Published online Dec 20, 2024. doi: 10.5662/wjm.v14.i4.95598
Revised: May 12, 2024
Accepted: June 3, 2024
Published online: December 20, 2024
Processing time: 103 Days and 15.9 Hours
While prior data showed an increasing incidence of hepatocellular carcinoma (HCC) in the United States, there are limited comprehensive and comparative data on the geographical variations of HCC trends in different demographic-specific populations.
To evaluate sex and age-specific incidence rates and time trends in different geographical regions in the United States.
Age-adjusted HCC incidence rates were collected from the United States Cancer Statistics (USCS) database which covers approximately 98% of the population in the United States. HCC rates were stratified by sex, age, and geographical region. annual percentage change (APC) and average APC (AAPC) were estimated using Joinpoint Regression. A pairwise comparison was conducted between sex-specific trends.
There were 467344 patients diagnosed with HCC in the United States in the USCS database between 2001 and 2020. The rates and trends varied by geographical region. When looking at the West region (115336 patients), incidence rates of HCC were overall increasing and also increasing in older adults. However, when evaluating younger adults, HCC incidence rates decreased in men but not in women with a sex-specific absolute AAPC-difference of 2.15 (P = 0.005). When evaluating the Midwest region (84612 patients), similar results were seen. While incidence rates were increasing in the overall population and in older adults as well, they were decreasing in younger men but not in women with a sex-specific absolute AAPC-difference of 1.61 (P < 0.001). For the Northeast region (87259 patients), the analysis showed similar results with decreasing HCC incidence rates in younger men but not counterpart women (Sex-specific AAPC-difference = 3.26, P < 0.001). Lastly, when evaluating the south (180137 patients), the results were also decreasing in younger men but not in women (Sex-specific AAPC-difference = 2.55, P < 0.001).
Nationwide analysis covering around 98% of the United States population shows an increasing incidence of HCC across all geographical regions, most notably in the South. While younger men experienced decreasing HCC incidence, younger women had a stable trend and this was noted across all regions as well. Our study offers insight into the epidemiology of HCC in different demographic groups across various United States geographical regions. While the reasons contributing to our findings are unclear, they can be related to sex and regional disparities in healthcare access and utilization. Future research is warranted to characterize the temporal change in HCC risk factors across different United States regions.
Core Tip: In this retrospective study of the United States Cancer Statistics database (which covers approximately 98% of the United States population), we analyzed sex and age-specific hepatocellular carcinoma (HCC) incidence across different United States regions between 2001-2020. HCC incidence rates were significantly increasing in the West, Midwest, Northeast, and South of the United States, most notably in the South. While younger men experienced decreasing HCC incidence, younger women had non-decreasing incidence, and this was noted across all regions. While this can be due to regional disparities in healthcare access/utilization, future research is needed to investigate regional HCC risk factors, especially in younger adults.
- Citation: Abboud Y, Malhotra R, Maan MHA, Mathew A, Abboud I, Pan CW, Alsakarneh S, Jaber F, Mohamed I, Kim D, Pyrsopoulos NT. Hepatocellular carcinoma national burden across different geographical regions in the United States between 2001 and 2020. World J Methodol 2024; 14(4): 95598
- URL: https://www.wjgnet.com/2222-0682/full/v14/i4/95598.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i4.95598
Hepatocellular carcinoma (HCC) accounts for around 80% of liver cancers and its incidence has tripled since 1980[1]. Although HCC can occur sporadically, almost 90% of cases can be attributed to underlying liver diseases such as hepatitis C virus (HCV), hepatitis B virus (HBV), alcohol-associated liver disease, and metabolic dysfunction associated steatotic liver disease (MASLD). With the routine vaccination against HBV and an increase in the proportion of HCV patients with sustained virologic response, coupled with increased prevalence of MASLD, epidemiological changes in HCC risk factors are expected in the coming years[2].
