Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.105462
Revised: March 21, 2025
Accepted: April 3, 2025
Published online: December 20, 2025
Processing time: 193 Days and 23.2 Hours
Irisin is a chief myokine released during physical activity and has garnered attention for its potential therapeutic effects on different metabolic and car
Core Tip: Physical inactivity, sarcopenia, and diabetes are interrelated conditions, each exacerbating the others. The impact of these issues is significant, particularly on the cardiovascular system. Irisin, a myokine with autocrine, paracrine, and endocrine functions, may play a key role in metabolic health and could serve as an adjunct to the pharmacological management of prediabetes and diabetes. The exact mechanisms through which irisin exerts its beneficial effects, and the optimal levels for the therapeutic potential, need further investigation. Moreover, assessing irisin levels in response to different exercise types and intensities could be valuable in customizing individualized exercise plans for patients with diabetes and related complications.
- Citation: Cigrovski Berkovic M, Cigrovski V, Ruzic L. Role of irisin in physical activity, sarcopenia-associated type 2 diabetes, and cardiovascular complications. World J Methodol 2025; 15(4): 105462
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/105462.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.105462
Diabetes mellitus is a widespread metabolic disease with a rising incidence that parallels the advancement of age. Although its pathophysiology is complex, type 2 diabetes mellitus (T2DM) and its early-stage precursor, prediabetes, are largely driven by unhealthy lifestyles, including low levels of physical activity and sedentary behavior, which are closely interrelated with insulin resistance (IR), a hallmark of T2DM[1]. Despite extensive research and efforts dedicated to developing individually tailored pharmacotherapy, the management strategies of T2DM remain challenging and often fail to achieve the desired effects, leading to chronic complications that drive unfavorable outcomes[2]. In this vicious cycle, one of the important players is sarcopenia, an age-related condition characterized by a reduction in muscle mass, strength, and function accentuated by physical inactivity and closely linked to physical disability, compromised glycemic control, poor quality of life, and finally, mortality[3]. Unfortunately, sarcopenia is often overlooked, especially in persons with high body mass index, which is often in T2DM. Currently, several diagnostic methods are available for this purpose, ranging from non-invasive, easily accessible, and harmless bio-electrical impedance analysis to less commonly used methods that may involve higher costs, limited accessibility, and potential risks. These include dual-energy X-ray absorptiometry, magnetic resonance imaging, and computed tomography, the latter of which is considered the gold standard for quantifying muscle mass[4].
In patients with T2DM, the incidence of sarcopenia is 2-3 times higher compared to patients without T2DM, and sarcopenia and insufficient physical activity are both independently correlated with the onset of T2DM. On the other hand, a 10% increase in skeletal muscle index decreases the HOMA-IR by 11% and the incidence of prediabetes by 12%[5]. Sarcopenia is extensively found in older adults with T2DM, where it correlates with high HbA1c and macrovascular and microvascular complications of diabetes[6]. Several studies have demonstrated an association between sarcopenia and an increased risk of atherosclerosis, the prevalence of coronary calcification, and major adverse cardiovascular events. Additionally, the presence of microvascular diabetic complications, such as retinopathy, nephropathy, and neuropathy, heightens the risk of developing sarcopenia. The mechanisms linking these factors include IR, chronic inflammation, and advanced glycation end-products, which are exacerbated by reduced mobility, a feature common among patients with comorbid T2DM[7].
Numerous studies and lifestyle intervention programs have shown that engaging in physical activity can provide significant metabolic benefits, often comparable to pharmacological treatments for individuals with prediabetes and early-stage diabetes[8]. Recent molecular research has highlighted that muscle contraction can serve as an underutilized therapy for individuals with metabolic disorders independently or in conjunction with other treatments[9,10]. Indeed, evidence from original studies with decades-long follow-ups, such as Da Quing and the Diabetes Prevention Program, supports lifestyle interventions, including physical activity, in diabetes reversal and prevention[11,12]. This therapeutic effect is mediated through the skeletal muscle either directly, by diminishing peripheral IR and increasing muscular glucose uptake, or indirectly, via its endocrine function, supported by the release of over 600 myokines like myostatin, interleukin-6, interleukin-15, myonectin, etc., with irisin potentially playing a central role in metabolic regulation[13-16].
