharmal SH, Cho J, Singh RG, Petrov MS. Implications of Tobacco Smoking and Alcohol Consumption on Ectopic Fat Deposition in Individuals After Pancreatitis. Pancreas. 2020;49:924-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Li S, Su L, Lv G, Zhao W, Chen J. Transabdominal ultrasonography of the pancreas is superior to that of the liver for detection of ectopic fat deposits resulting from metabolic syndrome. Medicine (Baltimore). 2017;96:e8060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Villarroya F, Domingo P, Giralt M. Drug-induced lipotoxicity: lipodystrophy associated with HIV-1 infection and antiretroviral treatment. Biochim Biophys Acta. 2010;1801:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Fernandes-Santos C, Evangelista Carneiro R, de Souza Mendonca L, Barbosa Aguila M, Mandarim-de-Lacerda CA. Rosiglitazone aggravates nonalcoholic Fatty pancreatic disease in C57BL/6 mice fed high-fat and high-sucrose diet. Pancreas. 2009;38:e80-e86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Wilson JS, Colley PW, Sosula L, Pirola RC, Chapman BA, Somer JB. Alcohol causes a fatty pancreas. A rat model of ethanol-induced pancreatic steatosis. Alcohol Clin Exp Res. 1982;6:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Takahashi M, Hori M, Ishigamori R, Mutoh M, Imai T, Nakagama H. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 2018;109:3013-3023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 42. | Ou HY, Wang CY, Yang YC, Chen MF, Chang CJ. The association between nonalcoholic fatty pancreas disease and diabetes. PLoS One. 2013;8:e62561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 43. | Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837-e841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 312] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 44. | Mirrakhimov AE. Nonalcoholic fatty pancreatic disease and cardio-metabolic risk: is there is a place for obstructive sleep apnea? Cardiovasc Diabetol. 2014;13:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Taylor R. Type 2 diabetes: etiology and reversibility. Diabetes Care. 2013;36:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 46. | Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506-2514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 877] [Cited by in RCA: 787] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 47. | Lingvay I, Esser V, Legendre JL, Price AL, Wertz KM, Adams-Huet B, Zhang S, Unger RH, Szczepaniak LS. Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab. 2009;94:4070-4076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 48. | Marks WM, Filly RA, Callen PW. Ultrasonic evaluation of normal pancreatic echogenicity and its relationship to fat deposition. Radiology. 1980;137:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Worthen NJ, Beabeau D. Normal pancreatic echogenicity: relation to age and body fat. AJR Am J Roentgenol. 1982;139:1095-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137:727-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 217] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Quinn SF, Gosink BB. Characteristic sonographic signs of hepatic fatty infiltration. AJR Am J Roentgenol. 1985;145:753-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Alzaid A, Aideyan O, Nawaz S. The size of the pancreas in diabetes mellitus. Diabet Med. 1993;10:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Majumder S, Philip NA, Takahashi N, Levy MJ, Singh VP, Chari ST. Fatty Pancreas: Should We Be Concerned? Pancreas. 2017;46:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Yoon JH, Lee JM, Lee KB, Kim SW, Kang MJ, Jang JY, Kannengiesser S, Han JK, Choi BI. Pancreatic Steatosis and Fibrosis: Quantitative Assessment with Preoperative Multiparametric MR Imaging. Radiology. 2016;279:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 55. | Idilman IS, Tuzun A, Savas B, Elhan AH, Celik A, Idilman R, Karcaaltincaba M. Quantification of liver, pancreas, kidney, and vertebral body MRI-PDFF in non-alcoholic fatty liver disease. Abdom Imaging. 2015;40:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 56. | Khoury T, Asombang AW, Berzin TM, Cohen J, Pleskow DK, Mizrahi M. The Clinical Implications of Fatty Pancreas: A Concise Review. Dig Dis Sci. 2017;62:2658-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Krill JT, Szafron D, Elhanafi S, Hussein MS, Patel K, Raijman I, Fisher W, El Serag HB, Othman MO. Endoscopic Ultrasound Finding of Diffuse Echogenicity in the Pancreas, Is It Relevant? Dig Dis Sci. 2022;67:3244-3251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Kim SY, Kim H, Cho JY, Lim S, Cha K, Lee KH, Kim YH, Kim JH, Yoon YS, Han HS, Kang HS. Quantitative assessment of pancreatic fat by using unenhanced CT: pathologic correlation and clinical implications. Radiology. 