HCC burden is unequally distributed with disparities occurring at various steps of the cancer care continuum including implementation of screening programs, access to specialist care, timely diagnosis, and treatment. HCC is usually rare under the age of 40 years, and its incidence increases with age before plateauing around the age of 70 years[3]. The incidence of HCC is disproportionately greater in males, with associated mortality reported to be three times higher than in women[4]. These differences are attributed to a variation in the prevalence of risk factors such as alcohol use, smoking, viral hepatitis, and MASLD which disproportionally affect men and women. The evolving trends in sex- and age-specific HCC incidence rates call for further exploration and analysis of data at a national level to guide future interventions.
American Cancer Society projects cancer deaths in 2024 from liver and bile duct cancer to be the highest in California (3580), followed by Texas (2960) and Florida (2180)[1]. Limited data comparing regional variation in HCC incidence show rates are highest in Texas, followed by Hawaii, New Mexico, and California; argued to be in part due to their racial/ethnic diversity[5]. While recent data showed an increasing overall incidence of HCC with variations based on age and sex[4], literature is scarce on demographic-specific trends across geographic regions. Understanding the epidemiological differences at a national level is imperative to identify regional variabilities in epidemiology and study their impact on HCC-associated morbidity and mortality. Therefore, we aimed to perform a comprehensive analysis of HCC incidence rates and time trends stratified by sex and age in different geographical regions in the United States using the United States Cancer Statistics (USCS) database.
We report a time trend comprehensive analysis of national incidence rates of HCC in the United States between 2001 and 2020 across various regions in the United States using publicly available and de-identified data from the USCS database which covers nearly 98% of the United States population[6]. HCC incidence rates were age-adjusted to the standard 2000 United States population using SEER Stat software [version 8.4.2, National Cancer Institute (NCI)]. The rates were categorized