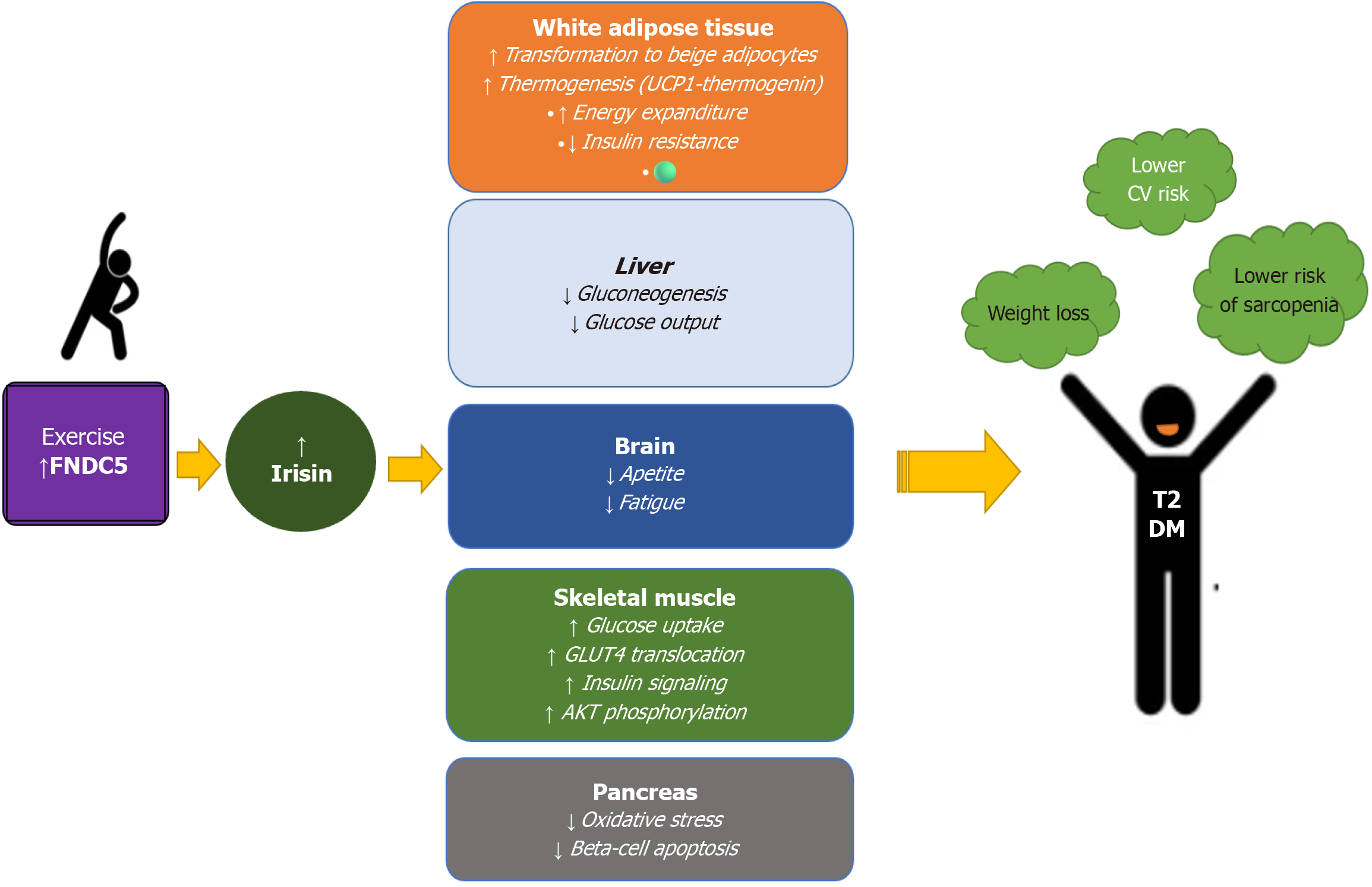
Skeletal muscle is crucial for movement and physical strength and functions as a vital endocrine organ. Recent research has uncovered its role in regulating metabolism and preventing metabolic diseases such as diabetes. When muscles contract, they release various signaling molecules known as myokines. One of the most significant ones for metabolic health is irisin, primarily (more than 72%) secreted by skeletal muscle, and in smaller amounts by heart, adipose tissue, and liver[17]. It is derived from a larger precursor, fibronectin type III domain-containing protein 5, a membrane-spanning protein cleaved to form irisin by an unknown protease[18]. Irisin synthesis and secretion are induced by acute bouts of exercise and peroxisome proliferator-activated receptor-γ coactivator 1-α, but irisin expression appears to be influenced by age, gender, and obesity[19]. Once secreted, irisin can regulate glucose homeostasis in skeletal muscle in an autocrine manner by stimulating glucose uptake via p38 mitogen-activated protein kinase-glucose transporter 4 translocation[20]. In addition, irisin can improve the expression of uncoupling protein 1 and promote the browning of white adipose tissue, increasing mitochondrial density, and potential for aerobic glycolysis with greater energy expenditure[21].
