Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.105386
Revised: April 18, 2025
Accepted: June 13, 2025
Published online: December 20, 2025
Processing time: 195 Days and 4.9 Hours
Chest physiotherapy and incentive spirometry, essential for pulmonary care, can exacerbate acute post-thoracotomy pain. Pain relief is, therefore, essential to facilitate early mobilization. This study evaluated the analgesic efficacy of unilateral continuous erector spinae block (ESB) compared to thoracic epidural analgesia (TEA) in terms of quality of pain relief and perioperative hemodynamic changes.
To compare the analgesic efficacy of continuous ultrasound-guided unilateral ESB and thoracic epidural in patients undergoing antero-lateral thoracotomy.
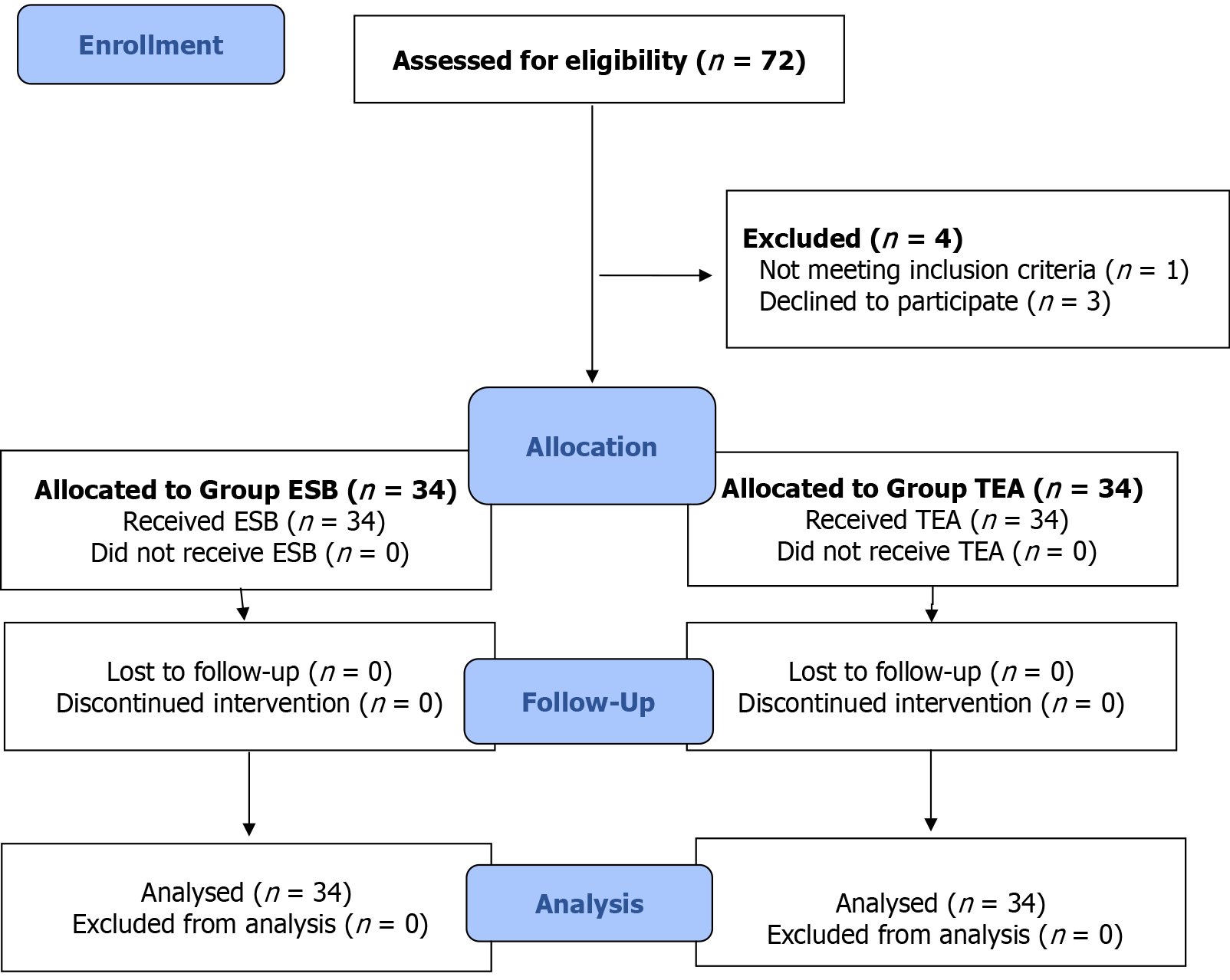
This prospective, observational study was conducted at a tertiary care hospital of central India. Sixty-eight adult patients of either gender, posted for elective thoracic surgeries requiring one lung ventilation, were allocated to either TEA (n = 34) or ESB (n = 34) group, based on the attending anesthesiologist’s expertise. Continuous data were analyzed by independent t-tests, and categorical data by χ2 tests.