by sex and age into younger and older adults (defined with an age cutoff 55 years)[4]. HCC incidence rates were also stratified by geographical region in the United States into West, Midwest, Northeast, and South. Time-trends were reported as annual percentage change (APC) and average APC (AAPC) and were generated using Joinpoint Regression Software (version 5.0.2, NCI) via the weighted Bayesian Information Criteria (BIC) method (which is a data-driven analytical method used to estimate trends over time that is recommended to be used in large databases)[7-9]. A pairwise comparison was done between the sex-specific trends using the tests of parallelism and coincidence with a two-sided P-value cutoff at 0.05[10].
During the study period of 2001-2020, there were 467344 patients diagnosed with HCC in the United States. The majority of the patients were men (74.0%) and were diagnosed in the South (38.5%). HCC incidence rates and time trends varied between the cohorts across different geographical regions.
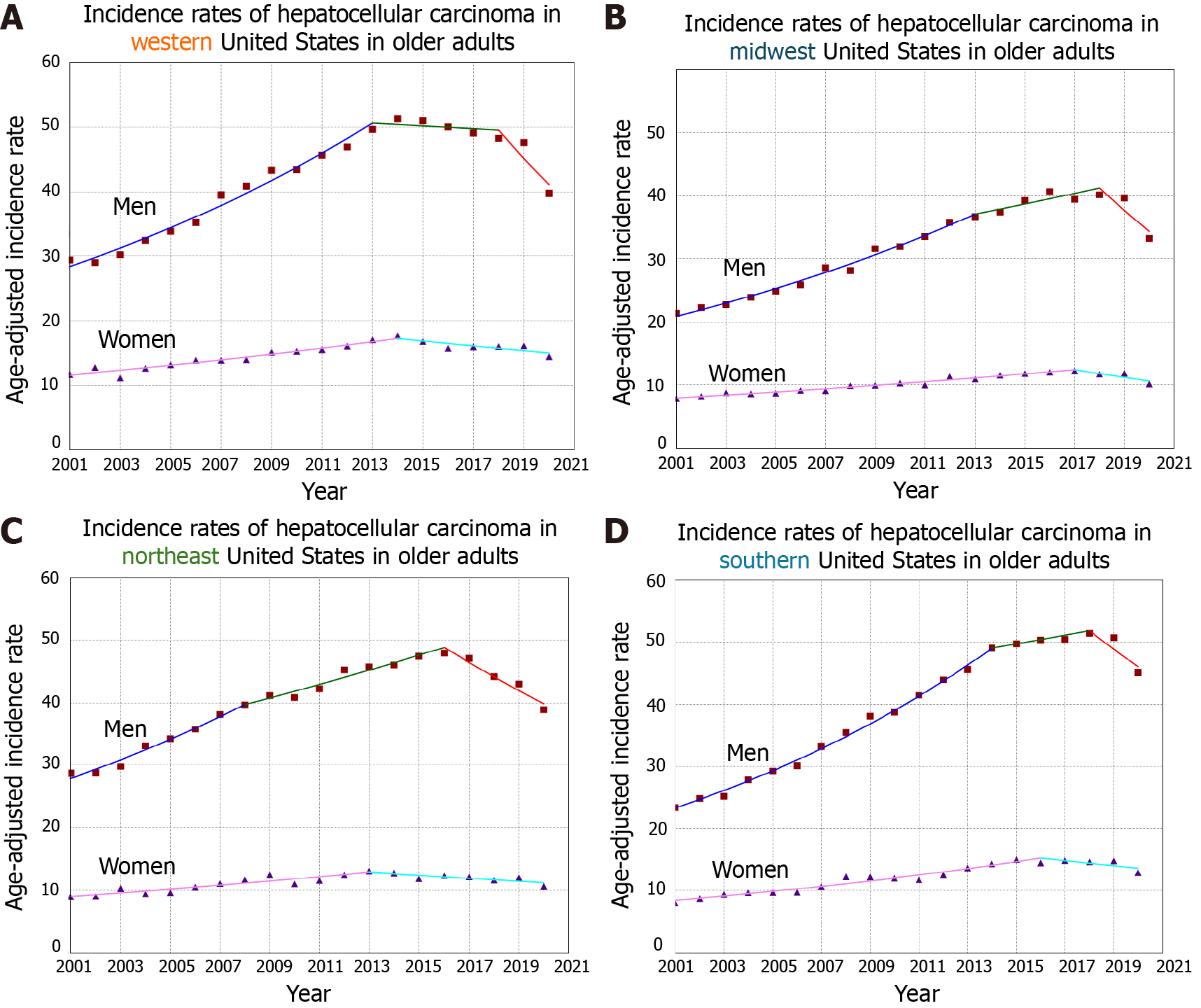
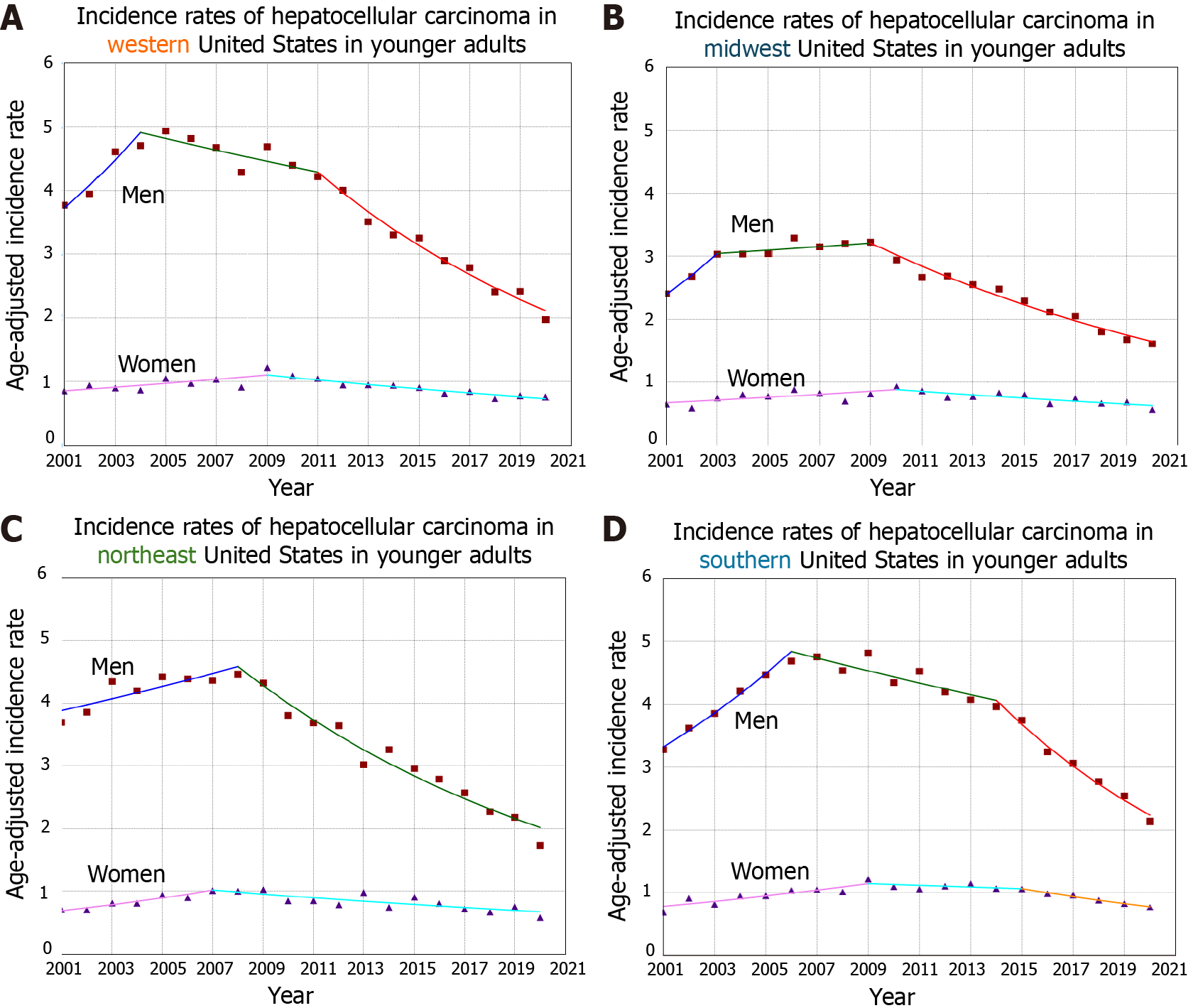
In the West regions (115336 patients; 26.9% women), HCC incidence rate per 100000 population significantly increased in the overall population from 5.57 to 6.48 between 2001 and 2020, and also in older adults (95152 patients; 28.2% women) from 19.66 to 26.31 between 2001 and 2020 (Figure 1). However, in younger adults (19160 patients; 19.8% women), while HCC incidence rates decreased in men from 3.77 to 1.97 between 2001 and 2020 (AAPC = -2.92, P < 0.001), the rates did not decrease in women (AAPC = -0.78, P = 0.10) with a significant difference between the sexes (Sex-specific absolute AAPC-difference = 2.15, P < 0.005) (Table 1 and Figure 2).
| Age group, year | Cases (n = 467344)1, (%) | Trends2 | Sex-specific AAPC difference (95%CI)3 | Pairwise comparison P values | ||||
| Time period | APC | AAPC | Sex-specific AAPC difference | Coincidence4 | Parallelism5 | |||
| West | ||||||||
| All ages | ||||||||
| Women | 31008 (6.64) | 2001-2014 | 2.66a | 0.96a | -0.02 (-0.86 to 0.82) | 0.95 | < 0.001 | 0.008 |
| 2014-2020 | -2.61a | |||||||
| Men | 84328 (18.04) | 2001-2009 | 4.42a | 0.94a | ||||
| 2009-2014 | 1.66a | |||||||
| 2014-2018 | -1.72 | |||||||
| 2018-2020 | -8.68a | |||||||
| Aged ≥ 55 years | ||||||||
