INTRODUCTION
Massive rotator cuff tears (RCTs), especially in elderly patients, are associated with significant functional decline and reduced quality of life. The management of this group of patients is challenging in orthopaedics[1]. Patient-related factors as well as factors related to the individual characteristics of the tear play a role in treatment decision making. Patient-related factors include patient age, presence of comorbidities, and level of functional demands, while the individual characteristics of the tear include parameters such as the size and chronicity of the tear, as well as the presence of fatty infiltration and/or muscle atrophy[2]. Following a massive RCT, the rotator cuff muscles undergo pathological changes, including fibrosis, atrophy, and fatty infiltration, which compromise postoperative functional recovery[3]. Muscle atrophy and fatty infiltration have been attributed to both disuse-related degeneration and mechanical factors, such as increased tension on the suprascapular nerve due to tendon retraction[4,5]. Additionally, oxidative stress has been implicated as a key contributor to rotator cuff muscle degeneration and atrophy following tendon rupture[6,7].
Biomechanical and histological investigations in animal models have confirmed that muscle atrophy and fatty infiltration, hallmark features of chronic massive RCT, significantly impair myotendinous unit function[3,8] Clinically, these degenerative changes are associated with poorer outcomes as well as increased rerupture rates after repair[9,10]. The poor prognosis of chronic massive RCT with concomitant significant muscle degeneration has led many surgeons to consider alternative methods of surgical treatment, such as latissimus dorsi tendon transfer or superior capsular reconstruction[11,12].
Recent research has increasingly focused on identifying factors that inhibit or decrease the rate of progression or even reverse muscle atrophy and fatty infiltration in patients with massive RCTs. This study protocol introduces a novel approach by investigating the potential therapeutic effects of two agents, alpha-tocopherol and the synthetic heparan sulfate mimetic OTR-4131, on mitigating muscle atrophy, fatty infiltration, and fibrosis in rotator cuff muscles following massive RCT. The protective and regenerative properties of alpha-tocopherol have been reported in studies involving the muscles of the lower limbs[13,14]. This effect is attributed to its ability to mitigate oxidative stress and prevent proteolytic degradation. Its antioxidant properties protect muscle cells from damage induced by reactive oxygen species while preserving the integrity of the extracellular matrix. Additionally, alpha-tocopherol downregulates the expression of genes involved in muscle proteolysis and promotes the expression of protective proteins, such as heat shock protein 72, which play a crucial role in muscle preservation and recovery. Similarly, OTR-4131 has demonstrated protective and regenerative effects in muscle injury models, likely due to its ability to stabilize the extracellular matrix, prevent excessive proteolytic activity, and enhance the bioavailability of growth factors essential for tissue repair[15].
This experimental protocol aims to outline the methodology for evaluating the effects of alpha-tocopherol and OTR-4131 on muscle atrophy and fatty infiltration following a massive RCT in a rat model, with the hypothesis that these treatments will mitigate these pathological changes and yield favorable histological and morphological outcomes compared to controls.
MATERIALS AND METHODS
Ethics approval
Approval for this randomized, prospective experimental protocol was granted by the Ethics and Bioethics Committee of the School of Medicine, Aristotle University of Thessaloniki. All procedures will comply with the revised European and National Regulations (European Community Council directive 86/609/EEC; Presidential Degree 56/2013). The study protocol is prepared according to the ARRIVE guidelines 2.0 for reporting animal research[16]. The study is conducted under the supervision and contribution of a veterinarian, who will be responsible for observance of the rules of hygiene in the premises and the rules of acclimatization, protection, and nutrition of the experimental animals.
Study design and population
For the purposes of this research, an experimental control trial will be conducted using 40 male Wistar rats aging 12 weeks. Wistar rats are selected as animal models due to their widespread use in musculoskeletal and rotator cuff research. This animal model has already been validated in previous studies for replicating degenerative changes similar to those seen in massive human RCTs[8,17]. Its significant similarities to humans in terms of the shoulder joint anatomy (presence of coracoacromial arch, presence of suprascapular and subscapular tendons) and the degenerative changes that occur in the rotator cuff muscles following the occurrence of massive RCTs make it an appropriate and reliable model for this type of experimental research[18]. To minimize inter-animal variability in response to interventions, rats will be weight-matched (± 10%) at the time of randomization. We used the 12-week age threshold, as this corresponds to early adulthood in rats[19]. At this developmental stage, rats have completed sexual maturation, offering a stable musculoskeletal and hormonal environment suitable for modeling degenerative changes following massive RCTs. Male rats will be chosen to avoid overlapping hormone-related confounding factors in the experiment.