Furthermore, irisin could protect muscles from deterioration and dysfunction, and thus prevent diabetic myopathy. A potential explanation is the reduction of hyperglycemia and inflammation, and consequently diminished IR, and improved insulin sensitivity[22]. In rodent models, irisin's myogenic role was shown through the induction of skeletal muscle hypertrophy and at the same time attenuation of atrophy by activating interleukin (IL)-6, satellite cells, and protein synthesis[23]. Moreover, a study by Park et al[24] showed that irisin may function as a potential promyogenic factor in postmenopausal sarcopenia. Irisin also acts on the adipose tissue, stimulating white adipocyte browning and thermogenesis and reducing visceral fat[25,26]. Moreover, experimental studies showed that irisin has anti-apoptotic actions on pancreatic beta-cells which can stimulate their proliferation, insulin biosynthesis, and secretion[27,28]. Although measurement of irisin concentration is still challenging and faces many discrepancies related to the me
Besides, studies have shown a link between low circulating irisin levels and muscle weakness and atrophy, suggesting its potential as a biomarker of sarcopenia[33,34], and sarcopenic obesity, a common finding in T2DM patients[35]. A study by Oguz et al[36] showed irisin levels were significantly lower in patients with T2DM with sarcopenia (10.07 ± 6.17 ng/mL) compared to those measured in patients with T2DM and no sarcopenia (13.67 ± 9.07 ng/mL), P = 0.038. In contrast, the lowest irisin levels were detected in patients with T2DM and sarcopenic obesity (7.66 ± 5.27 ng/mL), so the authors suggested an irisin value of < 9.49 ng/mL to be a cut-off for detecting sarcopenic obesity with sensitivity and specificity of 75.8% and 78.1%, respectively[36]. When serum irisin levels were compared between T2DM patients with and without vascular complications, prior had the lowest values, suggesting irisin could be used as a biomarker to further stratify diabetic patients according to their potential risk of developing micro and macrovascular complications[36-39].
In people with T2DM, sarcopenia increases the risk of developing cardiovascular complications [cardiovascular disease (CVD), hazard ratio (HR) = 1.89, heart failure (HR = 2.59), stroke (HR = 1.90), and myocardial infarction (HR = 1.56)], which might occur 14.5 years earlier than in those without sarcopenia[40]. The risk factor connecting T2DM and CVD is muscle strength, which is lower in the diabetic population, where the prevalence of sarcopenia ranges between 7% and 29.3% and is potentially associated with excess CVD risk[41,42]. The molecular mechanisms responsible for complications are still not fully understood. However, it seems that chronic inflammation and oxidative stress play significant roles in their development through the promotion of atherosclerosis[43]. Studies suggest that irisin can reduce markers of inflammation, such as tumor necrosis factor-alpha and IL-6, which are elevated in diabetes and contribute to CVD. Moreover, irisin could protect the heart by improving endothelial function and exerting anti-inflammatory and antioxidant properties[44]. In a study comparing 350 patients with coronary artery disease with 214 healthy participants, low irisin levels were characteristic of the prior, and inversely correlated with the severity of atherosclerosis expressed using a coronary atherosclerosis index[45]. A similar correlation was also found for carotid artery intima-media thickness. Both findings are mainly speculated to be driven by irisin's regulation of glucose and lipid metabolism, while another ex
Although research is still in the early stages, there is evidence that irisin might prevent or reduce the severity of diabetic microvascular complications- nephropathy and retinopathy by promoting cellular repair and reducing inflammation[44,48]. Preliminary studies show irisin might also exert neuroprotective mechanisms, and promote nerve regeneration[48]. The production of irisin is largely triggered by muscle contraction during exercise. Regular physical activity that strengthens the muscles is expected to improve their function as an endocrine organ. Consequently, muscle-derived irisin could serve as a promising therapeutic target. However until the evidence is gathered from the large-scale prospective cohort studies validating the causal relationship between irisin levels and diabetic complications, irisin's true potential to prevent or treat vascular complications is speculative. Understanding the effects of different types of exercise on an individual level is crucial for optimizing and enhancing metabolic health.
