Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.106591
Revised: April 12, 2025
Accepted: June 10, 2025
Published online: December 20, 2025
Processing time: 156 Days and 0.5 Hours
Neoadjuvant therapy can reduce the size of gastroesophageal tumors to the extent that they are no longer macroscopically visible. This may increase the risk of microscopic-positive resection margins. One potential method to reduce this uncertainty could be the preoperative endoscopic marking of proximal tumor margins with BioXmark®, a novel liquid fiducial marker. This study aimed to report the initial experiences of the first ten patients marked with BioXmark®.
To evaluate the visibility of BioXmark® on ultrasound after preoperative marking of the proximal resection line of an esophageal tumor.
The circumference of the esophagus was endoscopically marked preoperatively with a fiducial marker in four quadrants, 5 cm proximal to the tumor. During the surgery, the surgeon’s proposed proximal resection line was marked. Next, an ultrasound probe was used to identify the previously placed fiducial markers, and its placement was marked. The difference between the surgeon’s proposed resection line and the fiducial marker was measured intraoperatively and subsequently examined with respect to the resection margin and status.
BioXmark® was implanted in ten patients, 5 cm proximal to the tumor. The surgeon’s proposed resection line was positioned 2-6 cm proximally to the surgical marker line. Technical success of injecting the fiducial marker was achieved in all ten patients. In six patients, the marker was successfully identified intraoperatively on ultrasound. No peri- or postoperative adverse events related to BioXmark® implantation were found.
Excellent technical success with the implantation of the fiducial surgical marker was achieved, but limited intraoperative visibility on ultrasound was achieved. Further studies are required to optimize its clinical application.
Core Tip: This study explores the use of the liquid fiducial marker BioXmark® to enhance precision in cancer surgery. BioXmark® was injected 5 cm proximal to the tumor duringpreoperative nasal gastroscopy. The injected BioXmark® was subsequently identified in vivo using perioperative ultrasound. By improving intraoperative visualization of tumor margins, fiducial markers may increase resection precision and the likelihood of achieving an R0 resection, potentially reducing patient morbidity and mortality. The marker's technical feasibility and safety were demonstrated, although further studies are required to optimize its visibility and clinical application.
- Citation: Solstad TU, Mucha AW, Olsen AA, Grossjohann H, Achiam MP. Preoperative marking of the proximal resection margin in esophageal cancer with a surgical fiducial marker: First experiences. World J Methodol 2025; 15(4): 106591
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/106591.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.106591
Gastroesophageal cancer is one of the leading causes of cancer-related deaths worldwide, and its prevalence is increasing[1,2]. In Denmark, approximately 900 people are diagnosed annually, the majority of tumors being gastroesophageal junction adenocarcinomas (GEJ AC)[3]. The Danish national guidelines for the surgical treatment of GEJ AC is an R0 resection with a proximal resection margin at least 5 cm from the tumor, measured in vivo[4]. These recommendations are based on a large study that showed a significantly increased survival in patients with a proximal resection margin larger than 3.8 cm compared to those with a resection margin less than 3.8 cm[5]. This survival difference was identified only by observing patients who had received an R0 resection. Neoadjuvant chemotherapy often leads to tumor shrinkage, which may be so significant that the tumor is no longer macroscopically visible. Thus, the surgeon must digitally palpate the area where the tumor has been described, and measure and set the resection margin with an educated estimate. However, no studies have confirmed whether this subjective method achieves the recommended 5 cm margins. Precise tumor location is essential to achieve an R0 resection while ensuring optimal perfusion, and the current methods to achieve this are prone to error.
At Copenhagen University Hospital Rigshospitalet, we introduced a liquid fiducial marker to mark the proximal resection line 5 cm proximal to the tumor in vivo. The liquid fiducial marker was implanted during preoperative gastroscopy to ensure an acceptable resection margin. BioXmark® (Nanovi A/S, Lyngby, Denmark) is a liquid injectable and adherent fiducial marker used for radiographic marking of soft tissue. BioXmark® has shown promising results in image-guided adaptive radiotherapy for cancers of the lungs, bladder, rectum, and esophagus[6-10].
This study aimed to collect real-world data from the first ten patients in whom BioXmark® was used to preoperatively mark the proximal resection margin of GEJ tumors. To our knowledge, this is the first study on this application.
This was a quality assurance study on prospectively collected data from the first ten consecutive patients undergoing esophageal resection for GEJ tumors with tumor margins marked by BioXmark®. This study was conducted at a single Danish tertiary referral center for upper gastrointestinal malignancies, covering a population of 2.8 million inhabitants. As BioXmark® was implemented as a standard supplement to the standard operating procedure for esophagectomies at Rigshospitalet at the time and the present study was a quality assurance study, ethical committee approval was not required, no inclusion and exclusion criteria were determined, and no control group was included. At the time of referral to Rigshospitalet, all patients were asked to sign a consent form to review their data and health information for quality control. This study was approved as a quality assurance project by the hospital manager, The Danish Data Protection Agency (P-2021-800), and the Regional Committee for the Capital Region in Denmark (H-21041919). This study was registered at ClinicalTrials.gov (ID: NCT05069766).