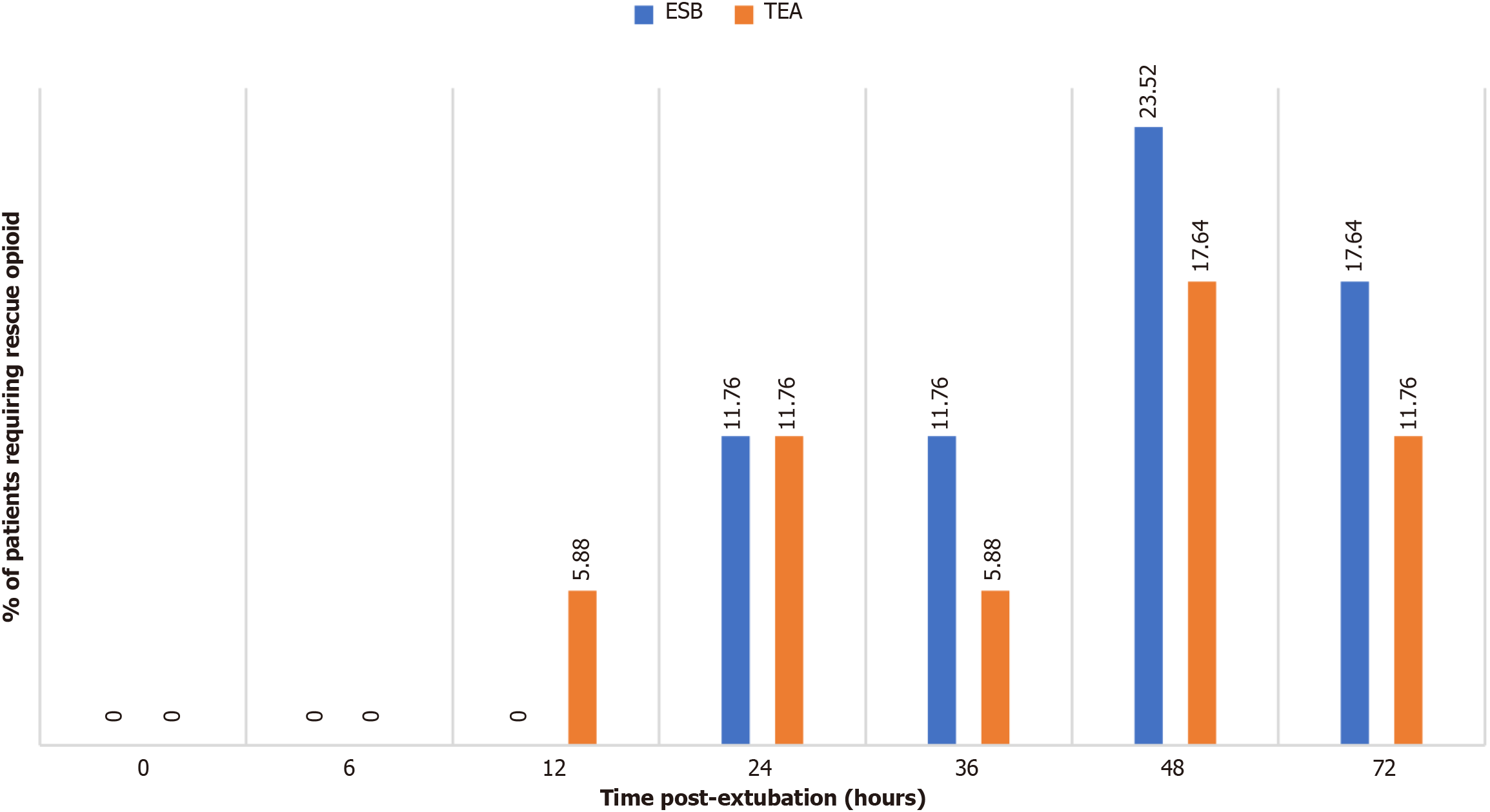
The proportion of patients requiring rescue opioids within 24 hours post-extubation was similar between the two group. Resting numerical rating scale scores (0 hour, 6 hours, and 72 hours post-extubation) were significantly higher in the ESB group compared to the TEA group [1.70 ± 1.03 vs 1.05 ± 0.77
The two techniques provided similar opioid-sparing effects following antero-lateral thoracotomy, though TEA exhibited a superior analgesic efficacy at the expense of increased hemodynamic instability requiring vasopressor support.
Core Tip: This prospective study compared the analgesic efficacy of unilateral continuous erector spinae block (ESB) vs thoracic epidural analgesia (TEA) in patients undergoing thoracotomy, evaluating pain scores and perioperative hemodynamics. While rescue opioid requirements within 24 hours post-extubation were comparable between the two groups, resting numerical rating scale scores at 0 hour, 6 hours, and 72 hours were significantly higher in the ESB cohort. Hemodynamically, the ESB group maintained higher mean arterial pressures, whereas TEA was associated with greater hypotensive episodes, necessitating significantly more vasopressor support to sustain mean arterial pressures ≥ 65 mmHg at 0 hours and 6 hours. Although TEA demonstrates superior analgesia, its use is limited by increased hemodynamic instability, suggesting ESB as a viable alternative when cardiovascular compromise is a concern.