Studies exploring the effects of physical exercise on irisin levels have yielded varying results suggesting exercise type, intensity, and duration, besides an individual's age, sex, and fitness level, might be important factors influencing irisin levels[49]. Aerobic exercise increases FNDC5 expression, which leads to the irisin release. Long-term moderate-intensity aerobic training increases irisin levels, which might be an explanation for the way it influences body composition and insulin sensitivity[50]. In addition, endurance training leads to a significant increase in irisin serum levels in middle-aged/older individuals, which inversely correlates with the reduction of visceral fat[51].
Resistance training and skeletal muscle contraction can activate PGC-1α and consequently increase irisin production[52,53]. Evidence suggests that a single bout of resistance exercise causes significantly greater elevations in plasma irisin compared to those after endurance exercise alone or after the same duration of combined resistance and endurance exercises[53]. A recent meta-analysis involving only data available from randomized control trials with a control group and including 921 participants (64% in the intervention group) suggests that physical exercise significantly increases circulating irisin and at the same time decreases insulin, glucose, and IR. For patients with prediabetes and T2DM, the best results can be achieved with resistance and combined training[54]. Although there is limited research on the effects of high-intensity training on irisin production and release, it seems it may increase irisin levels in T2DM patients, regardless of the interval or continuous modality of training[55]. Utilizing findings on physiological irisin levels in the human population is crucial for assessing exercise-based therapies, considering variations in ethnicity, age, weight, sex, and metabolic status. A key challenge is separating the metabolic benefits of exercise-induced irisin from the energy expenditure it brings. Achieving therapeutic irisin levels may require more than exercise alone. Current alternatives include antidiabetic agents like metformin and GLP-1RA, which boost FNDC5 expression, and inorganic nitrate, as shown in studies with beetroot juice[56,57]. Future research should clarify how irisin is released and cleared during different types of exercise. This, together with better defining basal irisin levels, is a prerequisite for its potential therapeutic use[58-60] (Figure 1).
Irisin is emerging as a promising molecule in the context of sarcopenia and diabetes. Experimental evidence of effects on insulin sensitivity, fat browning, inflammation, and diabetic complications suggest that it could offer a novel approach to managing and mitigating the long-term effects of diabetes, Despite promising results from animal models, clinical studies in humans are still limited and give conflictive results. The exact mechanisms through which irisin exerts its beneficial effects and the optimal levels for the therapeutic potential need further investigation. Moreover, future research is required to determine whether strategies to increase irisin levels—such as physical exercise, pharmacological agents, or supplementation—could serve as an effective adjunct to conventional diabetes treatment.