| Women | 26804 (5.74) | 2001-2014 | 3.10a | 1.36a | 0.61 (-0.42 to 1.65) | 0.24 | < 0.001 | 0.002 |
| 2014-2020 | -2.30a | |||||||
| Men | 68348 (14.62) | 2001-2013 | 4.95a | 1.97a | ||||
| 2013-2018 | -0.44 | |||||||
| 2018-2020 | -8.91a | |||||||
| Aged < 55 years | ||||||||
| Women | 3786 (0.81) | 2001-2009 | 3.26a | -0.78 | -2.15a (-3.65 to -0.64) | 0.005 | < 0.001 | < 0.001 |
| 2009-2020 | -3.61a | |||||||
| Men | 15383 (3.29) | 2001-2004 | 9.67a | -2.92a | ||||
| 2004-2011 | -1.91a | |||||||
| 2011-2020 | -7.54a | |||||||
| All ages | ||||||||
| Women | 3786 (0.810) | 2001-2009 | 3.26a | -0.78 | -2.15a (-3.65 to -0.64) | 0.005 | < 0.001 | < 0.001 |
| 2009-2020 | -3.61a | |||||||
| Men | 15383 (3.291) | 2001-2004 | 9.67a | -2.92a | ||||
| 2004-2011 | -1.92a | |||||||
| 2011-2020 | -7.54a | |||||||
| Midwest | ||||||||
| All ages | ||||||||
| Women | 22675 (4.851) | 2001-2017 | 2.42a | 1.12a | 0.50 (-0.54 to 1.55) | 0.34 | < 0.001 | 0.02 |
| 2017-2020 | -5.56a | |||||||
| Men | 61937 (13.252) | 2001-2012 | 3.90a | 1.62a | ||||
| 2012-2018 | 0.99 | |||||||
| 2018-2020 | -8.38a | |||||||
| Aged ≥ 55 years | ||||||||
| Women | 19403 (4.151) | 2001-2017 | 2.82a | 1.53a | 1.12a (0.04 to 2.19) | 0.04 | < 0.001 | < 0.001 |
| 2017-2020 | -5.08a | |||||||
| Men | 51228 (10.961) | 2001-2013 | 4.89a | 2.65a | ||||
| 2013-2018 | 2.15a | |||||||
| 2018-2020 | -8.72a | |||||||
| Aged < 55 years | ||||||||
| Women | 2942 (0.629) | 2001-2010 | 3.02a | -0.35 | -1.61 (-3.57 to 0.36) | 0.10 | < 0.001 | < 0.001 |
| 2010-2020 | -3.28a | |||||||
| Men | 10227 (2.188) | 2001-2003 | 12.75a | -1.95a | ||||
| 2003-2009 | 0.88 | |||||||
| 2009-2020 | -5.89a | |||||||
| Northeast | ||||||||
| All ages | ||||||||
| Women | 21729 (4.645) | 2001-2013 | 2.67a | 0.74 | 0.14 (-0.90 to 1.17) | 0.79 | < 0.001 | 0.2 |
| 2013-2020 | -2.48a | |||||||
| Men | 65530 (14.021) | 2001-2007 | 4.77a | 0.88a | ||||
| 2007-2016 | 1.25a | |||||||
| 2016-2020 | -5.49a | |||||||
| Aged ≥ 55 years | ||||||||
| Women | 18703 (4.001) | 2001-2013 | 3.01a | 1.13a | 0.76 (-0.26 to 1.78) | 0.14 | < 0.001 | < 0.001 |
| 2013-2020 | -2.00a | |||||||
| Men | 53688 (11.487) | 2001-2008 | 5.17a | 1.89a | ||||
| 2008-2016 | 2.62a | |||||||
| 2016-2020 | -4.97a | |||||||
| Aged < 55 years | ||||||||
