Published online Dec 20, 2024. doi: 10.5662/wjm.v14.i4.95685
Revised: June 28, 2024
Accepted: July 5, 2024
Published online: December 20, 2024
Processing time: 101 Days and 4.9 Hours
Point-of-care ultrasound (POCUS) is a limited ultrasound examination performed by the clinician at the bedside, emerging as a complement to physical examination across various medical specialties. In the field of nephrology, its integration has been gradual, primarily limited to guiding procedures like temporary dialysis catheter placement or, in some cases, diagnostic kidney ultrasounds. In reality, the assessment of hemodynamic status at the bedside holds immense value for nephrologists, yet there exists limited awareness among practitioners regarding its implementation. While there is a growing trend towards incorporating multi-organ POCUS training in fellowship programs, private practice nephrologists remain relatively uninformed. This discussion explores the untapped potential of POCUS as a valuable diagnostic tool in everyday nephrology practice, demon
Core Tip: The integration of point-of-care ultrasound (POCUS) into nephrology practice offers significant potential to streamline diagnosis and enhance patient care. Despite some barriers to implementation, such as time constraints and initial costs, the benefits of POCUS in improving diagnostic accuracy and practical outcomes are substantial. By leveraging available resources and gradually incorporating POCUS into practice, nephrologists can overcome these barriers and harness the full potential of this valuable diagnostic tool.
- Citation: Sinanan R, Moshtaghi A, Koratala A. Point-of-care ultrasound in nephrology: A private practice viewpoint. World J Methodol 2024; 14(4): 95685
- URL: https://www.wjgnet.com/2222-0682/full/v14/i4/95685.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i4.95685
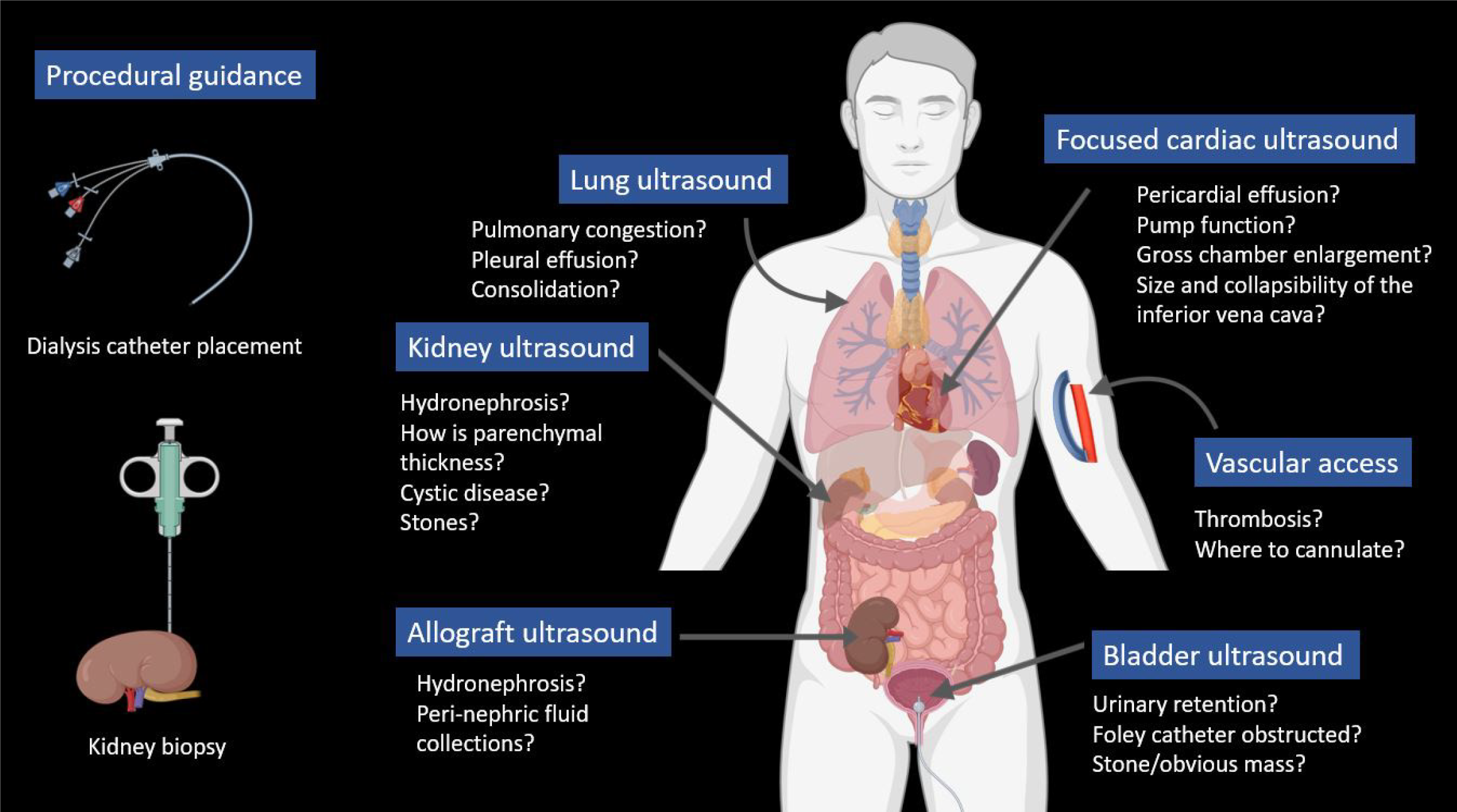
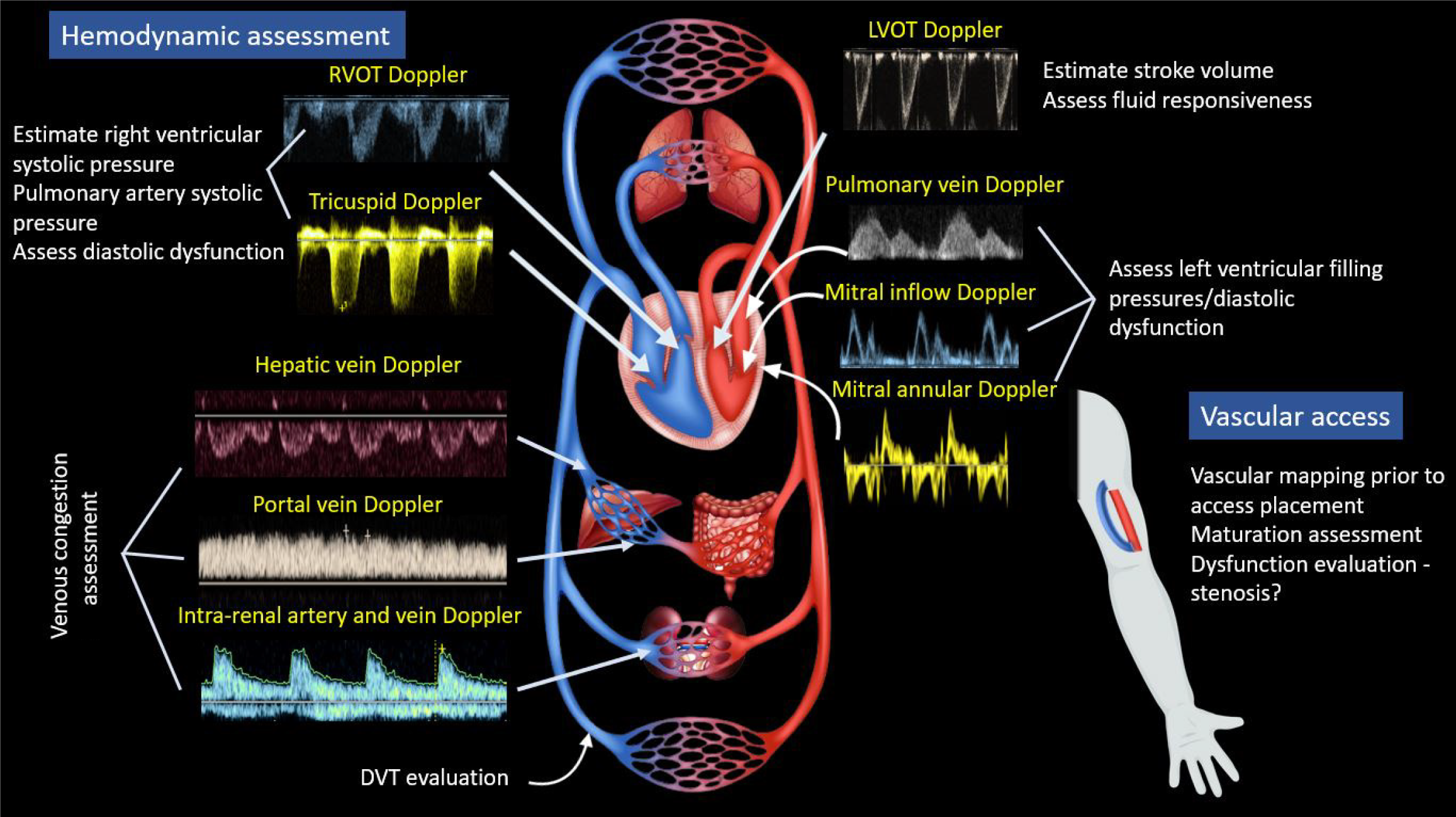
Physical examination has long been regarded as a vital component of bedside diagnosis and medical decision-making, involving the interpretation of physical findings alongside medical history and laboratory data. In recent decades, point-of-care ultrasonography (POCUS) has emerged as a valuable adjunct to physical examination across various medical specialties. POCUS entails clinician-performed focused ultrasound examinations at the bedside to address clinical queries, aiming to quickly establish diagnosis, narrow the differential, or guide a bedside procedure[1,2]. While the use of imaging guidance for procedures like central venous catheter placement is well-established, diagnostic POCUS remains relatively overlooked and poorly understood by many physicians. The