| 1. | Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1971] [Cited by in RCA: 2029] [Article Influence: 119.4] [Reference Citation Analysis (0)] |
| 2. | Haw JS, Galaviz KI, Straus AN, Kowalski AJ, Magee MJ, Weber MB, Wei J, Narayan KMV, Ali MK. Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern Med. 2017;177:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 270] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 3. | Lisco G, Disoteo OE, De Tullio A, De Geronimo V, Giagulli VA, Monzani F, Jirillo E, Cozzi R, Guastamacchia E, De Pergola G, Triggiani V. Sarcopenia and Diabetes: A Detrimental Liaison of Advancing Age. Nutrients. 2023;16:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 4. | Ackermans LLGC, Rabou J, Basrai M, Schweinlin A, Bischoff SC, Cussenot O, Cancel-Tassin G, Renken RJ, Gómez E, Sánchez-González P, Rainoldi A, Boccia G, Reisinger KW, Ten Bosch JA, Blokhuis TJ. Screening, diagnosis and monitoring of sarcopenia: When to use which tool? Clin Nutr ESPEN. 2022;48:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96:2898-2903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 585] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 6. | Shatila H, Ghazal N, Bukshaisha G, Al-Zeyara S, Khoury CFE, Bassil M. Risk and determinants of sarcopenia in people with diabetes: a case-control study from Qatar Biobank cohort. BMC Endocr Disord. 2024;24:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Purnamasari D, Tetrasiwi EN, Kartiko GJ, Astrella C, Husam K, Laksmi PW. Sarcopenia and Chronic Complications of Type 2 Diabetes Mellitus. Rev Diabet Stud. 2022;18:157-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 8. | Rintamäki R, Rautio N, Peltonen M, Jokelainen J, Keinänen-Kiukaanniemi S, Oksa H, Saaristo T, Puolijoki H, Saltevo J, Tuomilehto J, Uusitupa M, Moilanen L. Long-term outcomes of lifestyle intervention to prevent type 2 diabetes in people at high risk in primary health care. Prim Care Diabetes. 2021;15:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Thyfault JP, Bergouignan A. Exercise and metabolic health: beyond skeletal muscle. Diabetologia. 2020;63:1464-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (2)] |
| 10. | Pinto AC, Tavares P, Neves B, Oliveira PF, Vitorino R, Moreira-Gonçalves D, Ferreira R. Exploiting the therapeutic potential of contracting skeletal muscle-released extracellular vesicles in cancer: Current insights and future directions. J Mol Med (Berl). 2024;102:617-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, Zhang B, Feng X, Li H, Chen X, Cheng YJ, Gregg EW, Hu Y, Bennett PH, Li G; Da Qing Diabetes Prevention Study Group. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019;7:452-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 435] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 12. | Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 743] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 13. | Lee JH, Jun HS. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front Physiol. 2019;10:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 289] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 14. | Lundgren JR, Janus C, Jensen SBK, Juhl CR, Olsen LM, Christensen RM, Svane MS, Bandholm T, Bojsen-Møller KN, Blond MB, Jensen JB, Stallknecht BM, Holst JJ, Madsbad S, Torekov SS. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N Engl J Med. 2021;384:1719-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 327] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 15. | Cao X, Thyfault JP. Exercise drives metabolic integration between muscle, adipose and liver metabolism and protects against aging-related diseases. Exp Gerontol. 2023;176:112178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 16. | Qiu Y, Fernández-García B, Lehmann HI, Li G, Kroemer G, López-Otín C, Xiao J. Exercise sustains the hallmarks of health. J Sport Health Sci. 2023;12:8-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 149] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 17. | Perakakis N, Triantafyllou GA, Fernández-Real JM, Huh JY, Park KH, Seufert J, Mantzoros CS. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 453] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 18. | Korta P, Pocheć E, Mazur-Biały A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina (Kaunas). 2019;55:485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Ou-Yang WL, Guo B, Xu F, Lin X, Li FX, Shan SK, Wu F, Wang Y, Zheng MH, Xu QS, Yuan LQ. The Controversial Role of Irisin in Clinical Management of Coronary Heart Disease. Front Endocrinol (Lausanne). 2021;12:678309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belén Crujeiras A, Seoane LM, Casanueva FF, Pardo M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8:e60563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 484] [Article Influence: 37.2] [Reference Citation Analysis (1)] |