The primary outcome of this study was to describe our experience using a liquid fiducial marker to preoperatively mark the proximal resection line of the tumor margin in patients with GEJ tumors. Moreover, we aimed to describe the performance of BioXmark® in terms of visibility during surgery using ultrasound. The secondary outcome was to confirm the safety of BioXmark when used according to the label regarding adverse events related to implantation.
Clinical patient data and information about BioXmark® implantation and subsequent operation were retrieved from electronic health records (Sundhedsplatformen, Epic Systems Corporation, Verona, Wisconsin, United States). Patient records were evaluated by Andreas W Mucha and Trygve U Solstad, and unclear cases were discussed with August A Olsen and Michael P Achiam. Electronic patient records were individually reviewed, and information collected included age, sex, American Society of Anesthesiologists score, information regarding preoperative endoscopy examinations (tumor location, tumor distance from incisors), information about neoadjuvant treatment, and histological tumor type. Statistical analysis in this study was primarily descriptive, using means, standard deviations, and percentages to summarize the data.
BioXmark consists of iodinated sucrose acetate isobutyrate and sucrose acetate isobutyrate mixed with ethanol. After implantation, ethanol diffuses out of the marker and into the surrounding tissue, where it is metabolized, thus increasing marker viscosity. This results in a highly viscous and sticky gel-like marker[11]. BioXmark® can be implanted with standard endoscopic equipment and will form sticky and soft markers in vivo that are adaptive to the surrounding tissue. Owing to the proprietary iodine-based contrast agent, the marker is visible on numerous imaging modalities, including X-ray, ultrasound, and magnetic resonance imaging (MRI)[11].
Placement of the fiducial marker: Three upper gastrointestinal surgery specialists individually placed the fiducial marker in ten patients. The location of the GEJ tumor was preoperatively described in cm from the incisors as seen on the centimeter marking on the scope at an index gastroscopy. After induction, the specialist performed a nasal gastroscopy. The tumor was endoscopically identified based on the aforementioned preoperative measurements, and the scope was retracted 5 cm proximal to the tumor. Any segment of Barrett’s dysplasia or otherwise pathologic finding was considered, and the border of injection was moved accordingly. An injection of 1–2 mL isotonic saline was placed in the submucosal layer of the esophagus in four quadrants using a standard 10 mL syringe of isotonic saline and a 4 mm Novaject injection needle (Vytil, Bagnolet, France). Next, the saline syringe was switched to a 5 mL standard plastic syringe filled with 1 mL BioXmark®, which was used to fill the dead space of the injection needle. Subsequently, another vial of 1 mL BioXmark® was filled into a 1 mL Luer Lock micro-dosing syringe (Vlow Medical, JC Eindhoven, The Netherlands). The needle was retracted into the lumen, and the remaining isotonic saline was flushed into the lumen. At the visual cue of viscous liquid emerging from the tip of the injection needle inside the lumen, 60–100 μL of the fiducial marker was injected into each of the four submucosal saline deposits. A minimum volume of 60 μL per depot was recommended by the manufacturer. To ensure that a satisfactory depot was placed, defined as a clear visual bulge after injection, the amount of the fiducial marker required was adjusted on a case-by-case basis by the operating consultant surgeon. Finally, the injection needle and nasal scope were retracted, a nasogastric tube was placed, and standard hybrid robot-assisted or open Ivor-Lewis esophagectomy was performed.
Surgical procedure: A standard Ivor-Lewis esophagectomy technique, either as a hybrid or as an open procedure, was performed for each patient. The operation was initiated with the abdominal part, either open or robotic-assisted, and subsequently, a right thoracotomy was performed in all cases. Subtotal esophagectomy was performed with gastric pull-up and stapled gastroesophageal anastomosis. Lymphadenectomy was performed 2-field with D2 resection in the abdomen, dissection of the truncal celiac nodes, and en-bloc mediastinal lymphadenectomy. Paraesophageal and subcarinal lymph nodes were also removed, but cervical lymphadenectomy was not performed[12]. The patients received anesthetic management according to standard clinical practice.