Only animals suitable for surgical procedures and meeting laboratory animal care guidelines will be included. Exclusion criteria will apply to rats with pre-existing musculoskeletal abnormalities, conditions affecting the rotator cuff or surrounding musculature, signs of illness, stress, or injury during preoperative assessment, failure to recover appropriately from anesthesia, or postoperative complications. Additionally, any condition compromising animal welfare or the validity of experimental results will lead to exclusion as determined by the veterinary team. The rats will be housed in pairs of two in each cage, to minimize the chances of aggressive behavior, under a controlled temperature of 18-22 °C and humidity of 55%-60%, with controlled 12-hour intervals of light and darkness, at the accredited animal facility of the laboratory of anatomy, Histology and Embryology of Aristotle University of Thessaloniki’s veterinary school (accreditation number EL54BIO23). They will undergo a one-week acclimatization period before the initiation of the experiment to minimize stress and ensure baseline stability. Rats will have free access to water and food. No antibiotics will be administered, and male rats are chosen to avoid the effects of hormone-related factors in the experiment.
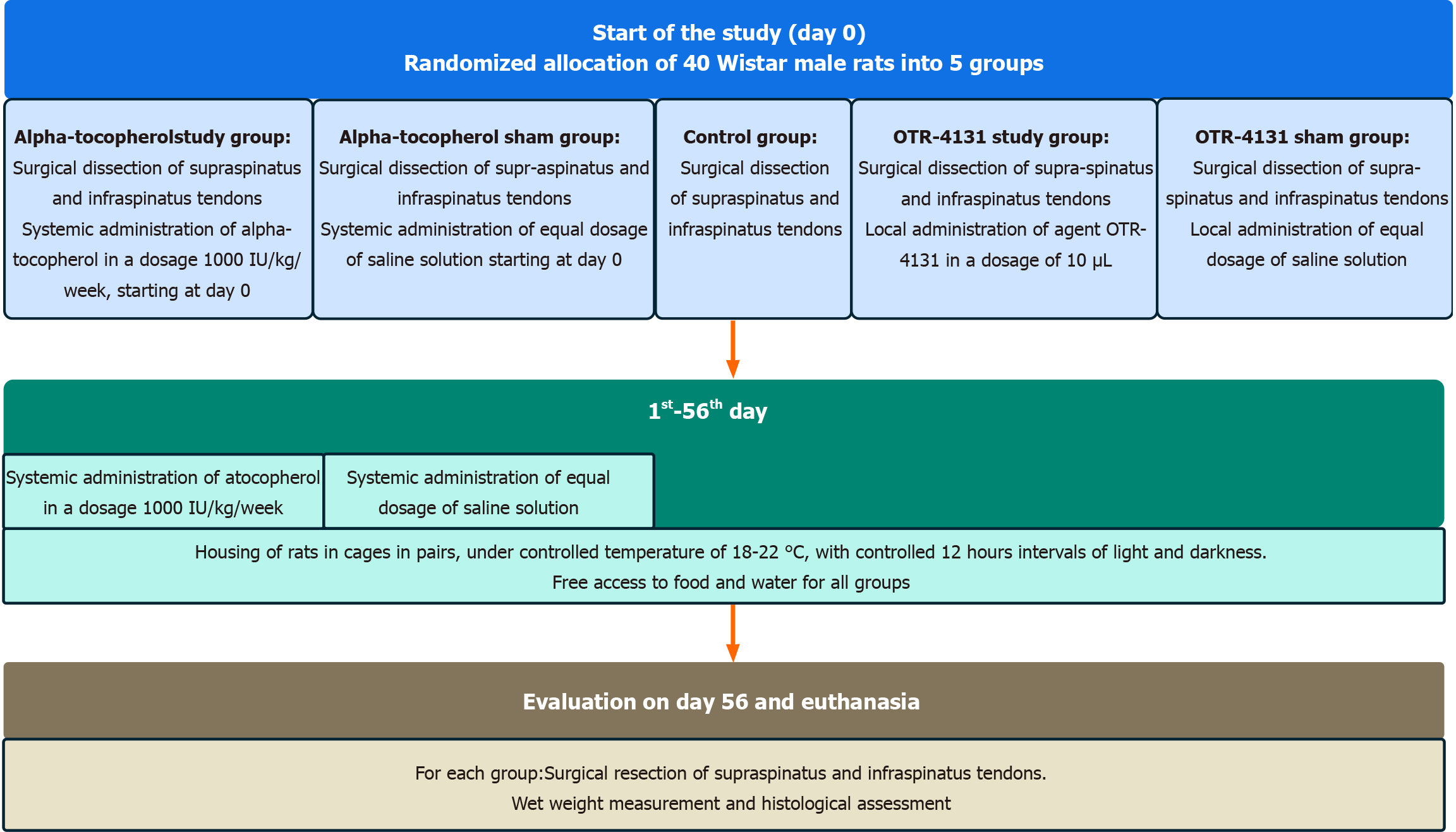
The rats will undergo surgical dissection of the rotator cuff of the right shoulder and will be randomized into five experimental groups by a computer algorithm (Google computer random number generator): (1) A group consisting of 8 male rats, in which no intervention, surgical or pharmaceutical, will be performed (Control Group); (2) A group consisting of 8 male rats, in which alpha-tocopherol will be administered systematically via intramuscular injection into the gluteus muscles on the first postoperative day and every 7th day thereafter for up to 8 weeks postoperatively (alpha-tocopherol study group); (3) A group consisting of 8 male rats, in which an equal dose of isotonic saline solution will be administered via the same route at the same time points during the experiment (alpha-tocopherol sham group); (4) A group consisting of 8 male rats, in which the agent OTR-4131 will be administered as a solution with a dose of 10 μL and a concentration of 1 μg/10 μL via intramuscular injection in each of the supraspinatus and infraspinatus muscles (OTR-4131 study group); and (5) A group consisting of 8 male rats, in which an equal dose of isotonic saline solution will be administered by intramuscular injection into each of the supraspinatus and infraspinatus muscles (OTR-4131 sham group). The endpoint of the study for all groups is set at 8 weeks after the initial surgery, at which time tissue harvesting and outcome assessments will be performed. An 8-week monitoring period was chosen as it represents a well-established timeframe for evaluating chronic degenerative changes following rotator cuff injury in rodent models[20].