2014;271:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 59. | Yamazaki H, Tauchi S, Kimachi M, Dohke M, Hanawa N, Kodama Y, Katanuma A, Yamamoto Y, Fukuhara S, Fukuma S. Independent association between prediabetes and future pancreatic fat accumulation: a 5-year Japanese cohort study. J Gastroenterol. 2018;53:873-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Yamazaki H, Tauchi S, Wang J, Dohke M, Hanawa N, Kodama Y, Katanuma A, Saisho Y, Kamitani T, Fukuhara S, Yamamoto Y. Longitudinal association of fatty pancreas with the incidence of type-2 diabetes in lean individuals: a 6-year computed tomography-based cohort study. J Gastroenterol. 2020;55:712-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 61. | Hori M, Takahashi M, Hiraoka N, Yamaji T, Mutoh M, Ishigamori R, Furuta K, Okusaka T, Shimada K, Kosuge T, Kanai Y, Nakagama H. Association of pancreatic Fatty infiltration with pancreatic ductal adenocarcinoma. Clin Transl Gastroenterol. 2014;5:e53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 62. | Leung PS. Common pancreatic disease. Adv Exp Med Biol. 2010;690:29-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Madro A. Malnutrition in Chronic Pancreatitis: Causes, Assessment Methods, and Therapeutic Management. Can J Gastroenterol Hepatol. 2020;2020:8875487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1352] [Article Influence: 169.0] [Reference Citation Analysis (49)] |
| 65. | Hori M, Mutoh M, Imai T, Nakagama H, Takahashi M. Possible involvement of pancreatic fatty infiltration in pancreatic carcinogenesis. JOP. 2016;17:166‐175. |
| 66. | Wang H, Maitra A, Wang H. Obesity, Intrapancreatic Fatty Infiltration, and Pancreatic Cancer. Clin Cancer Res. 2015;21:3369-3371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Rebours V, Gaujoux S, d'Assignies G, Sauvanet A, Ruszniewski P, Lévy P, Paradis V, Bedossa P, Couvelard A. Obesity and Fatty Pancreatic Infiltration Are Risk Factors for Pancreatic Precancerous Lesions (PanIN). Clin Cancer Res. 2015;21:3522-3528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (2)] |
| 68. | Mathur A, Hernandez J, Shaheen F, Shroff M, Dahal S, Morton C, Farrior T, Kedar R, Rosemurgy A. Preoperative computed tomography measurements of pancreatic steatosis and visceral fat: prognostic markers for dissemination and lethality of pancreatic adenocarcinoma. HPB (Oxford). 2011;13:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Annett S, Moore G, Robson T. Obesity and Cancer Metastasis: Molecular and Translational Perspectives. Cancers (Basel). 2020;12:3798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 70. | Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 71. | Assimacopoulos-Jeannet F. Fat storage in pancreas and in insulin-sensitive tissues in pathogenesis of type 2 diabetes. Int J Obes Relat Metab Disord. 2004;28 Suppl 4:S53-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Schepis T, De Lucia SS, Nista EC, Manilla V, Pignataro G, Ojetti V, Piccioni A, Gasbarrini A, Franceschi F, Candelli M. Microbiota in Pancreatic Diseases: A Review of the Literature. J Clin Med. 2021;10:5920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 73. | Acharya C, Cline RA, Jaligama D, Noel P, Delany JP, Bae K, Furlan A, Baty CJ, Karlsson JM, Rosario BL, Patel K, Mishra V, Dugampudi C, Yadav D, Navina S, Singh VP. Fibrosis reduces severity of acute-on-chronic pancreatitis in humans. Gastroenterology. 2013;145:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 74. | Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, Durgampudi C, Karlsson JM, Lee K, Bae KT, Furlan A, Behari J, Liu S, McHale T, Nichols L, Papachristou GI, Yadav D, Singh VP. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107ra110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 335] [Article Influence: 23.9] [Reference Citation Analysis (2)] |
| 75. | Acharya C, Navina S, Singh VP. Role of pancreatic fat in the outcomes of pancreatitis. Pancreatology. 2014;14:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68689] [Article Influence: 13737.8] [Reference Citation Analysis (201)] |