Locating BioXmark® and measuring distance to the tumor: Before esophageal transection, the surgeon marked the proposed proximal resection line using a sterile pen. This estimation was based on the macroscopic evaluation of tumor extension, digital palpation of the area, and guidelines stating that the resection must be placed at least 5 cm proximal to the tumor. Next, using a TE7 Portable Ultrasound System (Mindray Medical International Limited, Shenzhen, Guangdong, China) with a T-probe and sterile draping, the exterior of the esophagus was scanned in vivo. If the BioXmark® was identified, a sterile marker was used to mark the area. The distance from the surgeon's proposed resection line to the fiducial marker was measured. Resection was performed on the proposed resection line determined by the surgeon. The resected specimen from all ten patients was transported to a nearby sterile table and scanned ex vivo using an ultrasound probe. The exterior of the esophagus was scanned first; then, the resected esophagus was cut with scissors, and the lumen was scanned. If the fiducial marker could not be identified through ultrasound by the surgeon, the operation was continued as planned, with the surgeon performing the resection as originally proposed.
A total of ten patients with GEJ tumors undergoing open or robotic Ivor Lewis esophagectomy were included in the period from February 2022 to August 2022. The baseline characteristics of the patients are shown in Table 1. All ten patients received an R0 resection at the proximal border, with eight cases identified as adenocarcinomas, one mixed neuroendocrine non-neuroendocrine neoplasm, and one mucoepidermoid carcinoma. Seven tumors were palpable in vivo, with a mean tumor size of 32.7 mm. The mean microscopic distance to the proximal tumor resection line was 37.3 mm, while the mean macroscopic distance was 45.3 mm, respectively. Barrett’s dysplasia was present in two out of ten resected specimens. Table 2 provides detailed histopathology and surgical procedure specifications.
| Characteristic | Study population n = 10 |
| Tumor type: Adenocarcinoma | 8 (80) |
| Tumor type: Mixed neuroendocrine non-neuroendocrine neoplasm | 1 (10) |
| Tumor type: Mucoepidermoid carcinoma | 1 (10) |