current scope of POCUS in nephrology is wide, as illustrated in Figures 1 and 2. Although some nephrology fellowship programs are incorporating efforts to teach multi-organ POCUS[3], training in diagnostic POCUS and expertise among nephrologists beyond kidney ultrasound remain rudimentary at present. Particularly in private practice settings, which often have a heavier clinical workload compared to academic environments, the uptake of POCUS is additionally hindered by a lack of awareness and limited training opportunities. However, we firmly believe that the substantial clinical load in this setting positions POCUS as an even more advantageous tool to streamline diagnosis, reduce cognitive burden on the physician, and enhance patient care. To gain a better understanding of POCUS, it is essential to recognize its clinical utility. In this review, we will delve into real-life scenarios that demonstrate various POCUS applications, drawing from the experiences of a private practice nephrologist (author RS) and additional insights provided by an academic nephrologist (author AK). Author AM is RS’s trainee in a community hospital setting. Our aim is to convincingly present the case that integrating this highly effective bedside diagnostic modality into one's busy practice is a worthwhile investment.
The clinical cases presented below are not meant to provide exhaustive case presentations, but rather to emphasize the pivotal role played by POCUS in diagnosis and management for each scenario. We will endeavor to succinctly explain the significance of abnormal findings. Regarding equipment, all images shown were captured using a multipurpose handheld ultrasound device. Of note, the image quality and available features vary widely depending on the cost of the ultrasound device. While handheld devices (often connectible to tablets or cell phones) do not match the image quality of traditional cart-based ultrasound machines, they offer enhanced portability for private practice nephrologists who typically round in multiple clinical facilities and settings. The key is to understand the limitations of the equipment being used and the images obtained in a given patient; for instance, “Do these images sufficiently answer the focused clinical question(s) I am asking?”
We have an elderly female with stage 3b chronic kidney disease (CKD) and a history of chronic obstructive pulmonary disease (COPD) presenting for routine clinic follow-up. She complains of shortness of breath on exertion but is otherwise doing well. There is no chest pain. Her blood pressure is well-controlled on two antihypertensive medications, including a calcium channel blocker, and exhibits trace to 1 + edema.