To minimize potential confounders, all rats will be allocated randomly to their cages and coded by an individual not participating otherwise in the study. The same person will assign the coded animals to the surgeons and prepare the treatment solutions, ensuring that the surgeons remain blinded to the treatment groups. To maintain consistency in surgical technique, all procedures will be performed by the same two surgeons. Additionally, the pathologist conducting the histological analyses will be blinded to the group allocation of the specimens. These measures are designed to reduce bias and enhance the reliability of the study results. Humane endpoints for the study will include monitoring for signs of severe distress, pain, systemic illness, or infection. Animals will be observed twice daily for the first 48 hours postoperatively and once daily thereafter. Specific signs monitored include abnormal feeding behavior, reduced activity, signs of severe pain (e.g., vocalizations, hunching, or guarding), and evidence of infection such as redness, swelling, discharge, or delayed wound healing at the surgical site. In cases where infection is detected, appropriate veterinary care will be provided. If the condition does not improve despite intervention or results in significant distress, the animal will be humanely euthanized to prevent unnecessary suffering. The study process flowchart is presented in Figure 1. An overview of the experimental protocol is presented in Figure 2.
Figure 1 Study process flowchart.
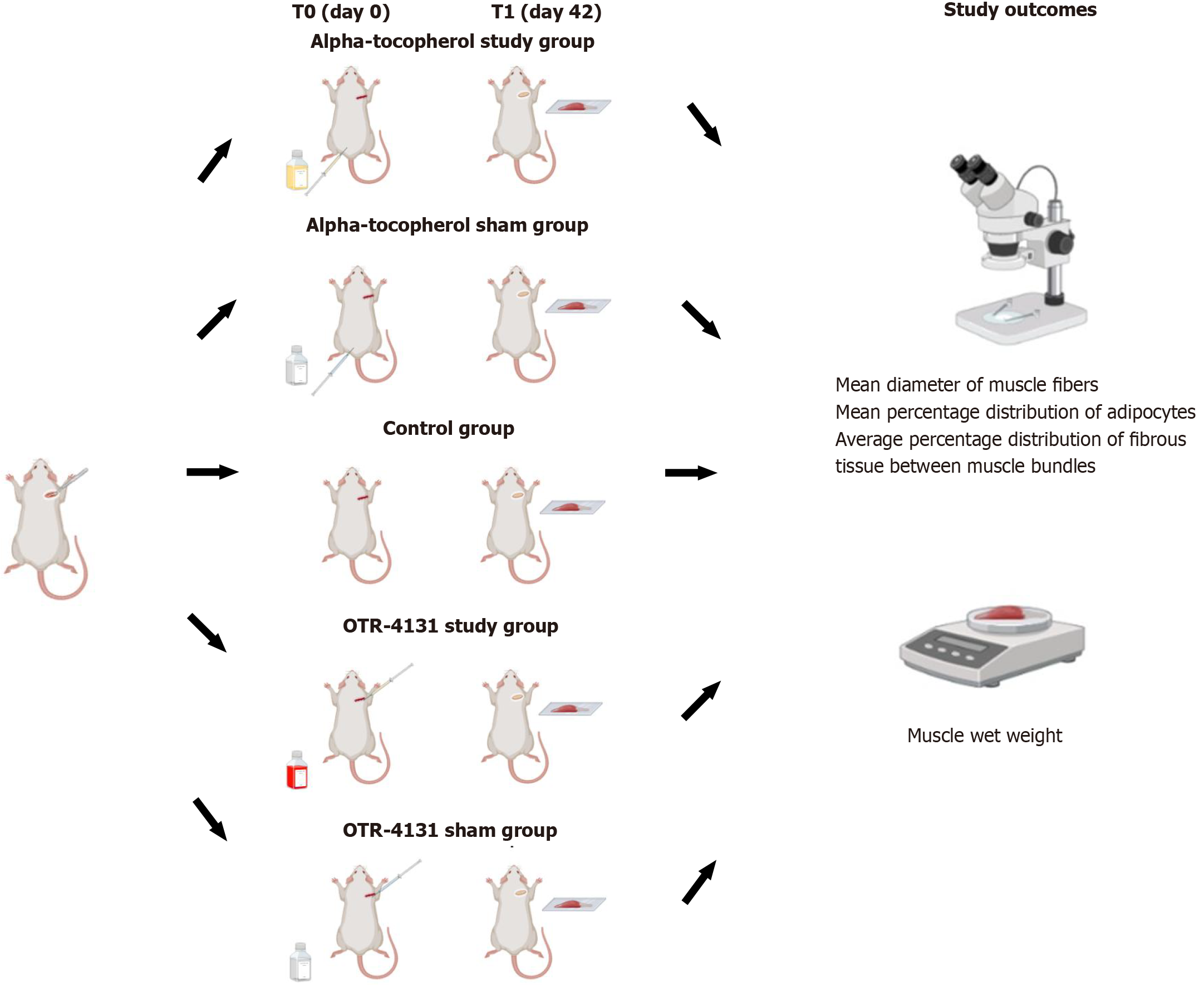
Figure 2 An overview of the experimental design.