- Citation: Jain A, Kaushal A, Kumar H, Karna ST, Ahmad Z, Trivedi S. Analgesic efficacy of continuous ultrasound-guided unilateral erector spinae block and thoracic epidural analgesia in patients undergoing antero-lateral thoracotomy. World J Methodol 2025; 15(4): 105386
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/105386.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.105386
Thoracotomy is widely recognized as one of the most painful surgical procedures[1]. Acute post-thoracotomy pain is aggravated by the constant chest wall movement of breathing[1]. Despite this, chest physiotherapy and incentive spirometry are encouraged to prevent postoperative atelectasis and retention of secretions. Inadequate pain management can lead to complications such as pulmonary issues (atelectasis, pneumonia, retention of bronchial secretions), car
The American Society of Anaesthesiology recommends multimodal perioperative pain management techniques[3]. Perioperative techniques for postoperative pain management include systemic opioids, peripheral regional analgesic techniques, and local anesthetic infiltration of incisions[3]. However, opioids can cause nausea, vomiting, pruritus, and respiratory depression, which may have detrimental effects on post-thoracotomy patients[4,5]. Various regional techniques such as thoracic epidural analgesia (TEA), paravertebral block, intercostal block, and intra-pleural analgesia have been widely used to manage post-thoracotomy pain[6-9]. While TEA remains the gold standard, it is associated with complications such as autonomic blockade and trauma to central neuraxial structures[10-12].
More recently, ultrasound-guided erector spinae block (ESB) has emerged as a promising technique for managing thoracic neuropathic pain, rib fractures, and postoperative pain following breast surgery[13]. This novel technique provides effective thoracic analgesia with a potentially safer profile than TEA or paravertebral blocks, as the injection site is farther from neurovascular structures and the pleura. Its efficacy in chronic thoracic pain management makes it an attractive alternative[14-16].
This study evaluated the analgesic efficacy of unilateral continuous ESB compared to TEA in terms of quality of pain relief and perioperative hemodynamic stability. By comparing these two regional analgesia techniques for post-thoracotomy pain, this study aimed to provide evidence for optimizing analgesic strategies.
The primary objective of this study was to compare the proportion of participants requiring rescue analgesia within 24 hours postoperatively. The secondary objectives included assessing static and dynamic pain scores at extubation, 6 hours, 12 hours, 24 hours, 36 hours, 48 hours, and 72 hours post-extubation, as well as evaluating postoperative hemodynamic instability between the two groups of patients undergoing antero-lateral thoracotomy.
This prospective, observational study was conducted at All India Institute of Medical sciences, Bhopal, India between January 2022 to November 2022. The institutional ethics committee approval was obtained and registration of trial with the Clinical Trial Registry-India (vide registration number CTRI/2021/12/038993, https://www.ctri.nic.in) was done. Sixty-eight adult patients of either gender belonging to American Society of Anaesthesiology physical status class II or III, posted for elective thoracic surgeries requiring one lung ventilation, were included in the study. Written informed consent was obtained from all the participants for the procedure and participation in the study and to use their data for research and educational purposes. The study was conducted in accordance with the 2013 declaration of Helsinki, and the reporting of the study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. Patients with anomalies of vertebral column, psychological disorders, or bleeding diathesis, on anti-coagulants, having blood or cerebro-spinal fluid tap during procedure, having opioid dependency or abuse, with a history of chronic pain on opioids, uncontrolled hypertension, or uncontrolled diabetes mellitus, and undergoing emergency surgery were excluded from the study.
Patients were allocated to either TEA (n = 34) or ESB (n = 34) group, based on the attending anesthesiologist’s expertise using a convenience sampling method. The patients were shifted inside the operation theatre and standard monitoring (non-invasive blood pressure, electrocardiogram, and pulse oximetry) was established. A wide bore intravenous (IV) cannula was secured and IV fluids were started at 8 mL/kg/hour. Continuous hemodynamic monitoring with a radial artery catheterization was done intraoperatively and postoperatively.
In the TEA group, a thoracic epidural catheter was inserted at T5-T6 level with the patient in the sitting position, using a 16G Touhy needle. After confirming the epidural space by the loss of resistance technique, the epidural catheter was threaded 4 cm into the epidural space. In the ESB group, ultrasound (USG)-guided unilateral erector spine block was performed in the lateral position with a 16G Touhy needle at T5-T6 level. After hydro-dissection with normal saline, an epidural catheter was threaded 4 cm into the erector spinae space. In both groups, the catheter was secured with 24G “Lock-it plus” epidural catheter securing device. After securing the catheter, general anaesthesia was induced. Thereafter, the patient was placed in the supine position and a loading dose of local anaesthetic (10 mL of 0.25% bupivacaine) was injected into the desired space, followed by a continuous infusion of 0.125% plain bupivacaine at a rate of 5-7 mL/hour till 48 hours post-extubation, and adjusted based on the patient’s numerical rating scale (NRS) pain scores.
General anaesthesia was induced with inj. glycopyrolate 4 mcg/kg, inj. midazolam 0.05 mg/kg, inj. fentanyl 2 mcg/kg, and inj. propofol 2-3 mg/kg IV. After confirming proper bag-mask ventilation, inj. vecuronium 0.1 mg/kg was administered to facilitate intubation. A disposable polyvinylchloride left-sided Robertshaw double-lumen tube was inserted. Correct placement of the double-lumen tube was confirmed by fibreoptic bronchoscopy and auscultation.