| Women | 2763 (0.591) | 2001-2007 | 6.63a | -0.13 | -3.26a (-5.08 to -1.43) | < 0.001 | < 0.001 | <0.001 |
| 2007-2020 | -3.10a | |||||||
| Men | 11462 (2.452) | 2001-2008 | 2.39a | -3.38a | ||||
| 2008-2020 | -6.60a | |||||||
| South | ||||||||
| All ages | ||||||||
| Women | 45975 (9.837) | 2001-2015 | 3.74a | 2.08a | 0.46 (-0.53 to 1.45) | 0.36 | < 0.001 | 0.01 |
| 2015-2020 | -2.42 | |||||||
| Men | 134162 (28.707) | 2001-2007 | 6.14a | 2.54a | ||||
| 2007-2014 | 3.66a | |||||||
| 2014-2018 | 0.05 | |||||||
| 2018-2020 | -6.50a | |||||||
| Aged ≥ 55 years | ||||||||
| Women | 38809 (8.304) | 2001-2016 | 3.99a | 2.53a | 1.12a (0.10 to 2.13) | 0.03 | < 0.001 | < 0.001 |
| 2016-2020 | -2.77 | |||||||
| Men | 108315 (23.176) | 2001-2014 | 5.89a | 3.65a | ||||
| 2014-2018 | 1.4 | |||||||
| 2018-2020 | -5.74a | |||||||
| Aged < 55 years | ||||||||
| Women | 6576 (1.407) | 2001-2009 | 4.77a | 0.5 | -2.01a (-3.77 to -0.24) | 0.02 | < 0.001 | < 0.001 |
| 2009-2015 | -1.2 | |||||||
| 2015-2020 | -6.00a | |||||||
| Men | 25030 (5.355) | 2001-2006 | 7.81a | -2.05a | ||||
| 2006-2014 | -2.16a | |||||||
| 2014-2020 | -9.46a | |||||||
When evaluating the Midwest (84612 patients; 26.8% women), similar results were seen where HCC incidence rates per 100000 population were significantly increasing in the overall population from 3.84 to 5.16 between 2001 and 2020, and also in older adults (70631 patients; 27.5% women) from 13.65 to 20.83 between 2001 and 2020 (Figure 1). However, in younger adults (13169 patients; 22.3% women), while HCC incidence rates were decreasing in men from 2.40 to 1.61 between 2001 and 2020 (AAPC = -1.95, P = 0.002), the rates did not decrease in women (AAPC = -0.35, P = 0.65) with an absolute sex-specific AAPC -difference of 1.61, P = 0.1 (Table 1 and Figure 2).
In the Northeast (87259 patients; 24.9% women), similar results were also seen where HCC incidence rates per 100000 population were increasing in the overall population from 5.01 to 5.74, and in older adults (72391 patients; 225.8% women) from 17.36 to 23.41, between 2001 and 2020 (Figure 1). However, in younger adults (14225 patients; 19.4% women), HCC rates were decreasing in men from 3.70 to 1.73 between 2001 and 2020 (AAPC= -3.38, P < 0.001), and this was not seen in women who experienced a stable trend (AAPC= -0.13, P = 0.87) with an absolute sex-specific AAPC-difference of 3.26, P < 0.001 (Table 1 and Figure 2).