| 21. | Li J, Yi X, Li T, Yao T, Li D, Hu G, Ma Y, Chang B, Cao S. Effects of exercise and dietary intervention on muscle, adipose tissue, and blood IRISIN levels in obese male mice and their relationship with the beigeization of white adipose tissue. Endocr Connect. 2022;11:e210625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 22. | Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L, Srbecky M, Imrich R, Kyselovicova O, Belan V, Jelok I, Wolfrum C, Klimes I, Krssak M, Zemkova E, Gasperikova D, Ukropec J, Ukropcova B. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592:1091-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 322] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 23. | Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, Sharma M, Kambadur R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun. 2017;8:1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 24. | Park HS, Kim HC, Zhang D, Yeom H, Lim SK. The novel myokine irisin: clinical implications and potential role as a biomarker for sarcopenia in postmenopausal women. Endocrine. 2019;64:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 25. | Rabiee F, Lachinani L, Ghaedi S, Nasr-Esfahani MH, Megraw TL, Ghaedi K. New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci. 2020;10:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 26. | Hernández-Ochoa EO, Vanegas C. Diabetic Myopathy and Mechanisms of Disease. Biochem Pharmacol (Los Angel). 2015;4:e179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, Yang LJ, Tang D. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 565] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 28. | Zhang T, Yi Q, Huang W, Feng J, Liu H. New insights into the roles of Irisin in diabetic cardiomyopathy and vascular diseases. Biomed Pharmacother. 2024;175:116631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Zhong X, Sun X, Shan M, Zhao X, Zhang R, Zhao Y, Yang Q. The production, detection, and origin of irisin and its effect on bone cells. Int J Biol Macromol. 2021;178:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 30. | Liu S, Du F, Li X, Wang M, Duan R, Zhang J, Wu Y, Zhang Q. Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic β cells. PLoS One. 2017;12:e0175498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Song H, Wu F, Zhang Y, Zhang Y, Wang F, Jiang M, Wang Z, Zhang M, Li S, Yang L, Wang XL, Cui T, Tang D. Irisin promotes human umbilical vein endothelial cell proliferation through the ERK signaling pathway and partly suppresses high glucose-induced apoptosis. PLoS One. 2014;9:e110273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Song R, Zhao X, Zhang DQ, Wang R, Feng Y. Lower levels of irisin in patients with type 2 diabetes mellitus: A meta-analysis. Diabetes Res Clin Pract. 2021;175:108788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Li Y, Xu Z. Association between irisin and metabolic parameters in nondiabetic, nonobese adults: a meta-analysis. Diabetol Metab Syndr. 2022;14:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 34. | Chang JS, Kim TH, Nguyen TT, Park KS, Kim N, Kong ID. Circulating irisin levels as a predictive biomarker for sarcopenia: A cross-sectional community-based study. Geriatr Gerontol Int. 2017;17:2266-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 35. | Zhao M, Zhou X, Yuan C, Li R, Ma Y, Tang X. Association between serum irisin concentrations and sarcopenia in patients with liver cirrhosis: a cross-sectional study. Sci Rep. 2020;10:16093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Oguz A, Sahin M, Tuzun D, Kurutas EB, Ulgen C, Bozkus O, Gul K. Irisin is a predictor of sarcopenic obesity in type 2 diabetes mellitus: A cross-sectional study. Medicine (Baltimore). 2021;100:e26529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Carmona-Maurici J, Rosa A, Azcona-Granada N, Peña E, Ricart-Jané D, Viñas A, López-Tejero MD, Domingo JC, Miñarro A, Baena-Fustegueras JA, Peinado-Onsurbe J, Pardina E. Irisin as a Novel Biomarker of Subclinical Atherosclerosis in Severe Obesity. Int J Mol Sci. 2023;24:8171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Hu W, Wang R, Li J, Zhang J, Wang W. Association of irisin concentrations with the presence of diabetic nephropathy and retinopathy. Ann Clin Biochem. 2016;53:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Hou Q, Song R, Zhao X, Yang C, Feng Y. Lower circulating irisin levels in type 2 diabetes mellitus patients with chronic complications: A meta-analysis. Heliyon. 2023;9:e21859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 40. | Aronis KN, Moreno M, Polyzos SA, Moreno-Navarrete JM, Ricart W, Delgado E, de la Hera J, Sahin-Efe A, Chamberland JP, Berman R, Spiro A 3rd, Vokonas P, Fernández-Real JM, Mantzoros CS. Circulating irisin levels and coronary heart disease: association with future acute coronary syndrome and major adverse cardiovascular events. Int J Obes (Lond). 2015;39:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 41. | Boonpor J, Pell JP, Ho FK, Celis-Morales C, Gray SR. In people with type 2 diabetes, sarcopenia is associated with the incidence of cardiovascular disease: A prospective cohort study from the UK Biobank. Diabetes Obes Metab. 