| 77. | Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33:88-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 78. | Akamatsu N, Sugawara Y, Komagome M, Shin N, Cho N, Ishida T, Ozawa F, Hashimoto D. Risk factors for postoperative pancreatic fistula after pancreaticoduodenectomy: the significance of the ratio of the main pancreatic duct to the pancreas body as a predictor of leakage. J Hepatobiliary Pancreat Sci. 2010;17:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, Lévy P, Sauvanet A, Ruszniewski P, Belghiti J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 80. | Zhang B, Yuan Q, Li S, Xu Z, Chen X, Li L, Shang D. Risk factors of clinically relevant postoperative pancreatic fistula after pancreaticoduodenectomy: A systematic review and meta-analysis. Medicine (Baltimore). 2022;101:e29757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 81. | Pinte L, Balaban DV, Băicuş C, Jinga M. Non-alcoholic fatty pancreas disease - practices for clinicians. Rom J Intern Med. 2019;57:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Gepner Y, Shelef I, Schwarzfuchs D, Zelicha H, Tene L, Yaskolka Meir A, Tsaban G, Cohen N, Bril N, Rein M, Serfaty D, Kenigsbuch S, Komy O, Wolak A, Chassidim Y, Golan R, Avni-Hassid H, Bilitzky A, Sarusi B, Goshen E, Shemesh E, Henkin Y, Stumvoll M, Blüher M, Thiery J, Ceglarek U, Rudich A, Stampfer MJ, Shai I. Effect of Distinct Lifestyle Interventions on Mobilization of Fat Storage Pools: CENTRAL Magnetic Resonance Imaging Randomized Controlled Trial. Circulation. 2018;137:1143-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 83. | Dreiling DA, Elsbach P, Schaffner F, Schwartz IL. The effect of restriction of protein and total calories on pancreatic function in obese patients. Gastroenterology. 1962;42:686-690. [PubMed] [DOI] [Full Text] |
| 84. | Al-Haddad M, Khashab M, Zyromski N, Pungpapong S, Wallace MB, Scolapio J, Woodward T, Noh K, Raimondo M. Risk factors for hyperechogenic pancreas on endoscopic ultrasound: a case-control study. Pancreas. 2009;38:672-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 85. | Souza-Mello V. Hepatic structural enhancement and insulin resistance amelioration due to AT1 receptor blockade. World J Hepatol. 2017;9:74-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Zhang W, Miao J, Li P, Wang Y, Zhang Y. Up-regulation of components of the renin-angiotensin system in liver fibrosis in the rat induced by CCL4. Res Vet Sci. 2013;95:54-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Erchinger F, Tjora E, Nordaas IK, Dimcevski G, Olesen SS, Jensen N, Dahl EE, Borch A, Nøjgaard C, Novovic S, Barauskas G, Ignatavicius P, Vujasinovic M, Lőhr M, Laukkarinen J, Parhiala M, Drewes AM, Engjom T. Pancreatic enzyme treatment in chronic pancreatitis: Quality of management and adherence to guidelines-A cross-sectional observational study. United European Gastroenterol J. 2022;10:844-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation. 2017;136:849-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 430] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 89. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG Outcome Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8748] [Article Influence: 795.3] [Reference Citation Analysis (2)] |
| 90. | Souza-Mello V, Gregório BM, Relvas-Lucas B, da Silva Faria T, Aguila MB, Mandarim-de-Lacerda CA. Pancreatic ultrastructural enhancement due to telmisartan plus sitagliptin treatment in diet-induced obese C57BL/6 mice. Pancreas. 2011;40:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 91. | Dutour A, Abdesselam I, Ancel P, Kober F, Mrad G, Darmon P, Ronsin O, Pradel V, Lesavre N, Martin JC, Jacquier A, Lefur Y, Bernard M, Gaborit B. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab. 2016;18:882-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 92. | Vanderheiden A, Harrison LB, Warshauer JT, Adams-Huet B, Li X, Yuan Q, Hulsey K, Dimitrov I, Yokoo T, Jaster AW, Pinho DF, Pedrosa I, Lenkinski RE, Pop LM, Lingvay I. Mechanisms of Action of Liraglutide in Patients With Type 2 Diabetes Treated With High-Dose Insulin. J Clin Endocrinol Metab. 2016;101:1798-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Gaborit B, Abdesselam I, Kober F, Jacquier A, Ronsin O, Emungania O, Lesavre N, Alessi MC, Martin JC, Bernard M, Dutour A. Ectopic fat storage in the pancreas using 1H-MRS: importance of diabetic status and modulation with bariatric surgery-induced weight loss. Int J Obes (Lond). 