Now, the questions for the nephrologist are - Is the exertional dyspnea of cardiac origin, or is it attributable to COPD? Could the edema signify congestive heart failure (CHF), or might it be related to her calcium channel blocker? Outpatient evaluation with an echocardiogram, chest X-ray (CXR), brain natriuretic peptide (BNP) measurement, stress testing, pulmonary function testing, etc., could entail weeks of waiting. Lung auscultation yields no notable findings making COPD exacerbation less likely, and her oxygen saturations are normal on room air.
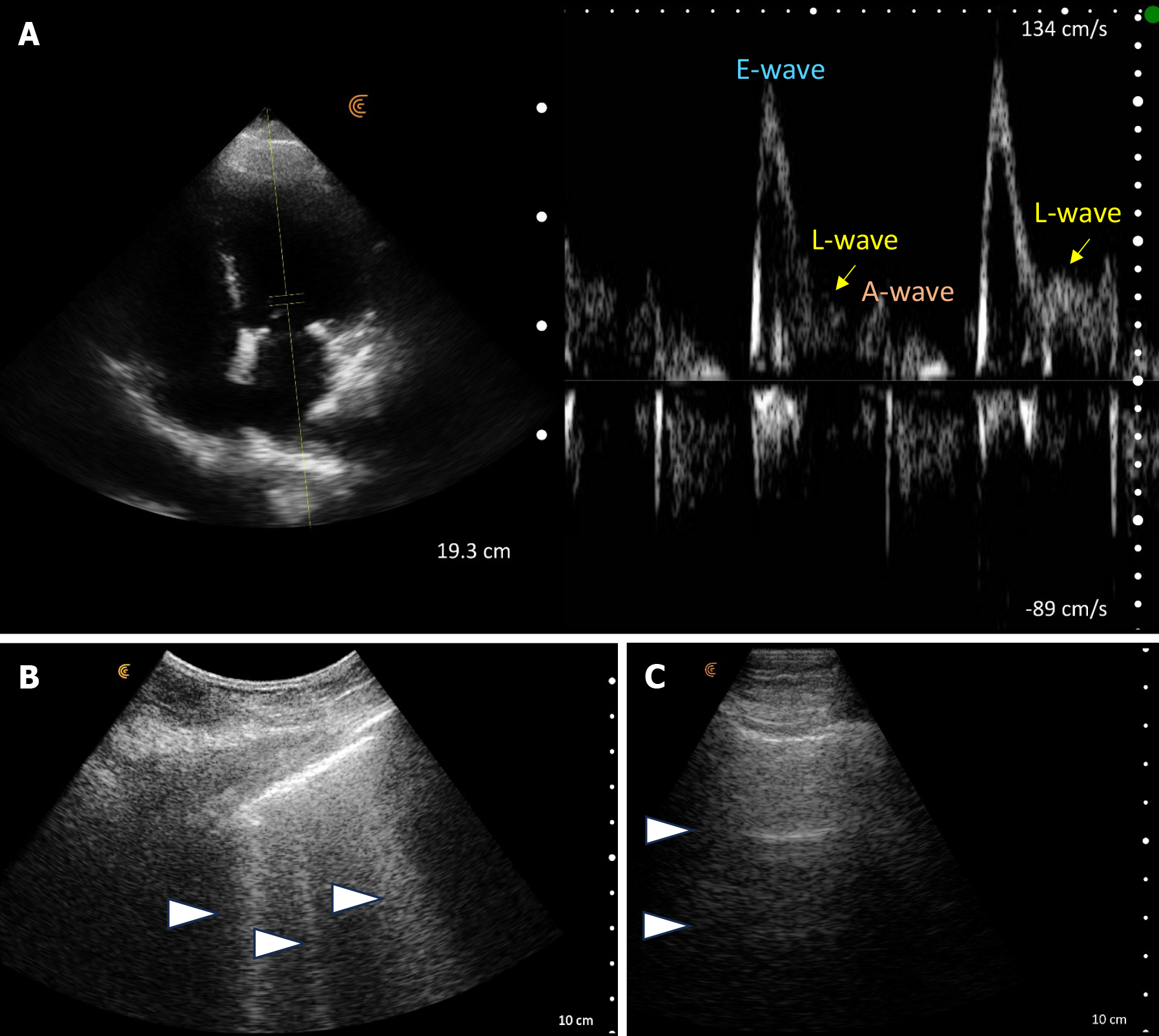
POCUS enhanced physical exam findings: Lung POCUS revealed diffuse B-lines, indicative of an interstitial pattern (often observed with pulmonary edema, pneumonia, fibrosis). Focused cardiac ultrasound depicted a qualitatively normal ejection fraction without gross regional wall motion abnormalities but demonstrated left ventricular hypertrophy and an abnormal mitral Doppler pattern of elevated E/A ratio > 2, along with an L-wave consistent with diastolic dysfunction and elevated left atrial pressure[4]. Based on these findings, a diagnosis of cardiogenic pulmonary edema [heart failure with preserved ejection fraction (HFpEF)] was made. In addition to cardiology referral, diuretics were promptly initiated, leading to rapid improvement. Subsequent POCUS assessment during follow up visit revealed a normal A-line pattern in the lungs, indicative of response to diuretics (Figure 3). It's worth mentioning that lung ultrasound demonstrates greater sensitivity compared to both physical examination and CXR in detecting cardiogenic pulmonary edema, which explains normal auscultation findings in our patient[5].
How did POCUS help? Obtaining conventional diagnostics such as CXR, BNP, and formal echocardiography, which provide a definitive diagnosis, may take days to weeks. However, with POCUS, the diagnosis of cardiogenic pulmonary edema and HFpEF was swiftly made at the bedside, saving valuable time. POCUS also facilitated objective evaluation of treatment response and potentially averted hospital admission for acute decompensated heart failure.