During one lung ventilation, all patients were ventilated with a tidal volume of 6 mL/kg with O2, air, and isoflurane and with a positive end-expiratory pressure of 5 cm of water. FiO2 was adjusted to maintain oxygen saturation up to 94% and respiratory rate was adjusted to keep the PCO2 and pH in the acceptable clinical range. Intraoperative analgesia was maintained with inj. paracetamol 15-20 mg/kg and inj, fentanyl in aliquots of 0.5 mcg/kg every 60 minutes guided by the hemodynamic parameters. Any episode of hypotension (a fall in systolic blood pressure of 20% or more of the baseline), if any, was noted and treated with inj. phenylephrine 50 mcg, followed by infusion of inj. nor-adrenaline at 0.05-0.15 mcg/kg/minute, to maintain a mean arterial pressure (MAP) of ≥ 65 mmHg. At the end of surgery, patients were extubated and shifted to Intensive Care Units for postoperative care.
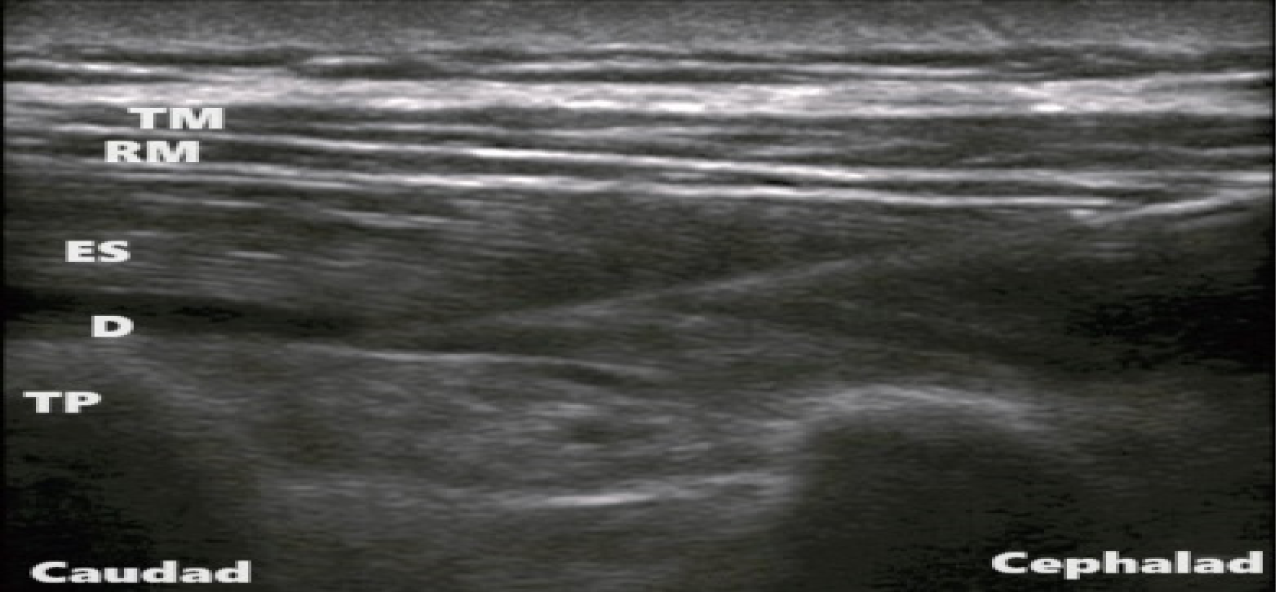
In the ESB group, USG-guided unilateral (depending upon side of thoracotomy) erector spinae plane block was performed under all aseptic precautions, in lateral position. A linear array, high frequency probe (5-13 MHz) of USG system was placed in longitudinal plane 3 cm lateral to the T6 spinous process corresponding to the T5 transverse process. Three muscles, i.e., the trapezius (uppermost), rhomboid major (middle), and erector spinae (lowermost), were identified superior to the hyper-echoic transverse process (Figure 1). Using in-plane approach, a 16 G Tuohy needle was inserted in caudal-cephalad direction, until the tip of needle was deep to the erector spinae muscle. Hydro-dissection below the muscle plane was done by injecting 5 mL of normal saline. An 18 G epidural catheter was then threaded 4 cm in cephalad direction.
In the TEA group, a thoracic epidural catheter was inserted under strict aseptic precautions, before induction of general anaesthesia. Local infiltration with 2% of lignocaine under the skin at T5-T6 intervertebral space was administered. An 18 G Tuohy needle was inserted at T5-T6 level to identify epidural space using loss of resistance technique. A 20 G catheter was threaded 4 cm in the epidural space.