| pT11 | 4 (40) |
| pT2 | 1 (10) |
| pT3 | 5 (50) |
| pN01 | 5 (50) |
| pN1 | 3 (30) |
| pN2 | 2 (20) |
| Tumor regression scoring according to Mandard, 2 | 3 (30) |
| Tumor regression scoring according to Mandard, 3 | 4 (40) |
| Tumor regression scoring according to Mandard, 4 | 1 (10) |
| Tumor regression scoring according to Mandard, 5 | 1 (10) |
| No neoadjuvant treatment | 1 (10) |
| Tumor size in millimeters | 32.7 ± 23.8 |
| Palpable tumor, yes | 7 (70) |
| Palpable tumor, no | 3 (30) |
| Microscopic distance to proximal tumor resection line, mm | 37.3 ± 17.9 |
| Macroscopic distance to proximal tumor resection line, mm | 45.3 ± 17.3 |
| R0 resection | 10 (100) |
| R1 resection | 0 (0) |
| Barrett’s dysplasia in resected tissue | 2 (20) |
| No Barrett’s dysplasia in resected tissue | 8 (80) |
The mean distance from the incisors to the proximal tumor border was 39.3 cm. In total, 40 fiducial markers were implanted, with four markers in each patient, corresponding to one marker in each of the four horizontal quadrants in the esophagus circumference. A mean volume of 82 µL BioXmark® was used per implantation. Table 3 provides further implantation details, including information on perioperative BioXmark® visibility.
| Characteristic | Study population n = 10 |
| Distance from incisors to proximal tumor border, cm | 39.3 ± 5.8 |
| Distance from surgeon’s proposed proximal resection line to BioXmark®, cm1 | 3.8 (± 1.1) |
| Number of implantations per patient | 4 |
| Amount of BioXmark® per implantation, μL | 82 ± 17.5 |
| BioXmark® visible on intraoperative ultrasound in vivo | 6 (60) |
| BioXmark® not visible or missing data on intraoperative ultrasound in vivo | 4 (40) |
| BioXmark® visible on intraoperative ultrasound ex vivo | 3 (30) |
| BioXmark® not visible or missing data on intraoperative ultrasound ex vivo | 7 (70) |
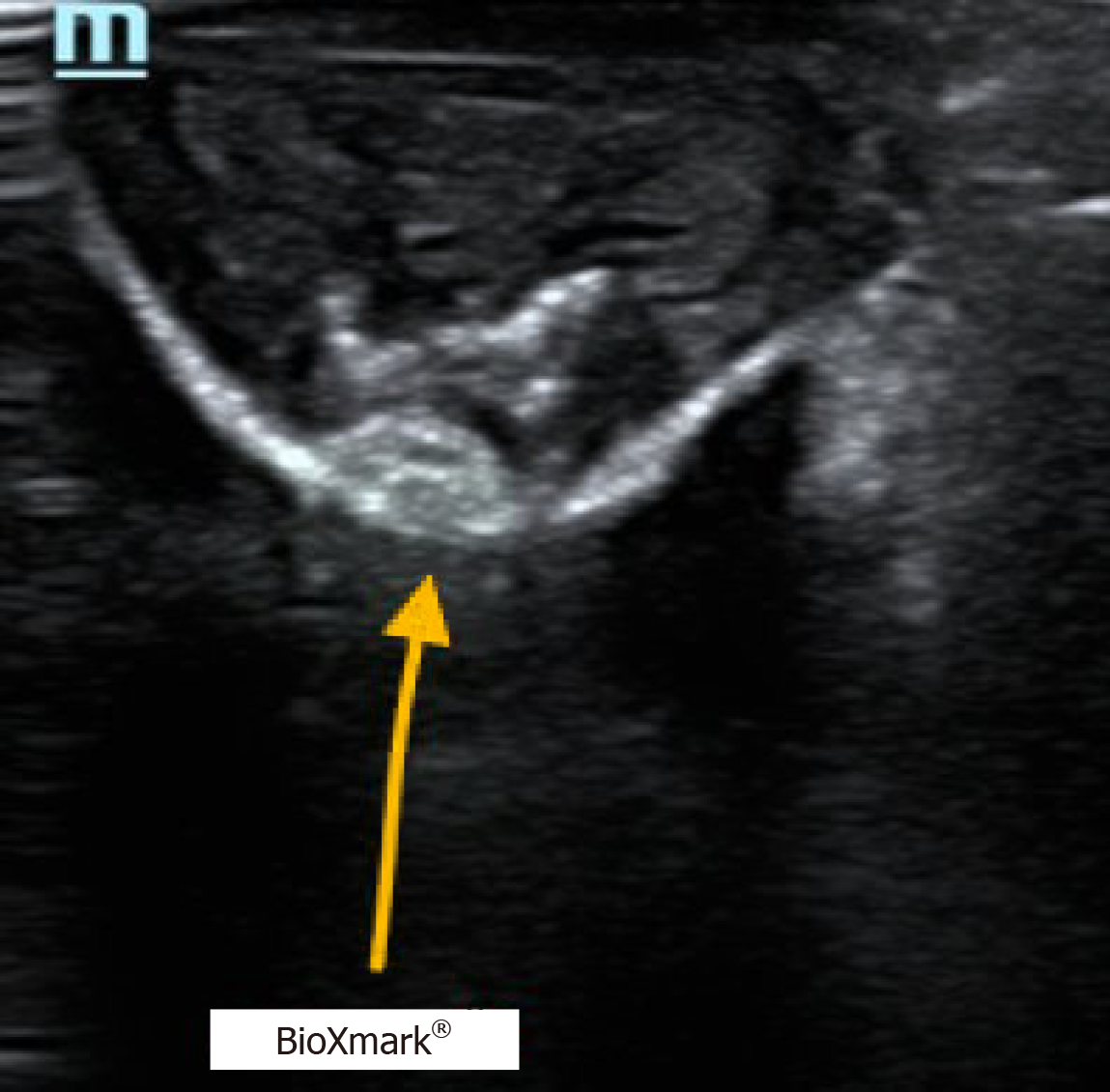
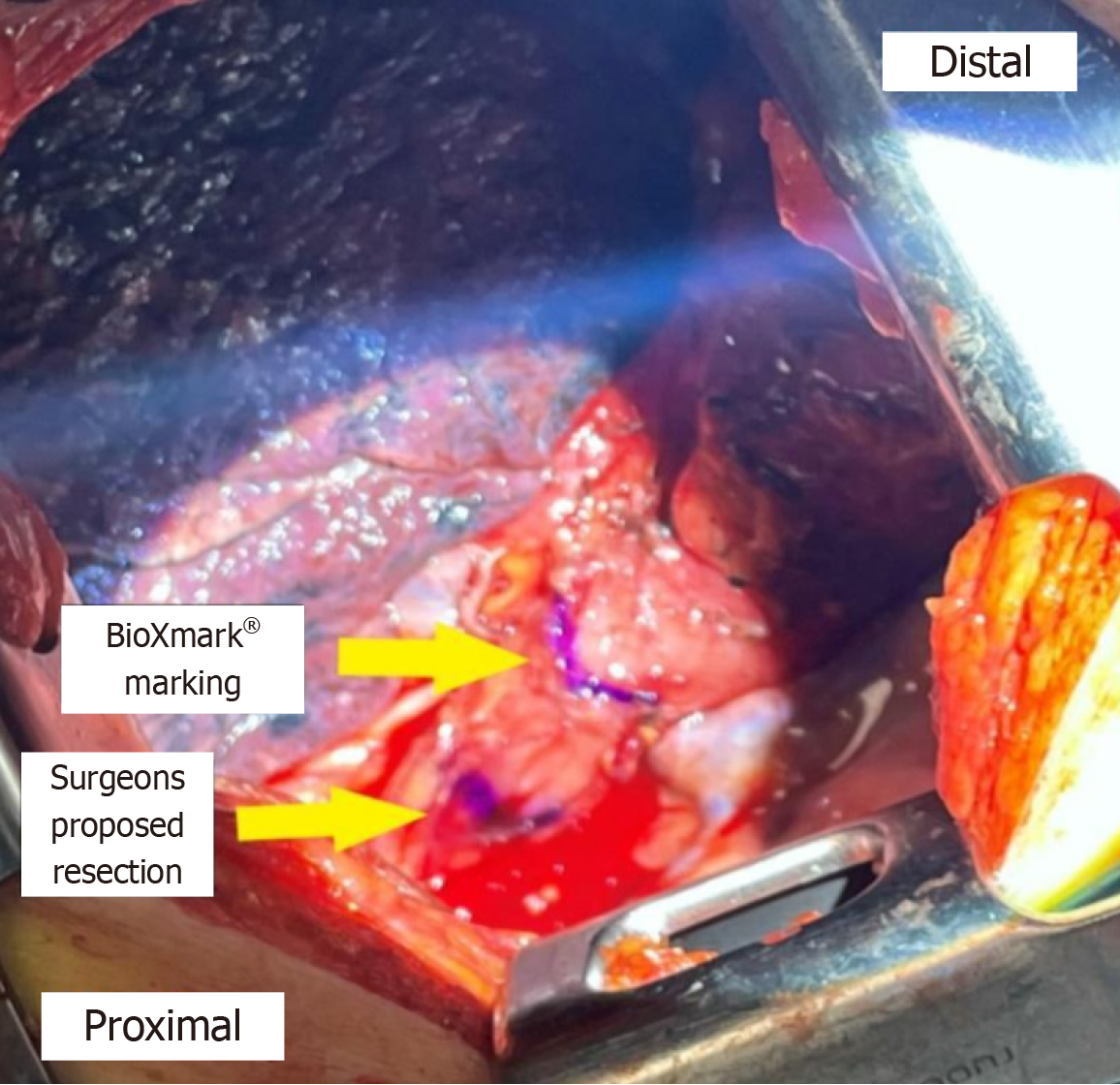
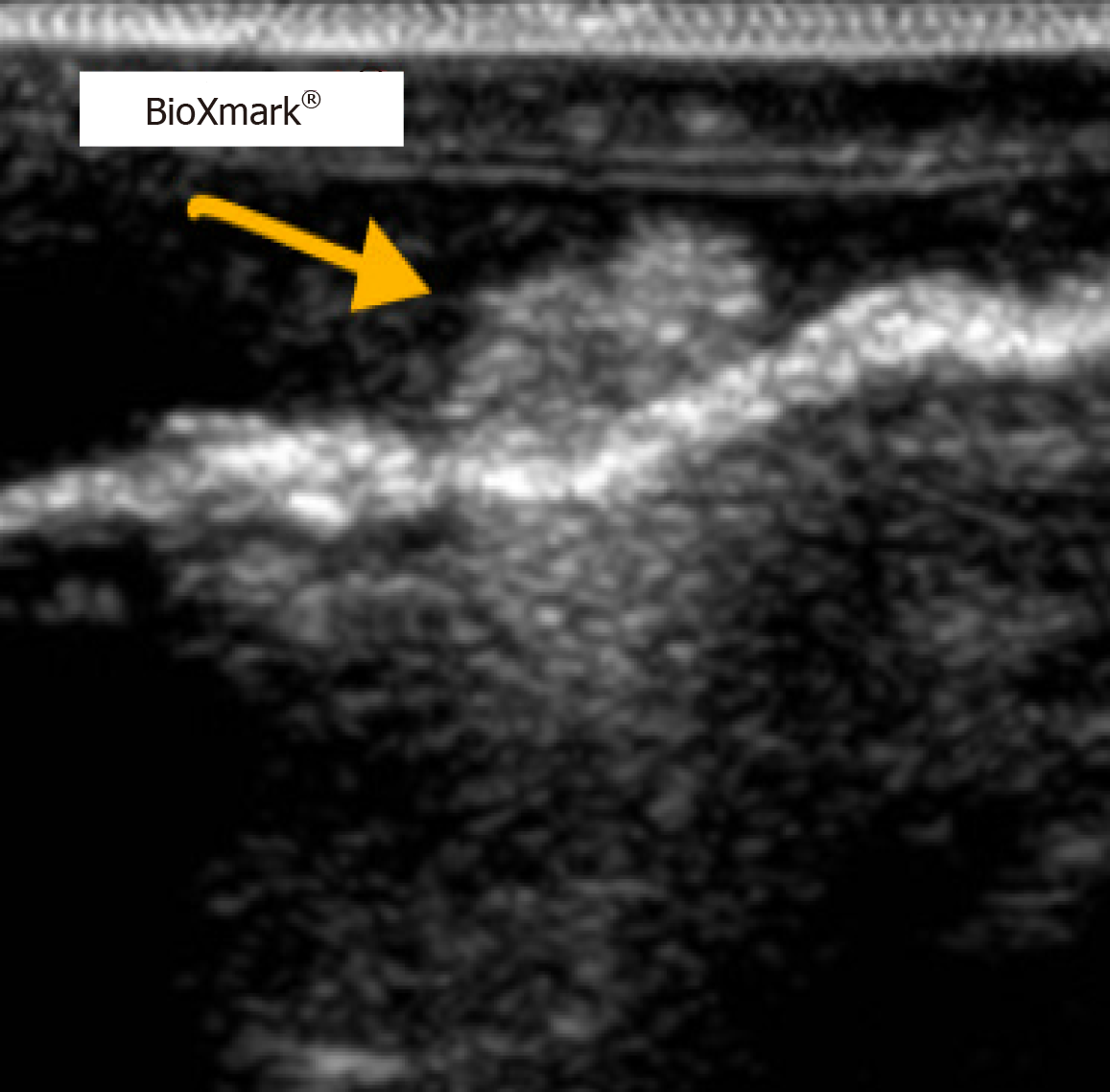
The surgeon’s proposed resection line was placed 2–6 cm proximally to the line marked by the fiducial marker. After thoroughly scanning the length of the esophagus’ exterior in vivo with the ultrasound probe, a hyperdense area of approximately 1.5 cm × 1.5 cm was found and concluded to be the fiducial marker. This line was marked with a sterile marker, and the differences between the two purple lines were measured (Figure 1). The hyperdense areas on ultrasound were determined to be the fiducial marker (Figures 2 and 3).