A middle-aged female, with a history of renal calculi, presents to the outpatient clinic with poorly localized abdominal pain and subjective fever. Urinalysis performed in the office revealed hematuria and pyuria. Despite her symptoms, she did not exhibit significant signs of illness during the office visit, and her serum creatinine was within her baseline range. The patient expressed reluctance to go to the emergency room (ER), which is understandable given the long wait times, inconvenience, high costs and her relatively stable condition. Moreover, outpatient imaging was not readily available, often requiring appointments scheduled several weeks out.
The dilemma arises: Should we refer a seemingly well patient with stable, near-normal kidney function to the ER? Or should we wait until diagnostic imaging is available as an outpatient, accepting the risk of potential clinical deterioration? Perhaps an alternative solution could involve POCUS.
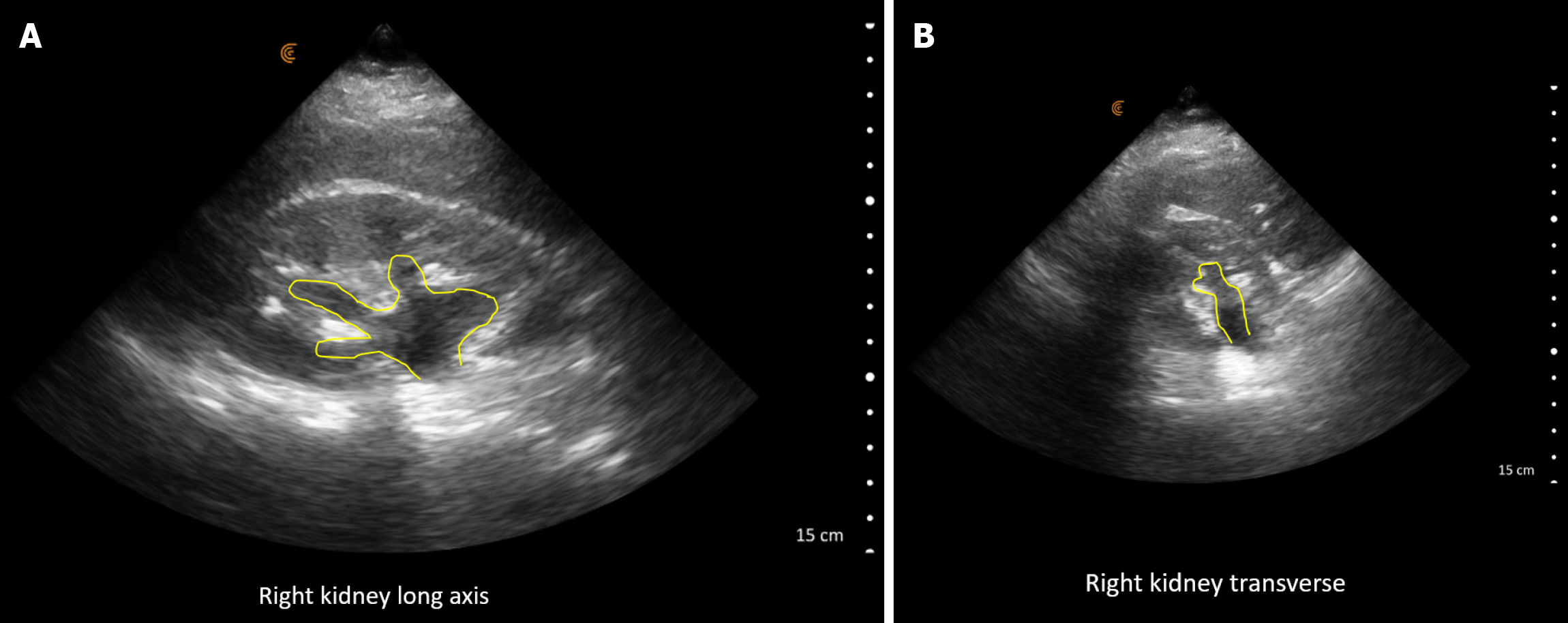
POCUS enhanced physical exam findings: Right hydronephrosis was identified on POCUS (Figure 4). The images were promptly reviewed with the on-call urologist, and an intervention was scheduled while awaiting confirmatory imaging. Subsequently, a computed tomography (CT) abdomen confirmed the presence of right hydronephrosis along with a proximal 5mm ureteric stone.
How did POCUS help? The timely detection of hydronephrosis via POCUS facilitated the immediate scheduling of surgical intervention, obviating the need to wait for a formal ultrasound, which could have resulted in treatment delays. Consequently, an unnecessary ER visit, or hospitalization was also averted.
This is the case of a middle-aged male with valvular heart disease who presented with weakness, lethargy, and bradycardia (heart rate in the 40s). He exhibited near-normal blood pressure and had no peripheral edema. Lung auscultation revealed clear breath sounds. Additionally, he was anuric, and his serum creatinine (SCr) had risen to 6.3 mg/dL from an apparently normal baseline, with associated hyperkalemia and markedly elevated serum transaminase levels. Serum lactate levels were found to be 14 mmol/L (ref: < 2). CXR did not demonstrate acute lung abnormalities and he was empirically initiated on aggressive intravenous fluids due to acute kidney injury (AKI), hyperkalemia and lactic acidosis. At this juncture, a POCUS examination was performed by the nephrology consultant.