Lastly, in the south (180137 patients; 25.5% women), similar results were also seen with increasing HCC rates per 100000 population in the overall population from 4.31 to 6.81 between 2001 and 2020, and also in older adults from 14.69 to 27.68 between 2001 and 2020 (Figure 1). However, younger adults (467344 patients; 20.8% women) experienced decreasing HCC incidence rates in men from 3.28 in 2001 to 2.14 in 2020 (AAPC = -2.05, P < 0.001), but not in women who had a stable trend (AAPC = -0.05, P = 0.95) with an absolute sex-specific AAPC-difference of 2.55, P < 0.001 (Table 1 and Figure 2).
Our nationwide analysis of data covering nearly all HCC patients in the United States shows increasing overall incidence of HCC in older adults across all geographical United States regions, most notably in the South. However, in younger adults, HCC incidence was decreasing in men but not in counterpart women, and that sex-specific difference was seen across all geographical regions in the United States.
As of 2020, primary liver cancer is the fifth most common cancer in men worldwide, the ninth most common cancer in women worldwide, and the sixth most common cancer diagnosed overall[11]. According to the NCI, in 2023, there were about 41000 diagnoses and about 29000 deaths related to primary liver cancer in the United States. A recent analysis using the USCS database showed increasing overall HCC incidence, and decreasing rates in younger men but not women[4]. Recent data also suggests that HCC incidence is trending away from male predominance in younger adults, with a decreasing male-to-female incidence ratio[5]. We add to prior literature showing that the greatest increase in overall HCC was in the South and the greatest decrease in HCC incidence in younger men was in the Northeast. We also show that the stable trend in younger women was noted across all United States regions. These differences are multifactorial, in large part due to the racial and geographical disparities in access to and quality of care. The findings of non-decreasing HCC incidence rate in younger females may be attributable to the changing prevalence of risk factors over recent years across all regions of the United States[4].