2015;39:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 94. | Steven S, Hollingsworth KG, Small PK, Woodcock SA, Pucci A, Aribisala B, Al-Mrabeh A, Daly AK, Batterham RL, Taylor R. Weight Loss Decreases Excess Pancreatic Triacylglycerol Specifically in Type 2 Diabetes. Diabetes Care. 2016;39:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 95. | Honka H, Koffert J, Hannukainen JC, Tuulari JJ, Karlsson HK, Immonen H, Oikonen V, Tolvanen T, Soinio M, Salminen P, Kudomi N, Mari A, Iozzo P, Nuutila P. The effects of bariatric surgery on pancreatic lipid metabolism and blood flow. J Clin Endocrinol Metab. 2015;100:2015-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 96. | Umemura A, Sasaki A, Nitta H, Baba S, Ando T, Kajiwara T, Ishigaki Y. Pancreas volume reduction and metabolic effects in Japanese patients with severe obesity following laparoscopic sleeve gastrectomy. Endocr J. 2017;64:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Hui SCN, Wong SKH, Ai Q, Yeung DKW, Ng EKW, Chu WCW. Observed changes in brown, white, hepatic and pancreatic fat after bariatric surgery: Evaluation with MRI. Eur Radiol. 2019;29:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Silva LLSE, Fernandes MSS, Lima EA, Stefano JT, Oliveira CP, Jukemura J. Fatty Pancreas: Disease or Finding? Clinics (Sao Paulo). 2021;76:e2439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Wagner R, Eckstein SS, Yamazaki H, Gerst F, Machann J, Jaghutriz BA, Schürmann A, Solimena M, Singer S, Königsrainer A, Birkenfeld AL, Häring HU, Fritsche A, Ullrich S, Heni M. Metabolic implications of pancreatic fat accumulation. Nat Rev Endocrinol. 2022;18:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 100. | Filippatos TD. Non-Alcoholic Fatty Pancreas Disease: A Diagnosis of Increasing Importance. Angiology. 2022;73:495-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 101. | El-badawy RM, Yousef AF, Elkholy HR, Sadoma AM. Studying the Association between Non-Alcoholic Fatty Pancreatic Disease and Non-Alcoholic Fatty Liver Disease. Benha Med J. 2020;37. [DOI] [Full Text] |
| 102. | van der Zijl NJ, Goossens GH, Moors CC, van Raalte DH, Muskiet MH, Pouwels PJ, Blaak EE, Diamant M. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab. 2011;96:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 103. | Uygun A, Kadayifci A, Demirci H, Saglam M, Sakin YS, Ozturk K, Polat Z, Karslioglu Y, Bolu E. The effect of fatty pancreas on serum glucose parameters in patients with nonalcoholic steatohepatitis. Eur J Intern Med. 2015;26:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 104. | Berger Z, Orellana F, Cocio R, Torres F, Simian D, Araneda G, Toledo P. Pancreatic steatosis: A frequent finding in a Chilean population. Rev Gastroenterol Mex (Engl Ed). 2023;88:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 105. | Weng S, Zhou J, Chen X, Sun Y, Mao Z, Chai K. Prevalence and factors associated with nonalcoholic fatty pancreas disease and its severity in China. Medicine (Baltimore). 2018;97:e11293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 106. | Targher G, Rossi AP, Zamboni GA, Fantin F, Antonioli A, Corzato F, Bambace C, Pozzi Mucelli R, Zamboni M. Pancreatic fat accumulation and its relationship with liver fat content and other fat depots in obese individuals. J Endocrinol Invest. 2012;35:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 107. | Bi Y, Wang JL, Li ML, Zhou J, Sun XL. The association between pancreas steatosis and metabolic syndrome: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35:e3142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 108. | Zhang CL, Wang JJ, Li JN, Yang Y. Nonalcoholic fatty pancreas disease: An emerging clinical challenge. World J Clin Cases. 2021;9:6624-6638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Ozturk K, Dogan T, Celikkanat S, Ozen A, Demirci H, Kurt O, Turker T, Yilmaz Y, Uygun A. The association of fatty pancreas with subclinical atherosclerosis in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2018;30:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Zhao ZZ, Xin LL, Xia JH, Yang SL, Chen YX, Li K. Long-term High-fat High-sucrose Diet Promotes Enlarged Islets and β-Cell Damage by Oxidative Stress in Bama Minipigs. Pancreas. 2015;44:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1515] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 112. | Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318 (Pt 1):1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1261] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/