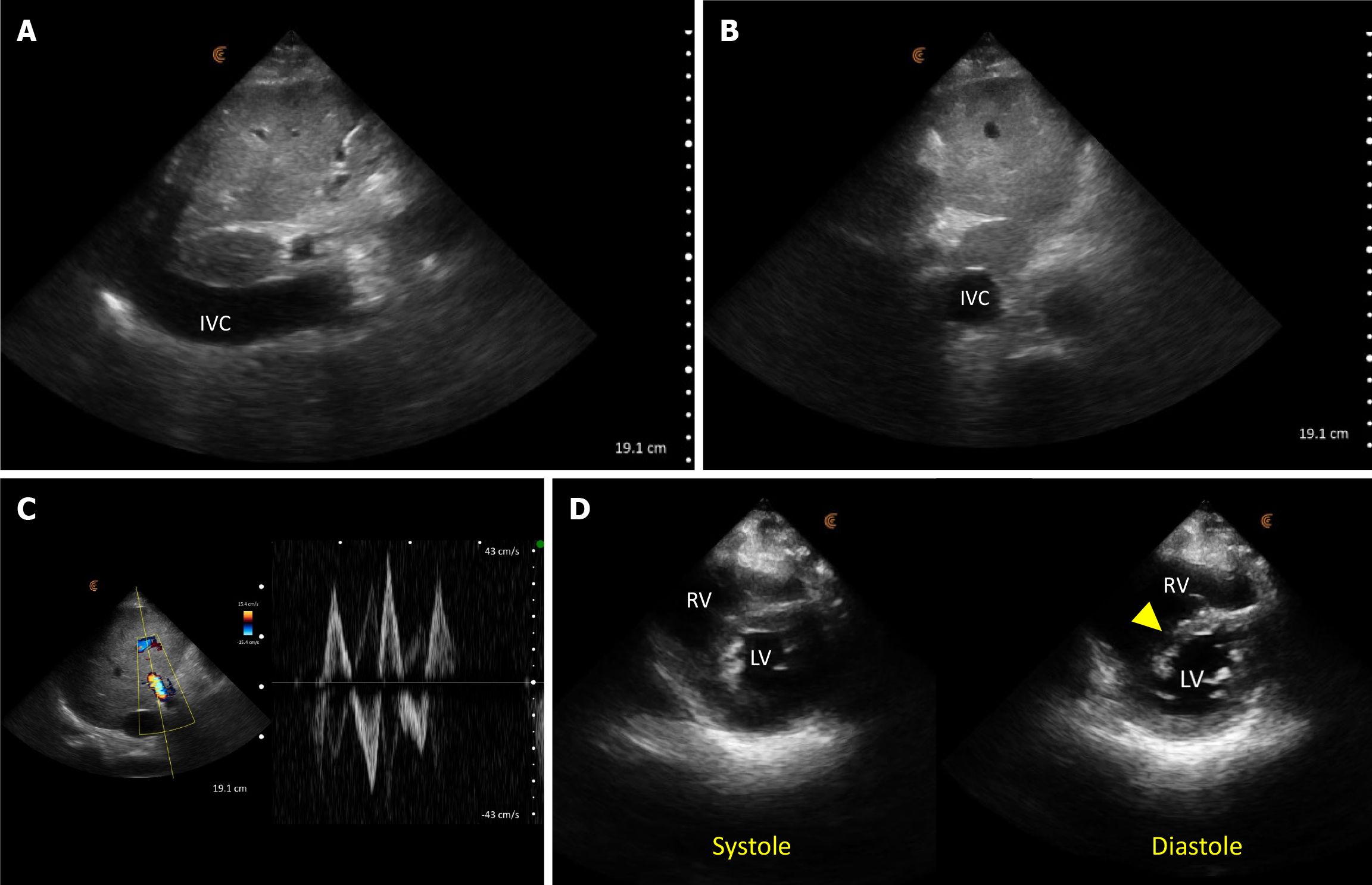
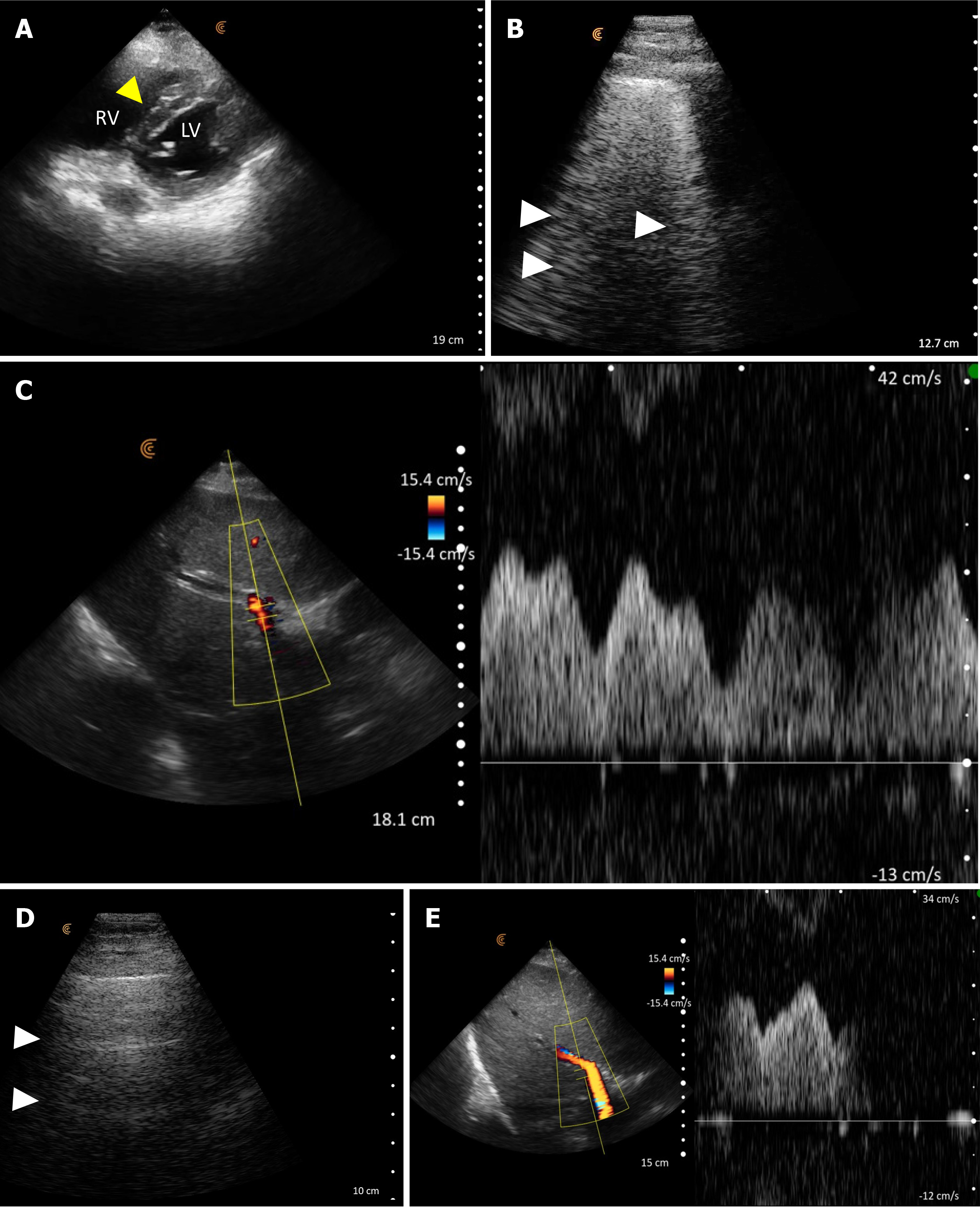
POCUS enhanced physical exam findings: The inferior vena cava (IVC) was plethoric, indicative of elevated right atrial pressure (RAP). Furthermore, the portal vein Doppler displayed a pulsatile waveform with flow reversal, consistent with severe venous congestion (Figure 5). While cardiac ultrasound was limited by the presence of electrocardiogram leads and transcutaneous pacing pads, we managed to identify a normal left ventricular ejection fraction and a dilated right ventricle with interventricular septal flattening, predominantly evident during diastole, which suggests volume overload. Continued volume administration can exacerbate this compression on the left ventricle leading to a drop in cardiac output and hypotension.