Fiducial markers in the form of hyperdense areas were identified in six of ten patients in vivo, corresponding to an in vivo success rate of 60%. In two of these six patients, fiducial markers were also found ex vivo. In the remaining four patients, the fiducial marker was not found ex vivo. In the last four patients, no fiducial markers could be identified in vivo or ex vivo.
No adverse events, device deficiencies, or clinically relevant worsening related to BioXmark® were observed in this study. These included the perioperative and postoperative events.
This is the first study to investigate the use of a liquid fiducial marker to improve resection margins in patients with esophageal cancer. Ten patients with gastroesophageal tumors were marked endoscopically with a fiducial marker 5 cm proximal to the tumor before surgery.
In this study, successful BioXmark® implantation was achieved in all ten patients, with the identification of the fiducial marker observed on perioperative ultrasound in six of ten cases. The surgeon consistently positioned the proposed resection line proximally to the recommended 5-cm distance from the tumor’s proximal boundary. The proposed resection line was placed 2–6 cm proximally in vivo to the line marked by the fiducial marker. Following Danish guidelines, the surgeon identified the tumor by digitally palpating the exterior of the esophagus, which is not a precise or reproducible method to achieve R0 resection. Despite the Danish guidelines calling for a 5-cm distance proximally from the tumor to resection, the standard procedure at Rigshospitalet typically involves resection at the level of the azygous vein or higher, if needed. The operating surgeon checks the preoperative scans, and unless these indicate otherwise, the resection is performed at the level of the azygous vein. Notably, in this study, the observed mean macroscopic resection line was 45 mm from the proximal edge of the tumor ex vivo, despite all resection lines being