A prior analysis of the SEER database of 43868 patients between 2000-2012 evaluating the most at-risk group in the United States (Southern region) compared to other regions, showed that blacks comprised a larger proportion of HCC patients in the South compared to other areas (32.4% vs 10.1%) and were diagnosed at a younger age, with more advanced stage at diagnosis and more metastases[12]. Furthermore, black patients were 58% less likely to receive liver transplant and 36% less likely to receive surgical therapy for HCC compared to patients of white race[13]. Of interest, this study also saw similar radial disparities in HCC outcomes in geographical regions outside the Southern United States. Another analysis of the SEER database from 1975-2017 showed that HCC mortality rates were highest in the South, followed by the West, Northeast, and Midwest[14]. These differences are multifactorial, in large part due to the racial and geographical disparities in access to and quality of care[15]. Our study builds on these prior studies, using a significantly larger sample size over a more recent period and stratifying the analysis by sex and age, demonstrating that the greatest increase in HCC incidence was in the South.
The introduction of direct-acting-antiviral treatment for HBV and HCV along with the increasing obesity epidemic in the United States has shifted the leading causes of HCC from viral hepatitis toward MASLD[16,17]. However, access to treatment has been a subject of public health concern, as fewer Medicaid and Medicare recipients with HCV receive timely treatment compared to those privately insured[18]. Furthermore, black Medicaid recipients are less likely to receive direct-acting antiviral therapy than white counterparts[18,19]. Viral hepatitis also disproportionately affects the black population. A prior study using the National Health and Nutrition Examination Survey database found that African Americans had a two to three-fold higher prevalence of chronic HBV than the general population between 1999 and 2008[20]. According to the United States Census Bureau in 2021, nine of the ten poorest states are in the South. These factors could explain the greater increase in HCC incidence in the South. These findings demonstrate a need for targeted public health intervention and multidisciplinary care in this high-risk, underserved, largely uninsured population.
MASLD is a leading cause of HCC in Western countries[21]. Sex differences in metabolic risk factors for HCC may explain the trends we observed. In the more recent years of our study period, in all regions but the Midwest, the HCC incidence rate in women older than 55 years of age has decreased less than that of men over 55 years of age. This could be explained in part by decreased estrogen levels in postmenopausal women. Estrogen may have protective effects on hepatic fibrinogenesis, causing later onset of HCC in the female population[22,23]. A single-center study reported significantly higher rates of MASLD in older women compared to younger women and older men[24]. Patients without screenable etiologies to cirrhosis are disproportionately identified later in the disease course; postmenopausal women may be part of this population[25,26]. Having said that, it is hard to blame a single risk factor for the observed findings, especially with the variation in environmental exposures, biologics, and socioeconomic status between different cohorts in the United States in different regions.
Our study has several strengths which include the stratified analysis by age and sex across different geographical regions, in addition to the large sample size (467344 patients), recent period (2001-2020), and the use of joinpoint regression with the BIC method and comparative analysis. With that in mind, our study is limited in the lack of clinical variables to assess for HCC risk factors. However, our manuscript is observational, and its epidemiological retrospective design is hypothesis-generating and aims to guide future efforts toward further investigations of the contributions leading to increasing HCC incidence across different United States populations and geographical locations. Our study suffer from other limitations inherent in large databases such as loss of records and coding reliability[27]. However, all the data used in our analysis were obtained from the USCS database which is the official source of cancer incidence data in the United States and undergoes many processes to ensure high-quality standardization and coding per the standards of the North American Association of Central Cancer Registries.
Our study offers insight into the epidemiology of HCC in different demographic groups across various United States geographical regions. While overall HCC incidence was increasing across all geographical regions, Southern states experienced the steepest increase. The non-decreasing trend in younger women was noted across different regions, compared to counterpart younger men who experienced a decreasing trend. The reasons contributing to our findings are unclear and can be related to sex and regional disparities in healthcare access and utilization. Future research is warranted to characterize the temporal change in HCC risk factors across different United States regions.