POCUS directed management: Given the above findings, intravenous fluids were discontinued, and the decision was taken to initiate continuous renal replacement therapy (CRRT). Following volume removal, there was an increase in urine output, and the patient reported feeling better. Within 48 hours, he was successfully weaned off CRRT, and his serum creatinine stabilized around 1.7 mg/dL. Additionally, there was an improvement in transaminase levels, which correlated with the normalization of the inferior vena cava and portal vein parameters (Figure 6).
How did POCUS help? POCUS revealed severe venous congestion, contrasting with the absence of volume overload evident during the clinical examination. This shifted the diagnosis towards congestive nephropathy and hepatopathy, as opposed to renal and liver injury stemming from decreased arterial flow due to hypovolemia. Consequently, POCUS prompted a change in management strategy, transitioning from administering intravenous fluids to initiating and subsequently via diuresis. This approach facilitated rapid clinical and biochemical improvement. Without POCUS, continued fluid administration would have exacerbated venous congestion and right ventricular overload. Additionally, POCUS served as a valuable tool for monitoring the response to therapy. It is important to recognize that the absence of pedal edema does not necessarily rule out venous congestion, and hemodynamics are dynamic – the lack of fluid overload upon initial assessment does not ensure its absence following volume loading.
A middle-aged woman with autoimmune hepatitis/cirrhosis was referred to the ER by her primary care physician due to laboratory results demonstrating a serum creatinine increase to 5.7 mg/dL from a normal baseline two months earlier. Her blood pressure was 81/43 mmHg, with a heart rate of 65 beats per minute. Upon