2024;26:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 42. | Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Cho YW, Newman AB; Health, Aging, and Body Composition Study. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30:1507-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 585] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 43. | Izzo A, Massimino E, Riccardi G, Della Pepa G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients. 2021;13:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 44. | Zhu D, Wang H, Zhang J, Zhang X, Xin C, Zhang F, Lee Y, Zhang L, Lian K, Yan W, Ma X, Liu Y, Tao L. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol. 2015;87:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 45. | Deng W. Association of Serum Irisin Concentrations with Presence and Severity of Coronary Artery Disease. Med Sci Monit. 2016;22:4193-4197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Saadeldin MK, Elshaer SS, Emara IA, Maged M, Abdel-Aziz AK. Serum sclerostin and irisin as predictive markers for atherosclerosis in Egyptian type II diabetic female patients: A case control study. PLoS One. 2018;13:e0206761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Lee MJ, Lee SA, Nam BY, Park S, Lee SH, Ryu HJ, Kwon YE, Kim YL, Park KS, Oh HJ, Park JT, Han SH, Ryu DR, Kang SW, Yoo TH. Irisin, a novel myokine is an independent predictor for sarcopenia and carotid atherosclerosis in dialysis patients. Atherosclerosis. 2015;242:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 48. | Emanuele E, Minoretti P, Pareja-Galeano H, Sanchis-Gomar F, Garatachea N, Lucia A. Serum irisin levels, precocious myocardial infarction, and healthy exceptional longevity. Am J Med. 2014;127:888-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Liu Y, Fu Y, Liu Z, Shu S, Wang Y, Cai J, Tang C, Dong Z. Irisin is induced in renal ischemia-reperfusion to protect against tubular cell injury via suppressing p53. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Mohammad Rahimi GR, Hejazi K, Hofmeister M. The effect of exercise interventions on Irisin level: a systematic review and meta-analysis of randomized controlled trials. EXCLI J. 2022;21:524-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 51. | Amanat S, Sinaei E, Panji M, MohammadporHodki R, Bagheri-Hosseinabadi Z, Asadimehr H, Fararouei M, Dianatinasab A. A Randomized Controlled Trial on the Effects of 12 Weeks of Aerobic, Resistance, and Combined Exercises Training on the Serum Levels of Nesfatin-1, Irisin-1 and HOMA-IR. Front Physiol. 2020;11:562895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Miyamoto-Mikami E, Sato K, Kurihara T, Hasegawa N, Fujie S, Fujita S, Sanada K, Hamaoka T, Tabata I, Iemitsu M. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLoS One. 2015;10:e0120354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Cosio PL, Crespo-Posadas M, Velarde-Sotres Á, Pelaez M. Effect of Chronic Resistance Training on Circulating Irisin: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int J Environ Res Public Health. 2021;18:2476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Wang C, Wang L, Liu J, Song J, Sun Y, Lin P, Liang K, Liu F, He T, Sun Z, Hou X, Chen L. Irisin modulates the association of interleukin-17A with the presence of non-proliferative diabetic retinopathy in patients with type 2 diabetes. Endocrine. 2016;53:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Ghanbari-Niaki A, Saeidi A, Ahmadian M, Gharahcholo L, Naghavi N, Fazelzadeh M, Mahjoub S, Myers S, Williams A. The combination of exercise training and Zataria multiflora supplementation increase serum irisin levels in postmenopausal women. Integr Med Res. 2018;7:44-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Leustean L, Preda C, Teodoriu L, Mihalache L, Arhire L, Ungureanu M. Role of Irisin in Endocrine and Metabolic Disorders—Possible New Therapeutic Agent? Appl Sci. 2021;11:5579. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Roberts LD, Ashmore T, McNally BD, Murfitt SA, Fernandez BO, Feelisch M, Lindsay R, Siervo M, Williams EA, Murray AJ, Griffin JL. Inorganic Nitrate Mimics Exercise-Stimulated Muscular Fiber-Type Switching and Myokine and γ-Aminobutyric Acid Release. Diabetes. 2017;66:674-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Parkin RA, Murray AJ. The therapeutic potential of irisin to mitigate the risk of metabolic syndrome in postmenopausal women. Front Reprod Health. 2024;6:1355922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 59. | Pekkala S, Wiklund PK, Hulmi JJ, Ahtiainen JP, Horttanainen M, Pöllänen E, Mäkelä KA, Kainulainen H, Häkkinen K, Nyman K, Alén M, Herzig KH, Cheng S. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol. 2013;591:5393-5400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 60. | Zhang Y, Xie C, Wang H, Foss RM, Clare M, George EV, Li S, Katz A, Cheng H, Ding Y, Tang D, Reeves WH, Yang LJ. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am J Physiol Endocrinol Metab. 2016;311:E530-E541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/