A bolus dose of 10 mL of 0.25% plain bupivacaine was administered after induction followed by a continuous infusion of 0.125% plain bupivacaine at 5-7 mL/hour till 48 hours post-extubation in both the study groups. The pain assessment was performed using a 11-point NRS, i.e., 0-10. The postoperative pain assessment was done at rest (static pain) and during spirometry (dynamic pain) using the 11-point NRS tool at 0 hour (extubation), 6 hours, 12 hours, 24 hours, 36 hours, 48 hours, and 72 hours post-extubation. Respiratory efforts in the postoperative period were assessed by the number of balls raised in the spirometer, which indicates the peak inspiratory flow rate. In the postoperative period, a pain score of 4 or more on NRS at rest was defined as breakthrough pain[17]. IV paracetamol 1 gm every 6 hours was administered to patients in both the study groups. Rescue analgesia was administered, at NRS > 4 at rest or on patient’s demand with inj. fentanyl 0.5 mcg/kg.
During the postoperative period, any episode of hypotension (a fall in systolic blood pressure of 20% or more of the baseline), if any, was noted and treated with inj. nor-adrenaline infusion to maintain a MAP of 65 mmHg or more.
The proportion of patients requiring rescue opioid within 24 hours postoperatively, episodes of hemodynamic instability, and any other complications associated with each block were compared between the two groups. The primary outcome of this study was to compare the proportion of participants requiring rescue analgesia within 24 hours postoperatively. The secondary outcomes were to assess the static and dynamic pain scores at extubation, post-extubation at 6 hours, 12 hours, 24 hours, 36 hours, 48 hours, and 72 hours and any hemodynamic instability postoperatively between the two groups of patients undergoing antero-lateral thoracotomy.
Sakae et al[17] compared erector spinal plane block and epidural block techniques for postoperative analgesia for open cholecystectomies. They found that 43% of patients in the ESB group needed rescue opioid within 24 postoperative hours, whereas only 13% required the same in the TEA group. Using G × Power 3 software for Windows (University of Kiel, Kiel, Germany) for calculation of the sample size, and based on the differences in proportion of patients requiring rescue opioid within 24 hours postoperatively between the ESB and TEA groups in the study done by Sakae et al[17], a sample size of 34 participants in each group was required to achieve an alpha-error of 0.05 and with 80% as the power of study.
Data analyses were conducted using Statistical Package for the Social Sciences (International Business Machines Corporation, NY, United States) for Windows, version 21. The data analysts were blinded to group assignment. Variables including age, time taken to perform the block, breath holding time, pain scores, and MAP, presented as the mean ± SD, were compared using the independent Student’s t-test. Categorical variables such as gender, balls lifted in spirometer, and proportion of participants requiring rescue opioids, expressed as numbers (%), were analyzed using the χ2 test. A P-value of less than 0.05 was considered statistically significant.
Seventy-two patients were assessed for eligibility (Figure 2). Demographic and baseline parameters, including the number of balls lifted in the spirometer and breath-holding time, were comparable between the two study groups (Table 1). The proportion of patients requiring rescue opioids within 24 hours post-extubation was similar between the two study groups (4/34 vs 4/34, P = 1) (Figure 3). The time taken to perform the block was significantly longer in the ESB group as compared to the TEA group (16.58 ± 3.66 vs 13.84 ± 2.88, P = 0.001) (Table 1). The resting NRS scores at 0 hours, 6 hours, and 72 hours post-extubation were significantly higher in the ESB group as compared to the TEA group (1.70 ± 1.03 vs 1.05 ± 0.77, P = 0.004, 1.64 ± 0.98 vs 1.20 ± 0.88, P = 0.050, and 3.20 ± 1.07 vs 2.61 ± 0.92, P = 0.013, respectively) (Table 2). A significantly higher dynamic NRS was observed throughout the postoperative period in the ESB group as compared to the TEA group (Table 2).
| Parameter | ESB (n = 34) | TEA (n = 34) | P value |
| Age (year) | 46.1 ± 11.28 | 47.08 ± 12.07 | 0.750 |
| Gender (male:female) | 20/14 | 24/10 | 0.310 |
| Body mass index (kg/m2) | 24.7 ± 1.6 | 25.1 ± 1.8 | 0.520 |