This figure was created with BioRender.com.
Sample size justification
The sample size was calculated using data from previous studies on muscle fiber thickness (cross sectional area), which is an indirect histological marker for assessing muscle atrophy[21,22]. To detect a difference of 835 μm2 in mean muscle fiber thickness between the control group (mean ± SD: 1838 ± 644 μm2) and the injected group (mean ± SD: 2673 ± 421 μm2), it was estimated that 8 rats per group would be needed. Taking into account a mortality loss rate of 10%, the final sample size was set at 9 rats per group. The power was set at 80% and the significance level at 5%. Sample size was calculated using the statistical program G*Power 3.1.9.4.
Intervention agents and administration
Alpha-tocopherol: Alpha-tocopherol (Vitamine E Nepalm, 100 mg/2 mL, Greece) will be dissolved in 0.1 mL of sesame oil (Chemco Sesame Seed Oil Refined, 100 mL, Athens, Greece). Systemic administration of alpha-tocopherol via intramuscular injection into the muscles of the back (gluteal region) will be performed, in a dosage of 1000 IU/kg/week. The intramuscular route was selected to ensure slow systemic release and reliable bioavailability. This administration dosage and type has been used in previous animal studies with rats investigating muscle tissue protection and antioxidant effects and has proven to be effective and safe[23,24]. Moreover, the dosage intake is well below the toxicity levels[25].
OTR-4131: The heparan sulfate mimetic OTR-4131 (provided by OTR3 Company, Paris, France) will be used in the form of intramuscular injection. The injection will take place immediately after the surgically induced RCT. A single injection will be used for each muscle, namely the supraspinatus and infraspinatus. To evaluate its effect 10 μL of 1 μg/μL solution will be used. The injection volume and concentration were determined based on prior studies in rats, where comparable local administration supported muscle regeneration without systemic toxicity[15,26].
Experimental procedure
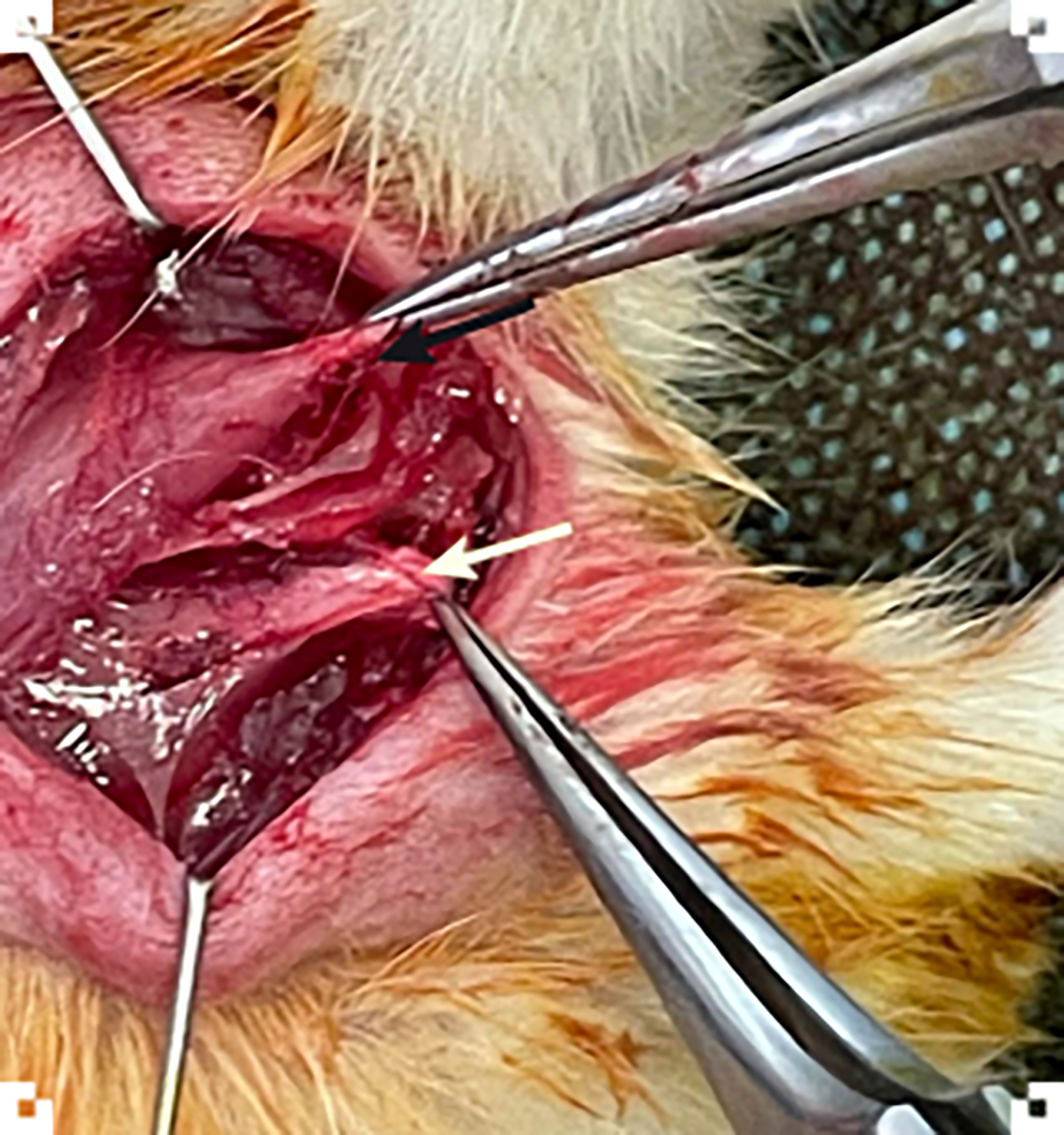