physical examination, there were no crackles, no pedal edema, and her abdomen appeared mildly distended. In the context of cirrhosis and AKI, the admitting physician ordered empiric intravenous fluids followed by midodrine, octreotide, and albumin. However, there was no improvement observed in renal function or clinical status after 24 hours of this treatment regimen.
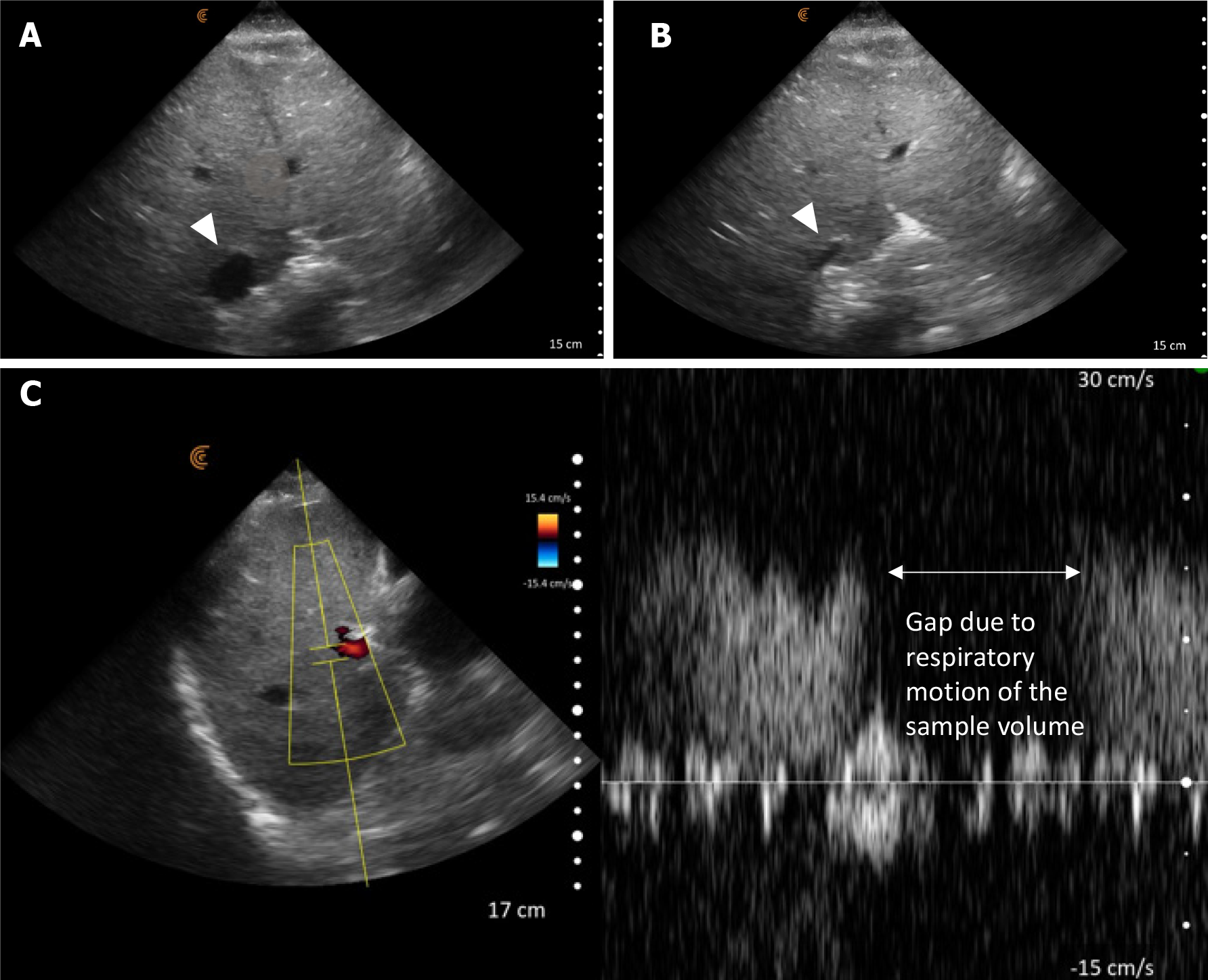
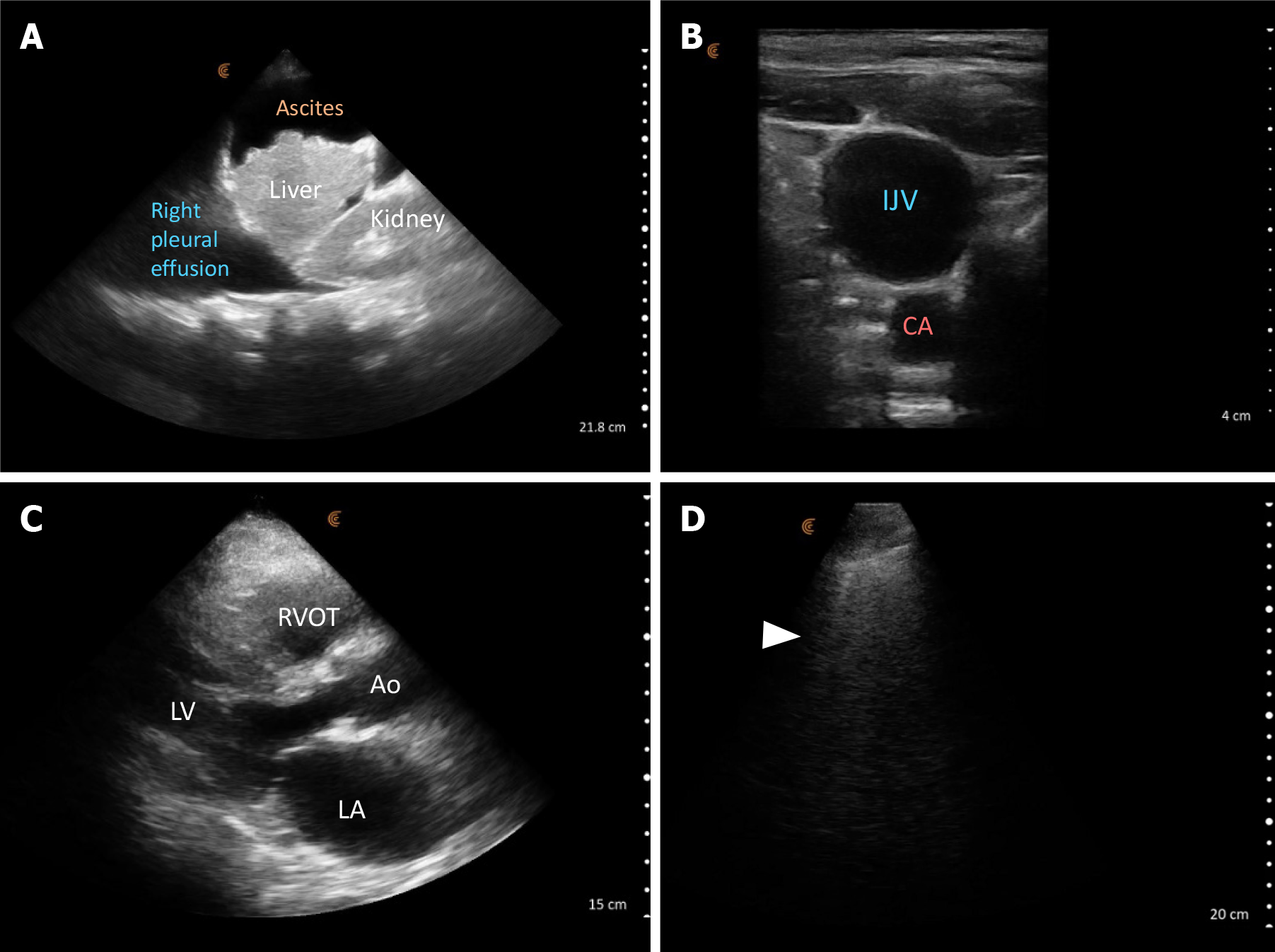
POCUS enhanced physical exam findings: POCUS revealed several crucial findings that were missed or not detectable during the initial examination: Focused cardiac ultrasound revealed a normal left ventricular ejection fraction but enlargement of both left and right atria suggestive of elevated cardiac filling pressures. Lung POCUS identified bilateral small pleural effusions and scattered B-lines suggestive of increased extravascular lung water. Abdominal assessment unveiled moderate to severe ascites accompanied by a shrunken liver. Ultrasound of the internal jugular vein showed that is distended with a collapse point at the angle of the jaw, consistent with significantly elevated jugular venous pressure/RAP (Figure 7). The collapse point is analogous to highest point of venous pulsations on the inspection method.
POCUS guided management: Based on the POCUS findings, a diagnosis of cirrhotic cardiomyopathy resulting in decreased renal perfusion was made. Volume expansion was halted, and diuretics were initiated. Additionally, a paracentesis was scheduled to relieve the intraabdominal pressure-related renal hypoperfusion. These interventions resulted in a substantial improvement in renal function and clinical condition. A formal echocardiogram later confirmed biatrial enlargement and reported an elevated pulmonary artery systolic pressure of 50-60 mmHg, further supporting the diagnosis.