placed proximally to the fiducial marker. However, tissue shrinkage after resection is important. One study found that the proximal resection margins of GEJ tumors can shrink to 32% after formalin fixation[13]. The overall length from the in vivo specimen to the fixed specimen may decrease by 50%, and despite maximal stretching of the resected unfixed specimen, restoration of only 86% of its original in vivo length is possible[13]. This indicates that the aforementioned strategy of resecting at the level of the azygous vein should satisfy the 5-cm distance from the proximal tumor border to resection when considering tissue shrinkage.
Generally, when determining the resection margin in a cancer operation, a surgeon must consider two viable options. If the resection is made too close to the tumor, the risk of R1 resection increases and is associated with increased morbidity and mortality rates. Finding the right distance to the tumor is a challenge, with the recommendations varying worldwide and with the added risk of “skip” metastases[5]. Even with R0 resection, the survival rates decrease if the resection margin is less than 3.8 cm proximal to the tumor[5]. Conversely, if the resection is too extensive, the risk of a poorly perfused conduit increases, and postoperative complications such as anastomotic leakage increase, potentially lowering long-term survival rates[14,15]. Hypothetically, marking of the proposed 5 cm distance from the most proximal part of the tumor could be a useful intraoperative tool when determining the most optimal place to transect the esophagus. This is especially relevant in more extensive resections, where perfusion worsens due to the length of the gastric conduit. One study demonstrated the superiority of indocyanine green fluorescence angiography (ICG-FA) in assessing intraoperative perfusion in GEJ cancer, which can be used to increase the chance of sufficient perfusion[16]. ICG-FA is a promising tool for assessing perfusion but has not yet been used as a standard to identify cancer tissue. However, if one could combine the knowledge of the optimally perfused tissue by ICG-FA and the optimal transection line with a fiducial marker, the number of safer and effective resections could increase.