At T0 (day 0), rats in the control and study groups will undergo general anesthesia, with the administration of ketamine (50 mg/kg) and xylazine (5 mg/kg). The surgical area will be shaved and sterilized with an antiseptic solution. Each rat will be placed in the prone position and secured on a surgical board to ensure stability during the procedure. A longitudinal skin incision, approximately 1-1.5 cm in length, will be made over the right shoulder joint and careful dissection through the subcutaneous tissue will be performed to expose the underlying structures. The deltoid muscle will be identified, and its fibers separated longitudinally. Similarly, muscle fibers from the lateral trapezoid muscle will also be separated longitudinally, in line with the spinus process, for adequate visualization of supraspinatus and infraspinatus tendons. The supraspinatus and infraspinatus muscles and their tendons will be identified, with the supraspinatus muscle-tendon unit located cranially, in the supraspinous fossa, and the infraspinatus located more caudally, in the infraspinous fossa (Figure 3). For experimental purposes, both tendons will be transected creating a model for studying RCTs. A Penrose drain will be placed around the tendon edges to prevent spontaneous reattachment. The deltoid and trapezoid muscles will be repositioned and the skin incision will be closed with fine sutures. The procedures aim to simulate the pathological environment of massive RCTs. In the OTR-4131 group, during the operation, the animals will receive an intramuscular injection in the supraspinatus and infraspinatus muscles of either OTR-4131 solution (OTR-4131 study group) or corresponding saline solution (OTR-4131 sham group). In the alpha-tocopherol group, the animals will receive after the operation an intramuscular injection of either alpha-tocopherol (study group) into the gluteus muscle or corresponding saline solution (control group) starting on operation day and subsequently every 7th day until the 8th postoperative week. After surgery, animals will be released into their housing cages and will be monitored daily for 8 weeks.
Figure 3 Image from the pilot surgery showing the identification of the supraspinatus (black arrow) and infraspinatus tendons (white arrow).