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 6058] [Article Influence: 3029.0] [Reference Citation Analysis (4)] |
| 2. | El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Hepatology. 2014;60:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 503] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 3. | Zhang X, El-Serag HB, Thrift AP. Sex and Race Disparities in the Incidence of Hepatocellular Carcinoma in the United States Examined through Age-Period-Cohort Analysis. Cancer Epidemiol Biomarkers Prev. 2020;29:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Abboud Y, Ismail M, Khan H, Medina-Morales E, Alsakarneh S, Jaber F, Pyrsopoulos NT. Hepatocellular Carcinoma Incidence and Mortality in the USA by Sex, Age, and Race: A Nationwide Analysis of Two Decades. J Clin Transl Hepatol. 2024;12:172-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Ajayi F, Jan J, Singal AG, Rich NE. Racial and Sex Disparities in Hepatocellular Carcinoma in the USA. Curr Hepatol Rep. 2020;19:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | USCS. U.S. Cancer Statistics Public Use Research Database, 2022 Submission (2001-2020). United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Available from: https://www.cdc.gov/cancer/uscs/public-use/. |
| 7. | Kim HJ, Yu B, Feuer EJ. Selecting the number of change-points in segmented line regression. Stat Sin. 2009;19:597-609. [PubMed] |
| 8. | Kim J, Kim HJ. Consistent Model Selection in Segmented Line Regression. J Stat Plan Inference. 2016;170:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Gillis D, Edwards BPM. The utility of joinpoint regression for estimating population parameters given changes in population structure. Heliyon. 2019;5:e02515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Kim HJ, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60:1005-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 244] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68596] [Article Influence: 13719.2] [Reference Citation Analysis (201)] |
| 12. | Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and Geographic Disparities in Hepatocellular Carcinoma Outcomes. Am J Prev Med. 2018;55:S40-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Zak Y, Rhoads KF, Visser BC. Predictors of surgical intervention for hepatocellular carcinoma: race, socioeconomic status, and hospital type. Arch Surg. 2011;146:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Yao Z, Dai C, Yang J, Xu M, Meng H, Hu X, Lin N. Time-trends in liver cancer incidence and mortality rates in the U.S. from 1975 to 2017: a study based on the Surveillance, Epidemiology, and End Results database. J Gastrointest Oncol. 2023;14:312-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 15. | Baicker K, Chandra A, Skinner JS. Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med. 2005;48:S42-S53. [PubMed] |
| 16. | Bhattacharya D, Aronsohn A, Price J, Lo Re V; AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2023 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 17. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1833] [Article Influence: 229.1] [Reference Citation Analysis (0)] |
| 18. | Thompson WW, Symum H, Sandul A; DHSc, Gupta N, Patel P, Nelson N, Mermin J, Wester C. Vital Signs: Hepatitis C Treatment Among Insured Adults - United States, 2019-2020. MMWR Morb Mortal Wkly Rep. 2022;71:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 19. | O'Brien TR, Devesa SS, Koshiol J, Marrero JA, Shiels MS. Decreasing incidence of hepatocellular carcinoma among most racial groups: SEER-22, 2000-2019. Cancer Med. 2023;12:19960-19967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 20. | Roberts H, Kruszon-Moran D, Ly KN, Hughes E, Iqbal K, Jiles RB, Holmberg SD. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016;63:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 4019] [Article Influence: 502.4] [Reference Citation Analysis (2)] |
| 22. | Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, Suzuki A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 23. | Farinati F, Sergio A, Giacomin A, Di Nolfo MA, Del Poggio P, Benvegnù L, Rapaccini G, Zoli M, Borzio F, Giannini EG, Caturelli E, Trevisani F; Italian Liver Cancer group. Is female sex a significant favorable prognostic factor in hepatocellular carcinoma? Eur J Gastroenterol Hepatol. 2009;21:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Wu EM, Wong LL, Hernandez BY, Ji JF, Jia W, Kwee SA, Kalathil S. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Ahmed Mohammed HA, Yang JD, Giama NH, Choi J, Ali HM, Mara KC, Harmsen WS, Wiesner RH, Leise MD, Therneau TM, Roberts LR. Factors Influencing Surveillance for Hepatocellular Carcinoma in Patients with Liver Cirrhosis. Liver Cancer. 2017;6:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Palmer LB, Kappelman MD, Sandler RS, Hayashi PH. Surveillance for hepatocellular carcinoma in a Medicaid cirrhotic population. J Clin Gastroenterol. 2013;47:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Limitations and biases of the Surveillance, Epidemiology, and End Results database. Curr Probl Cancer. 2012;36:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/