The decision of where to perform the resection is typically based on national or international guidelines, such as the Danish guidelines[4]. However, these guidelines neglect to consider the effect of neoadjuvant treatment on tumor size and the intraoperative identification of tumor margins. Furthermore, patient-specific conditions, such as esophageal length, concurrent Barrett’s disease, and other diseases may affect where one would set the resection margin. In our study, Barrett’s dysplasia was present in two out of ten histopathological specimens. Barrett’s dysplasia is not palpable; therefore, an increased risk of an insufficient proximal resection margin exists. Individuals diagnosed with Barrett's esophagus, whether with low-grade or high-grade dysplasia, face an elevated annual risk of developing esophageal adenocarcinoma[17,18]. These patients should have their Barrett’s esophagus segment resected or undergo regular endoscopic surveillance with biopsies to monitor histopathological changes.
In this study, neither method, resecting at the line of the fiducial marker or resecting at the azygous vein, consistently yielded the recommended 5-cm margin. Although BioXmark® may aid in defining margins beforehand, its ability to secure a specified distance remains uncertain. Attempts to predetermine a 5-cm margin with BioXmark® often resulted in the intraoperative ultrasound identifying a closer proximity to the tumor. Similarly, adhering to the azygous vein resection strategy resulted in a mean resection line of 45 mm, highlighting the difficulty of consistently meeting the desired margin. Addressing this discrepancy between the intended and observed margins is crucial because of its direct impact on R1 resection rates and subsequent patient outcomes. It is imperative to explore refinements or alternative approaches to enhance the precision of these methodologies to minimize R1 resection rates and ultimately improve overall patient survival[5].
The technical feasibility of injecting BioXmark® into organ tissues and its use in radio-guided therapy has been well documented[6-10], and we did not have any difficulties with BioXmark® injection in our study. As we did not have photos or literature available to compare with our findings, we attempted to identify BioXmark® on perioperative ultrasound. In four patients, we could not identify markers in vivo or ex vivo. This was attributed to migration of the fiducial marker in vivo, the small amount injected, or challenges in properly identifying the marker via ultrasound. Another reason could be incorrect use of the product, as we injected saline prior to injection of the fiducial marker, and complete solidification could not be ensured because the marker was placed directly before the start of the operation. This could have affected the product’s properties and should be carefully considered in future studies. Saline injections were administered to prevent transesophageal placement of the marker by lifting the submucosa. Although several studies have been published on the use of BioXmark®, none have investigated the use of BioXmark® during surgery or its visibility on ultrasound within a brief period after placement.
One study investigated the use of BioXmark® in a prospective feasibility study of 20 patients with rectal cancer[6]. Their findings demonstrated the positional stability of BioXmark® on computed tomography (CT) for use in image-guided radiotherapy, albeit with poor visibility on MRI. Similar results were observed in another study of image-guided radiotherapy for bladder cancer[7]. This study found that BioXmark® is easy to inject, visible on CT, positionally stable, and safe. One study on ten patients with esophageal cancer found that the implantation of BioXmark® was technically feasible, with appropriate visibility on CT, cone beam CT, and MRI, with excellent positional stability[8].
A limitation of this study is the short interval between implantation and ultrasound. Hypothetically, the difficulty in locating BioXmark® in this study could be due to insufficient time for the marker to harden or the use of a saline deposit. BioXmark® is a viscous substance that hardens in vivo as ethanol diffuses out of the marker; however, no information is available on the kinetics or duration of this process. Thus, the risk of marker migration or flattening into a thin sheet in vivo is likely high. This was a quality assurance study aimed at evaluating the first cases of a newly introduced procedure, and the specialists performing the ultrasound identification of the fiducial marker were surgeons, not radiologists. Furthermore, the surgeons had no prior experience of visualizing and identifying the fiducial marker. Moreover, the problem of insufficient time for markers to harden was first discovered after placing the fiducial marker. Endoscopic ultrasound could have been used to provide real-time imaging of the injection location; however, due to logistical difficulties, this was not possible. The fiducial marker BioXmark® is not intended for immediate ultrasound identification after injection, which could further explain the difficulties in locating the marker. A prospective study is currently underway, wherein BioXmark® is administered prior to chemotherapy. This approach enables the surgeon to macroscopically visualize the tumor during marker injection, thereby significantly enhancing the precision of marker placement. Additionally, the extended interval between injection and perioperative ultrasound identification should facilitate marker hardening, potentially improving visibility on perioperative ultrasound.