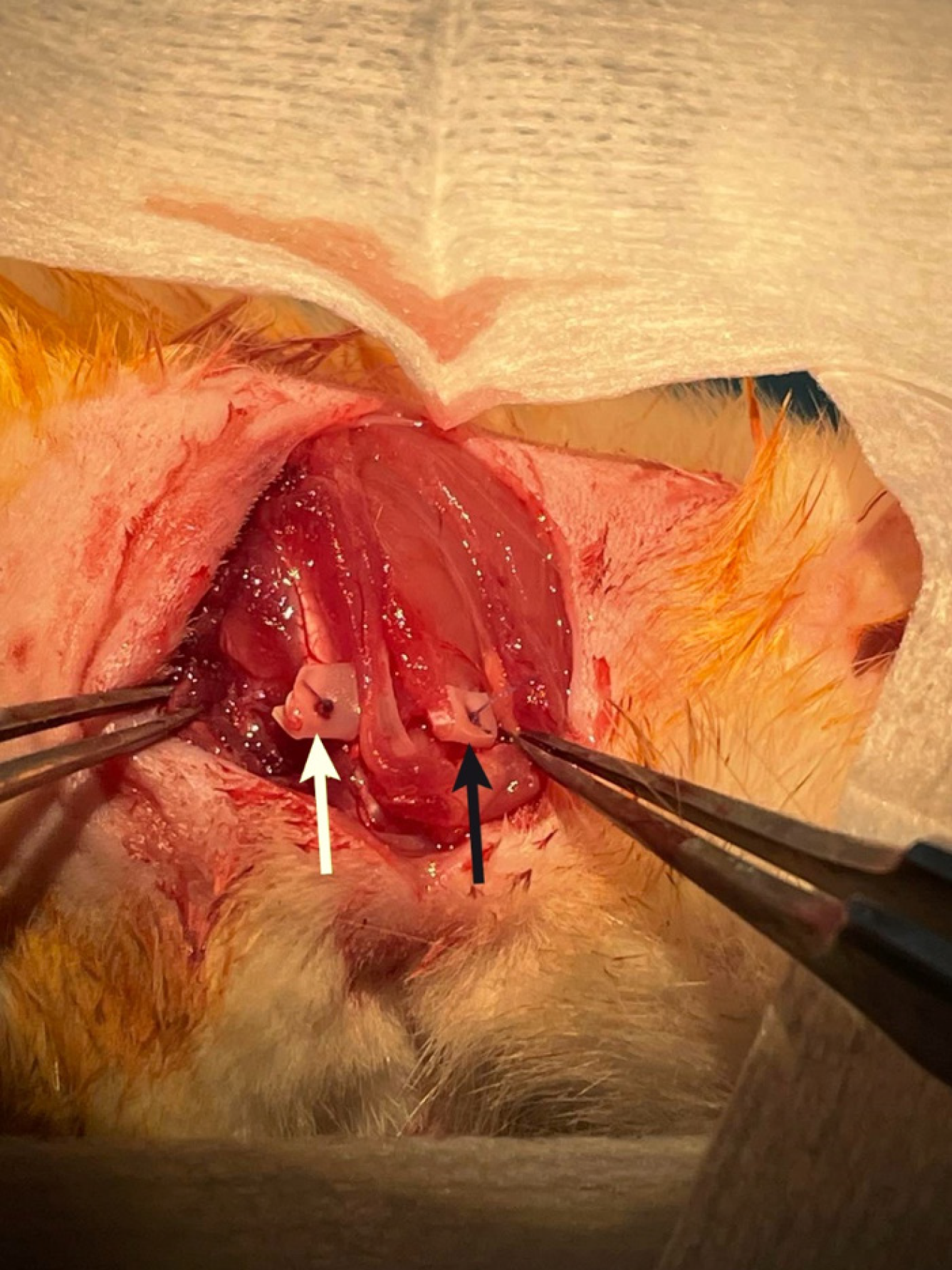
At T1 (day 56), a reoperation under similar anesthesia will be performed for the purpose of harvesting the infraspinatus and supraspinatus tendon-muscle units. A new incision will be made in line with the previous shoulder incision and extended medially toward the scapula. The subcutaneous tissue will be carefully dissected to reveal the trapezoid and deltoid muscles, which will again be split longitudinally in line with spinus process and retracted, in order to visualize the full extent of the infraspinatus and supraspinatus muscles (Figure 4). Both muscles will be identified and subperiosteally elevated from their origin on the scapula. Following muscle harvesting, the muscle-tendon specimens will be weighed, placed in a wooden tongue depressor with their edges pinned down to secure muscles length retention (Figure 5) and wrapped in saline-soaked gauze to maintain their moisture. After transport to the pathology laboratory, the specimen will be cryopreserved in liquid nitrogen (-196 °C). This rapid freezing will ensure the tissue’s structural integrity by preventing ice crystal formation that could otherwise damage the muscle. Following cryopreservation, 1 mm-thick sections will be cut from the specimen and stored for further histological analysis to assess the fat infiltration and muscle atrophy. The pathologist who will perform the measurements will be blinded regarding the origin of each specimen. This study does not constitute a clinical trial; therefore, clinical trial number is not applicable.
Figure 4 Image from the pilot surgery during the second procedure, illustrating the complete visualization of supraspinatus (black arrow) and infraspinatus (white arrow) muscles prior to harvesting.
Figure 5 Specimens of infraspinatus (left) and supraspinatus (right) muscle units obtained during the pilot surgery.
Euthanasia
After the second surgical procedure and tissue harvesting, rats will be euthanized by use of a CO2 euthanasia chamber, following the American Veterinary Medical Association Guidelines for the euthanasia of animals: 2020 edition[27].