| ASA physical status (II/III) | 28/6 | 31/3 | 0.280 |
| Balls lifted in spirometry preoperatively (1/2/3) | 15/14/5 | 10/18/6 | 0.450 |
| BHT (seconds) | 20.4 ± 2.04 | 20.35 ± 2.41 | 0.910 |
| Time taken to perform the block (minutes) | 16.58 ± 3.66 | 13.84 ± 2.88 | 0.001a |
| Type of surgery (lobectomy/ decortication/ pneumonectomy) | 12/14/8 | 12/15/7 | 0.100 |
| Intraoperative fentanyl consumed (mcg) | 196.41 ± 5.68 | 195.07 ± 6.74 | 0.370 |
| Intraoperative fluid administered (mL) | 1469.35 ± 28.64 | 1481.05 ± 29.64 | 0.100 |
| Intraoperative phenylephrine used (mcg) | 0.950 ± 0.3985 | 1.139 ± 1.1853 | 0.380 |
| Postoperative nor-adrenaline used (mg) | 3.49 ± 1.51 | 3.61 ± 0.96 | 0.680 |
| Time post-extubation (hours) | ESB (n = 34) | TEA (n = 34) | P value |
| Resting NRS | |||
| 0 | 1.70 ± 1.03 | 1.05 ± 0.77 | 0.004a |
| 6 | 1.64 ± 0.98 | 1.2 ± 0.88 | 0.055 |
| 12 | 2.17 ± 0.93 | 2.0 ± 1.10 | 0.480 |
| 24 | 2.76 ± 1.32 | 2.20 ± 1.32 | 0.080 |
| 36 | 2.64 ± 1.43 | 2.52 ± 1.18 | 0.710 |
| 48 | 3.29 ± 1.19 | 2.88 ± 1.25 | 0.170 |
| 72 | 3.2 ± 1.07 | 2.61 ± 0.92 | 0.010a |
| Dynamic NRS | |||
| 0 | 3.0 ± 1.3 | 1.7 ± 1.0 | 0.0001a |
| 6 | 3.23 ± 1.23 | 1.9 ± 1.05 | 0.0001a |
| 12 | 3.7 ± 1.08 | 2.8 ± 1.2 | 0.003a |
| 24 | 4.29 ± 1.46 | 2.9 ± 1.4 | 0.0001a |
| 36 | 4.23 ± 1.53 | 3.2 ± 1.2 | 0.0045a |
| 48 | 4.64 ± 1.34 | 3.58 ± 1.30 | 0.0015a |
| 72 | 4.52 ± 1.16 | 3.5 ± 0.99 | 0.0002a |
| Number of balls lifted in spirometry after surgery (1/2/3) | |||
| 0 | 10/22/2 | 2/25/7 | 0.015a |
| 6 | 8/16/10 | 1/11/22 | 0.002a |
| 12 | 10/10/14 | 1/7/26 | 0.003a |
| 24 | 6/16/12 | 1/9/24 | 0.008a |
| 36 | 4/18/12 | 1/12/21 | 0.060 |
| 48 | 4/18/12 | 1/9/24 | 0.020a |
| 72 | 4/16/14 | 1/6/27 | 0.005a |
The proportion of patients lifting one ball in spirometer was significantly more in the ESB group at 6 hours (8/34 vs 1/34, P = 0.012), 12 hours (10/34 vs 1/34, P = 0.003), and 24 hours (6/34 vs 1/34, P = 0.045) post-extubation as compared to the TEA group. The proportion of patients lifting two balls in spirometer at 48 hours (18/34 vs 9/34, P = 0.025) and 72 hours (16/34 vs 6/34, P = 0.009) post-extubation was higher in the ESB group. The proportion of patients lifting three balls in spirometer was significantly higher in the TEA group at 6 hours (10/34 vs 22/34, P = 0.003), 12 hours (14/34 vs 26/34, P = 0.003), 24 hours (12/34 vs 24/34, P = 0.003), 36 hours (12/34 vs 21/34, P = 0.029), 48 hours (12/34 vs 24/34, P = 0.003), and 72 hours (14/34 vs 27/34, P = 0.001) post-extubation.
The post-extubation MAPs were significantly higher in the ESB group as compared to the TEA group, and the proportion of patients requiring inotropic support to maintain a MAP of 65 mmHg was significantly higher in the TEA group at 0 hours (14/34 vs 0/34, P = 0.001) and 6 (14/34 vs 2/34, P = 0.001) hours post-extubation (Table 3). Total nor-adrenaline used postoperatively was higher in the TEA group (3.61 ± 0.96) as compared to the ESB group (3.49 ± 1.51), but statistically insignificant (P = 0.680).
| Time post-extubation (hours) | ESB (n = 34) | TEA (n = 34) | P value |
| MAP | |||
| 0 | 68.4 ± 3.15 | 58.8 ± 5.02 | < 0.0001a |
| 6 | 68.4 ± 6.16 | 60.2 ± 6.04 | < 0.0001a |
| 12 | 70.4 ± 5.58 | 61.2 ± 4.11 | < 0.0001a |
| 24 | 69.5 ± 5.08 | 63.14 ± 3.4 | < 0.0001a |
| 36 | 71.7 ± 4.8 | 64.7 ± 2.0 | < 0.0001a |
| 48 | 72.4 ± 4.36 | 64.58 ± 2.68 | < 0.0001a |
| 72 | 72.0 ± 4.36 | 65.8 ± 2.78 | < 0.0001a |
| Proportion of patients with postoperative inotropic requirement | |||
| 0 | 0/34 | 14/34 | 0.001a |
| 6 | 2/34 | 14/34 | 0.0012a |
| 12 | 2/34 | 8/34 | 0.08 |
| 24 | 2/34 | 6/34 | 0.260 |
| 36 | 0/34 | 0/34 | 1.00 |
| 48 | 0/34 | 0/34 | 1.00 |
| 72 | 0/34 | 0/34 | 1.00 |
This study provides robust data on the comparative effectiveness of ESB and TEA in thoracotomy patients. However, its external validity is influenced by patient demographics, procedural expertise, resource availability, and institutional practices. TEA’s superior analgesic efficacy and facilitation of pulmonary function recovery may render it the preferred technique in well-equipped centres. However, ESB offers a viable alternative in scenarios where hemodynamic stability is prioritized or where critical care resources are limited. Further studies in diverse populations and varied clinical settings are warranted to enhance the generalizability of these findings.