In conclusion, we found limited visibility of BioXmark® on intraoperative ultrasound after preoperative injection and neoadjuvant treatment. Despite the ease of its technical implantation, locating the fiducial marker was successful in only six patients. Further studies are needed to explore the potential clinical applications of fiducial liquid markers in cancer surgery. Fiducial markers have myriad applications in clinical settings, with potential uses across both medical and surgical specialties. Initial costs with implementation should be compared with the possible benefits such as reduced reoperation rates, improved patient outcomes, and ultimately reduced use of healthcare resources.
| 1. | Schlottmann F, Casas MA, Molena D. Evidence-based approach to the treatment of esophagogastric junction tumors. World J Clin Oncol. 2022;13:159-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Zheng YH, Zhao EH. Recent advances in multidisciplinary therapy for adenocarcinoma of the esophagus and esophagogastric junction. World J Gastroenterol. 2022;28:4299-4309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Dansk EsophagoGastrisk Cancer Gruppe database (DEGC) Årsrapport 2020. Available from: https://degc.dk/wp-content/uploads/2021/10/DEGC-arsrapport-2020-offentlig.pdf. |
| 4. | Dansk EsophagoGastrisk Cancer Gruppe database. Kirurgisk behandling - af karcinom i esophagus inkl. gastroesophageale overgang. Available from: https://degc.dk/wpcontent/uploads/2020/12/DEGC_kir_beh_esophagus_AdmGodk141120.pdf. |
| 5. | Barbour AP, Rizk NP, Gonen M, Tang L, Bains MS, Rusch VW, Coit DG, Brennan MF. Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg. 2007;246:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Opbroek TJS, Willems YCP, Verhaegen F, de Ridder R, Hoge C, Melenhorst J, Bakers F, Grabsch HI, Buijsen J, Van Limbergen EJ, Canters RAM, Berbée M. BioXmark® liquid fiducials to enable radiotherapy tumor boosting in rectal cancer, a feasibility trial. Clin Transl Radiat Oncol. 2023;38:90-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | de Ridder M, Gerbrandy LC, de Reijke TM, Hinnen KA, Hulshof MCCM. BioXmark® liquid fiducial markers for image-guided radiotherapy in muscle invasive bladder cancer: a safety and performance trial. Br J Radiol. 2020;93:20200241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Machiels M, Voncken FEM, Jin P, van Dieren JM, Bartels-Rutten A, Alderliesten T, Aleman BMP, van Hooft JE, Hulshof MCCM. A Novel Liquid Fiducial Marker in Esophageal Cancer Image Guided Radiation Therapy: Technical Feasibility and Visibility on Imaging. Pract Radiat Oncol. 2019;9:e506-e515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Rydhög JS, Mortensen SR, Larsen KR, Clementsen P, Jølck RI, Josipovic M, Aznar MC, Specht L, Andresen TL, Rosenschöld PM, Persson GF. Liquid fiducial marker performance during radiotherapy of locally advanced non small cell lung cancer. Radiother Oncol. 2016;121:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | de Blanck SR, Scherman-Rydhög J, Siemsen M, Christensen M, Baeksgaard L, Irming Jølck R, Specht L, Andresen TL, Persson GF. Feasibility of a novel liquid fiducial marker for use in image guided radiotherapy of oesophageal cancer. Br J Radiol. 2018;91:20180236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Nanovi A/S. Introduction to BioXmark®. Unknown publication date. Available from: https://nanovi.com/bioxmark/introduction/. |
| 12. | Kofoed SC, Calatayud D, Jensen LS, Helgstrand F, Achiam MP, De Heer P, Svendsen LB; Danish Esophageal, Cardia and Stomach Cancer Group. Intrathoracic anastomotic leakage after gastroesophageal cancer resection is associated with increased risk of recurrence. J Thorac Cardiovasc Surg. 2015;150:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Siu KF, Cheung HC, Wong J. Shrinkage of the esophagus after resection for carcinoma. Ann Surg. 1986;203:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Aahlin EK, Olsen F, Uleberg B, Jacobsen BK, Lassen K. Major postoperative complications are associated with impaired long-term survival after gastro-esophageal and pancreatic cancer surgery: a complete national cohort study. BMC Surg. 2016;16:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Yamashita S, Sheth RA, Niekamp AS, Aloia TA, Chun YS, Lee JE, Vauthey JN, Conrad C. Comprehensive Complication Index Predicts Cancer-specific Survival After Resection of Colorectal Metastases Independent of RAS Mutational Status. Ann Surg. 2017;266:1045-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Nerup N, Svendsen MBS, Svendsen LB, Achiam MP. Feasibility and usability of real-time intraoperative quantitative fluorescent-guided perfusion assessment during resection of gastroesophageal junction cancer. Langenbecks Arch Surg. 2020;405:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Singh S, Manickam P, Amin AV, Samala N, Schouten LJ, Iyer PG, Desai TK. Incidence of esophageal adenocarcinoma in Barrett's esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:897-909.e4; quiz 983.e1, 983.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 18. | Rastogi A, Puli S, El-Serag HB, Bansal A, Wani S, Sharma P. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 299] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/