Outcomes
Muscle atrophy of the rotator cuff muscles will be assessed by the following methods: (1) Measurement of muscle wet weight, immediately after tissue harvesting[28]; and (2) Following specimen fixation in 4% formalin, measurement of the mean diameter of the muscle fibers in transverse cross-section (cross sectional area) will be performed after hematoxylin-eosin staining and with use of Image J software (Image J, MD, United States)[3,28]. The presence and degree of fatty infiltration will be assessed by measuring the mean percentage distribution of adipocytes in images obtained after oil red staining and use of Image J software[8,17]. Finally, the presence and degree of fibrosis will be assessed by measuring the average percentage distribution of fibrous tissue between muscle bundles after Masson trichrome staining and use of Image J software[8].
Statistical analysis
The primary outcomes are continuous quantitative variables. Normality of data distribution will be assessed using the Kolmogorov-Smirnov test. For variables demonstrating a normal distribution, group comparisons will be performed using an independent samples t-test to evaluate differences in mean values between the intervention and control groups. In cases where the data do not conform to a normal distribution, the Mann-Whitney U test will be employed as a non-parametric alternative. Statistical analysis will be conducted using SPSS software (IBM Corp., Armonk, NY, United States), and a P value of < 0.05 will be considered indicative of statistical significance.
RESULTS
As this study is an experimental protocol, no data are currently available. The intervention groups are expected to exhibit better preservation of muscle structure, with higher muscle wet weight and larger muscle fiber diameter compared to the sham groups. Histological analysis with hematoxylin and eosin staining may indicate improved muscle fiber integrity, suggesting reduced atrophy in the treatment groups. Fatty infiltration is anticipated to be less pronounced in the alpha-tocopherol and OTR-4131 groups, as assessed by oil red O staining. In contrast, the sham groups may demonstrate increased intramuscular fat deposition, indicative of progressive muscle degeneration. Fibrosis, evaluated using Masson’s trichrome staining, is expected to be lower in the treatment groups, particularly in the OTR-4131 group, as this agent is hypothesized to stabilize the extracellular matrix and enhance the bioavailability of growth factors. The control group, in which no surgical intervention or pharmacological treatment will occur, is expected to maintain normal muscle morphology without signs of atrophy, fatty infiltration, or fibrosis. Comparisons between the intervention, sham, and control groups will provide insight into the extent of muscle degeneration following a massive RCT and the protective effects of the tested agents. The results of this study will contribute to a better understanding of the progression of muscle degeneration after RCTs and the potential therapeutic applications of alpha-tocopherol and OTR-4131. Detailed findings, including histological and morphological outcomes, will be presented in future publications.
DISCUSSION
RCTs are among the most common causes of shoulder pain and disability, with an incidence of approximately 30% in patients over 60 years[22]. They impose a significant economic burden, with more than 75000 surgeries performed annually[21]. Moreover, massive RCTs are associated with functional impairments, including reduced shoulder abduction, decreased range of motion due to stiffness, and diminished shoulder strength[29]. Depending on the size of the tear, RCTs are classified as small, medium or massive. Although no clear definition of massive RCT has been established in the literature, criteria such as a tear greater than 5 cm in maximum diameter and a tear involving two or more tendons are commonly used[2,30]. After a massive RCT, several pathologic changes occur in the rotator cuff muscles, including fibrosis, muscle atrophy and fatty infiltration, that can lead to poor recovery after surgery and increased rate of recurrence[31].
An increasing research interest has emerged towards investigation of interventions that mitigate the development of muscle atrophy, fatty infiltration and fibrosis after repair of massive RCTs. Several studies have examined the contribution of oxidative stress to muscle homeostasis, as well as muscle injury under different circumstances (crushing, overuse, muscle unloading and disuse, age)[32-35]. Oxygen free radicals are produced both at rest and during muscle contraction. Although low and normal levels of oxygen free radicals are necessary for normal force production in skeletal muscle, high levels can lead to oxidative damage to lipid and myocyte proteins[34]. In addition, oxygen free radicals regulate a number of signaling pathways as well as the expression of many genes which results in increased expression of proteolytic enzymes, increased activation of proteases and inhibition of protein synthesis in muscle. This combination of proteolysis and reduction in protein synthesis leads to loss of muscle protein[36,37]. The regulation of other gene products, such as antioxidant enzymes, stress proteins and mitochondrial electron transport proteins, is also affected by oxidative stress[36]. As a response to oxidative stress, myocytes produce antioxidant enzymes, such as peroxisome dismutase, catalase, glutathione peroxidase, γ-glutamylcysteine synthase and heme oxygenase-1, which do not only neutralise redundant oxygen free radicals, but also intervene in the various stages of muscle regeneration: They influence the inflammatory response after injury, enhance the viability and proliferation of muscle satellite cells and myoblasts, and influence their differentiation[38]. Finally, antioxidant enzymes also regulate the processes that accompany muscle regeneration, they induce angiogenesis and reduce fibrosis[33]. The role of oxidative stress has also been studied in rotator cuff pathology and appears to be involved in both the degeneration observed at the enthesis level and the degeneration and atrophy of rotator cuff muscles[39-41].