In thoracotomy patients, when regional analgesia is insufficient, opioids become the primary option for pain management. However, opioid use is associated with several side effects, including respiratory depression, nausea, vomiting, pruritus, delayed bowel function, and opioid dependence[4,5]. Thus, minimizing opioid consumption is crucial[18]. TEA, a well-established approach for thoracotomy pain, effectively reduces opioid requirements[4,5,17-19]. Forero et al[13,20] demonstrated that ESB is a simple and safe alternative for thoracic analgesia in both chronic neuropathic pain as well as acute postsurgical or post-traumatic pain. Hamilton et al[21] explained that by depositing local anaesthetic deep to the erector spinae muscle, i.e., in close proximity to the costotransverse foramina, ESB blocks both the dorsal and ventral rami of the thoracic spinal nerves at origin, and a continuous catheter inserted in this plane relieves pain in patient with multiple unilateral rib fractures. Thus, ESB can reduce the need of opioids in management of thoracotomy pain.
Singh et al[18] compared unilateral USG-guided ESB with TEA in paediatric thoracotomy patients and found comparable postoperative opioid-sparing effect. Similarly, our study demonstrated no significant difference in rescue opioid requirements between the ESB and TEA groups following antero-lateral thoracotomy.
However, the time required for block execution differed between the two studies. Singh et al[18] reported a shorter block performance time for ESB compared to TEA, while in our study, the ESB group had a significantly longer block performance time. This discrepancy can be attributed to several factors. In our adult population, acquiring a satisfactory ultrasound image for ESB often required more time due to anatomical variability, increased tissue depth, and patient habitus. In contrast, epidural placement, being a more routine and familiar procedure, was generally quicker. In pediatric patients, as studied by Singh et al[18], the relatively superficial anatomy may facilitate ultrasound visualization, but the difficulty in reliably palpating landmarks and the need for additional care during positioning can prolong epidural placement.
In our study, the resting NRS scores were significantly higher in the ESB group at 0 hours, 6 hours, and 72 hours and were comparable between the two groups at all other time-points. The dynamic NRS scores were significantly higher in the ESB group at all time-points post-extubation. These results are consistent with the study done by van den Broek et al[22]. Similarly, Nagaraja et al[4] observed that TEA was more effective than ESB in cardiac surgery patients, particularly in reducing movement-associated pain. Also, Hong et al[23] conducted a study comparing ESB to TEA for postoperative pain in video-assisted thoracic surgery and demonstrated that patients in the ESB group reported more pain and experienced fewer complications, such as nausea, dizziness, and hypotension. However, Singh et al[18] reported comparable pain scores between ESB and TEA in pediatric thoracotomy patients, suggesting that ESB may be equally effective in specific populations. This discrepancy could be attributed to differences in surgical approach (open thoracotomy vs video-assisted thoracoscopic surgery), patient age, or local anesthetic dosing. Our study, focusing on adult antero-lateral thoracotomy, supports the notion that TEA remains superior for dynamic pain control, possibly due to its more extensive blockade of thoracic dermatomes.
In the present study, the postoperative respiratory efforts were assessed by the number of balls raised in the spirometer, which indicates the peak inspiratory flow rate. The proportion of patient lifting three balls was significantly higher in the TEA group, suggesting a better pain control and potentially a better lung recruitment in the TEA group. This aligns with Joshi et al[9] who emphasized that effective analgesia is critical for preventing postoperative pulmonary complications such as atelectasis.
Multiple studies have consistently shown that TEA is associated with a higher risk of hypotension compared to ESB[18,19,24]. This trend has been observed across various surgical contexts - including thoracic trauma, hepatic surgery, and pediatric thoracotomy - where TEA often results in significant hemodynamic disturbances, likely due to its more extensive sympathetic blockade[18,19,24]. In contrast, ESB by targeting the dorsal rami of spinal nerves and sparing significant autonomic fibers, tends to preserve hemodynamic stability. Our findings align with this pattern, as the TEA group in our study exhibited significantly lower MAPs and higher postoperative inotropic requirements compared to the ESB group. These results reinforce the emerging view that ESB may offer a safer hemodynamic profile, particularly in patients at risk of instability.
Given these findings, the choice between ESB and TEA should be individualized based on patient factors and institutional resources. TEA may be preferable in centers with advanced hemodynamic monitoring, particularly for patients undergoing extensive thoracic resections where dynamic pain control is paramount, while ESB offers a safer alternative in resource-limited settings or for patients at high risk of hypotension (e.g., elderly, those with cardiac comorbidities).
This study has several limitations. First, patient allocation to ESB or TEA was based on anesthesiologist preference rather than randomization, introducing potential selection bias. Second, the lack of blinding for clinicians and patients may have influenced pain assessments and postoperative management. Third, the single-center design and limited sample size may restrict generalizability to other settings with differing patient demographics or surgical practices. Fourth, using a fixed local anesthetic infusion rate for all patients, without titration based on individual pain responses may have affected outcomes; a patient-controlled analgesia system or variable dosing could have optimized pain control. Finally, although all patients underwent antero-lateral thoracotomy, variations in surgical technique, tissue trauma, or surgeon experience may have confounded the comparison between ESB and TEA.
Both continuous unilateral ESB and TEA provide effective opioid-sparing postoperative analgesia for patients undergoing antero-lateral thoracotomy. While TEA demonstrates superior analgesic efficacy compared to ultrasound-guided unilateral ESB, it is associated with a higher incidence of postoperative hypotension and greater inotropic requirements.
| 1. | McGovern I, Walker C, Cox F. Pain relief after thoracotomy. Br J Anaesth. 2007;98:844; author reply 844-844; author reply 845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (97)] |
| 2. | Rogers ML, Henderson L, Mahajan RP, Duffy JP. Preliminary findings in the neurophysiological assessment of intercostal nerve injury during thoracotomy. Eur J Cardiothorac Surg. 2002;21:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (105)] |
| 3. | American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 969] [Article Influence: 69.2] [Reference Citation Analysis (107)] |
| 4. | Nagaraja PS, Ragavendran S, Singh NG, Asai O, Bhavya G, Manjunath N, Rajesh K. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann Card Anaesth. 2018;21:323-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (108)] |
| 5. | Xu ZZ, Li X, Zhang Z, Liu ZY, Song LL, Li XY, Zhang H. Ultrasound-guided erector spinae plane block versus thoracic paravertebral block on postoperative analgesia after laparoscopic nephroureterectomy: study protocol of a randomized, double-blinded, non-inferiority design trial. Trials. 2021;22:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Ochroch EA, Gottschalk A. Impact of acute pain and its management for thoracic surgical patients. Thorac Surg Clin. 2005;15:105-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Morris CJ, Bunsell R. Intrapleural blocks for chest wall surgery. Anaesthesia. 2014;69:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Schnabel A, Reichl SU, Kranke P, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2010;105:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 284] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Joshi GP, Bonnet F, Shah R, Wilkinson RC, Camu F, Fischer B, Neugebauer EA, Rawal N, Schug SA, Simanski C, Kehlet H. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008;107:1026-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 415] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 10. | Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006;104:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Giebler RM, Scherer RU, Peters J. Incidence of neurologic complications related to thoracic epidural catheterization. Anesthesiology. 1997;86:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 192] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Karmakar MK. Thoracic paravertebral block. Anesthesiology. 2001;95:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 372] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 13. | Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med. 2016;41:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1366] [Article Influence: 136.6] [Reference Citation Analysis (0)] |
| 14. | Yao Y, Fu S, Dai S, Yun J, Zeng M, Li H, Zheng X. Impact of ultrasound-guided erector spinae plane block on postoperative quality of recovery in video-assisted thoracic surgery: A prospective, randomized, controlled trial. J Clin Anesth. 2020;63:109783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Tulgar S, Ahiskalioglu A, De Cassai A, Gurkan Y. Efficacy of bilateral erector spinae plane block in the management of pain: current insights. J Pain Res. 2019;12:2597-2613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 16. | Huang W, Wang W, Xie W, Chen Z, Liu Y. Erector spinae plane block for postoperative analgesia in breast and thoracic surgery: A systematic review and meta-analysis. J Clin Anesth. 2020;66:109900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Sakae TM, Yamauchi LHI, Takaschima AKK, Brandão JC, Benedetti RH. [Comparison between erector spinal plane block and epidural block techniques for postoperative analgesia in open cholecystectomies: a randomized clinical trial]. Braz J Anesthesiol. 2020;70:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Singh S, Andaleeb R, Lalin D. Can ultrasound-guided erector spinae plane block replace thoracic epidural analgesia for postoperative analgesia in pediatric patients undergoing thoracotomy? A prospective randomized controlled trial. Ann Card Anaesth. 2022;25:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Zubair M, Adil Khan M, Khan MNA, Iqbal S, Ashraf M, Saleem SA. Comparison of Continuous Thoracic Epidural With Erector Spinae Block for Postoperative Analgesia in Adult Living Donor Hepatectomy. Cureus. 2022;14:e23151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Forero M, Rajarathinam M, Adhikary S, Chin KJ. Continuous Erector Spinae Plane Block for Rescue Analgesia in Thoracotomy After Epidural Failure: A Case Report. A A Case Rep. 2017;8:254-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Hamilton DL, Manickam B. Erector spinae plane block for pain relief in rib fractures. Br J Anaesth. 2017;118:474-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 22. | van den Broek RJC, Postema JMC, Koopman JSHA, van Rossem CC, Olsthoorn JR, van Brakel TJ, Houterman S, Bouwman RA, Versyck B. Continuous erector spinae plane block versus thoracic epidural analgesia in video-assisted thoracoscopic surgery: a prospective randomized open-label non-inferiority trial. Reg Anesth Pain Med. 2025;50:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Hong JM, Kim E, Jeon S, Lee D, Baik J, Cho AR, Cho JS, Ahn HY. A prospective double-blinded randomized control trial comparing erector spinae plane block to thoracic epidural analgesia for postoperative pain in video-assisted thoracic surgery. Saudi Med J. 2023;44:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Mostafa SF, Eid GM. Ultrasound guided erector spinae plane block versus thoracic epidural analgesia in traumatic flail chest, a prospective randomized trial. J Anaesthesiol Clin Pharmacol. 2023;39:250-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/