Apha-tocopherol is a water-soluble vitamin that has been extensively studied for its antioxidant properties[42]. Apha-tocopherol reduces oxidative stress by inducing an increase in the concentration of endogenous antioxidant enzymes and by reducing the levels of lactate in the blood, glutathione S-transferase, by-products of lipid peroxidation (thiobarbituric acid reactive substances) in plasma and liver, and levels of carbonyl protein in liver and muscles. In addition, alpha-tocopherol suppresses the expression of mediators of inflammation including nuclear factor-κB, tumor necrosis factor-α, interleukin-1, interleukin-6, interleukin-8, inducible nitric oxide synthase, cyclooxygenase 2 and the transcription factor signal transducer and activator of transcription 3. Finally, alpha-tocopherol regulates the expression of hypoxia-inducible factor-1, which is also associated with inflammation[43]. The effects of alpha-tocopherol in skeletal muscle have also been investigated under various circumstances, including diabetes, exercise, ischemia-reperfusion and muscle atrophy induced by limb unloading. Studies in animal models have shown that exogenous vitamin E supplementation enhances antioxidant defenses, reduces oxidation, suppresses oxidative stress, increases protein synthesis, and reduces protein degradation[14,44]. Servais et al[14] studied the effect of alpha-tocopherol on muscle atrophy induced by disuse in the gastrocnemius muscle in Wistar rats and found that it displays a protective effect through regulation of proteolytic enzyme gene expression and to a lesser extent through its antioxidant activity. Finally, Morikawa et al[45] studied the effect of another antioxidant, vitamin C, on degeneration of the rotator cuff tendon in in an experimental model of superoxide dismutase 1-deficient mice with induced supraspinatus tendon entheses degeneration. The authors found improvement in the histological parameters examined (collagen fiber orientation, chondrocyte number and percentage of oriented chondrocytes, polarized light refraction area and metachromatic area).
Heparan sulfate proteoglycans (HSPGs) are complex molecules consisting of a protein core attached to heparan sulfate glycosaminoglycan chains. These biomolecules play a critical role in the regulation of growth factors and the bioavailability of heparin-binding proteins, which are vital to tissue repair and regeneration[46]. Among these, fibroblast growth factor-2 (FGF-2) has garnered significant attention for its involvement in muscle regeneration. FGF-2 forms a ternary complex with its receptors and HSPGs, which is essential for proper FGF-2 signaling during muscle repair[47]. Increased expression of FGF-2 is noted in skeletal muscle during regeneration, and its infusion has been shown to enhance muscle recovery in models of injury and disease, such as dystrophic and reinnervated muscles[48,49]. Conversely, inhibition of FGF-2 signaling can lead to impaired muscle repair, further highlighting the critical role of HSPGs in the regeneration process. Synthetic analogues of heparan sulfate, such as OTR-4131, have been developed to mimic the functions of natural HSPGs while offering additional benefits, such as increased resistance to enzymatic degradation. This resistance allows OTR-4131 to preserve the structural integrity of matrix proteins and protect growth factors from proteolytic breakdown. This property is crucial in muscle repair, as it creates a microenvironment conducive to tissue regeneration[50,51]. In muscle injury models, OTR-4131 has demonstrated its capacity to promote muscle regeneration by facilitating extracellular matrix remodeling and increasing the availability of growth factors like FGF-2. While other agents aimed at reducing fatty infiltration and muscle atrophy act through different pathways, OTR-4131’s mechanism is unique in its focus on protecting the extracellular matrix and enhancing growth factor-mediated repair[52,53].
In this experimental study, we hypothesized that administration of alpha-tocopherol or agent OTR-3141 affects the development of muscle atrophy and fatty infiltration after massive tear of the rotator cuff of the shoulder in an experimental Wistar rat model. The use of a rat model provides a controlled and reproducible system, leveraging similarities in musculoskeletal structure and healing mechanisms that can be extrapolated to other species, including humans. However, species-specific differences in muscle physiology, immune response, and metabolic pathways may limit the direct generalizability of the results. While the experimental conditions allow precise assessment of the interventions’ effects, these controlled environments may not fully replicate the complexity of clinical scenarios. If successful, the results could inform further preclinical studies in larger animal models and, eventually, clinical trials in humans to assess the translational relevance of these findings.
The present study presents specific limitations. First, although the anatomy and function of the rotator cuff muscles in rats resemble those of humans, interspieces differences may limit the interprentation of the results[54,55]. Secondly, there are inherent limitations, as in most experimental studies, regarding the exact dosage as well as the route of administration of the investigated substance, as these parameters cannot be directly extrapolated to humans. Thirdly, RCTs in animals show an increased healing potential which contrasts with the corresponding findings in humans[55]. An additional limitation is the difference in the rupture site, as in humans, RCTs usually happen after a closed injury to a tendon that probably already has some degree of degeneration, in contrast to the surgical transection of the tendon that is performed in this experiment. Finally, the safety and possible long-term side effects of the administered substance cannot be assessed, as all animals will be sacrificed at the end of the experimental study.