How did POCUS help? Cardiac dysfunction often goes unnoticed in cirrhotic patients due to preserved systolic function. The left ventricle is usually hyperdynamic due to the high output cardiac state induced by splanchnic vasodilation, which can be misinterpreted as volume depletion when taken in isolation. This high output cardiac state eventually leads to elevated cardiac filling pressures and congestion, termed high output cardiac ‘failure’, a condition that remains underrecognized in cirrhosis[6]. Some authors describe this intricate interaction between the heart, kidneys, and cirrhosis-induced circulatory dysfunction as hepatocardiorenal syndrome[7]. In our case, POCUS altered the differential diagnosis from hypovolemia/hepatorenal syndrome to congestive nephropathy in the presence of cardiac dysfunction. This change in diagnosis prompted a shift in treatment strategy from continued volume expansion to diuresis and paracentesis. POCUS helped prevent prolonged hospitalization for AKI treatment, avoided the potential harm of volume overload, and likely mitigated the need for dialysis.
An elderly man with a history of CHF presented with symptoms of reduced oral intake, encephalopathy, and hypoxia. Notably, he had a recent hospitalization for heart failure exacerbation a few weeks prior. At the time of presentation, he was normotensive and afebrile. Auscultation revealed crackles in the right lower zone, while the rest of the physical examination was unremarkable. Laboratory results showed an elevated serum creatinine of 2.4 mg/dL compared to his normal baseline. Other notable findings included a normal white cell count and procalcitonin level but with an elevated C reactive protein (CRP) of 129 mg/dL. COVID19 testing was negative. The CXR reported "cardiomegaly, mild interstitial opacities bilaterally, and small pleural effusions consistent with congestive heart failure”. Although the physical examination findings were nonspecific, the chest X-ray report and the patient's recent hospitalization for pulmonary edema raised concerns for the primary service. As a result, they initiated intravenous furosemide, and the elevated CRP was attributed to a possible urinary tract infection.
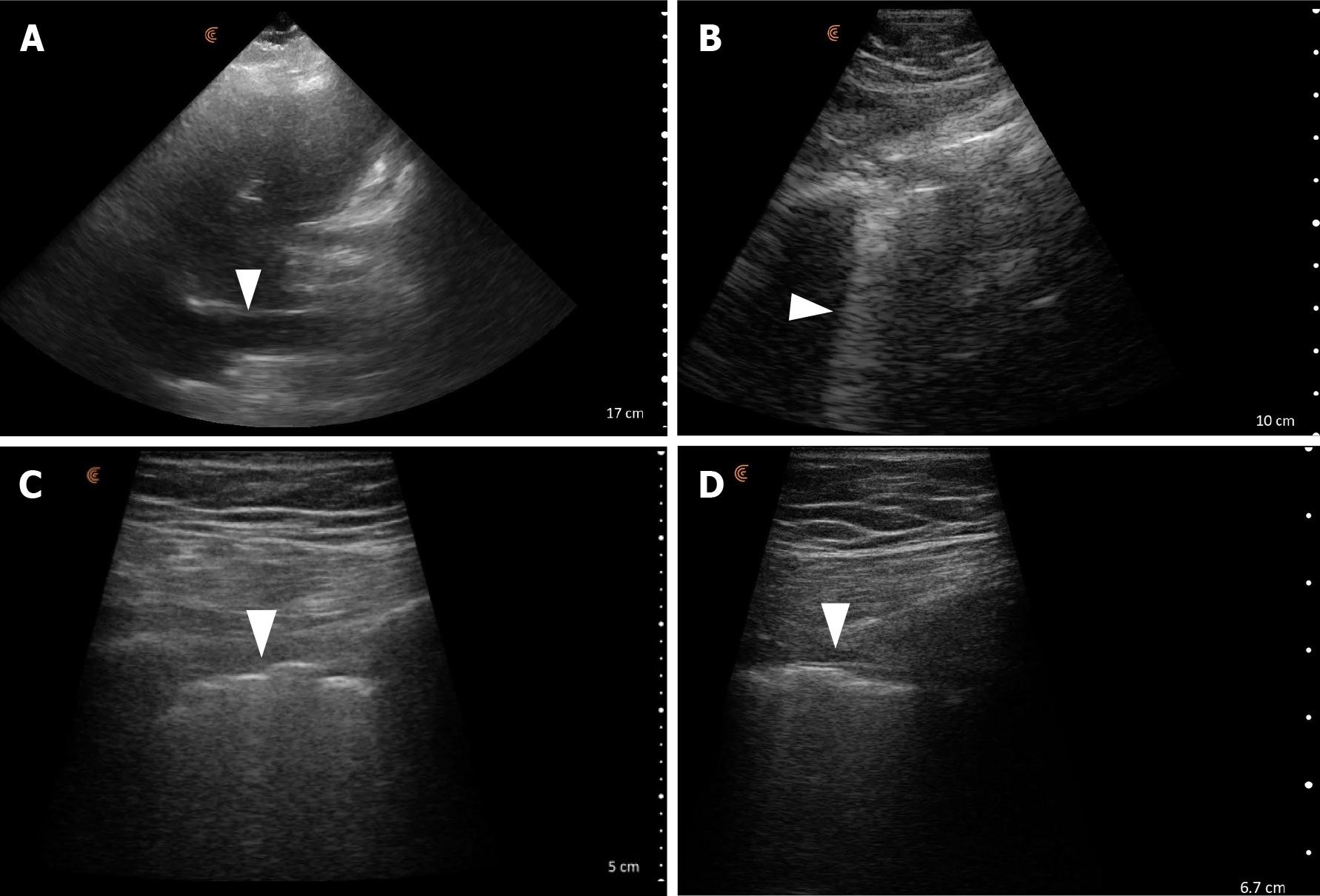
POCUS enhanced physical exam findings: POCUS demonstrated a collapsible IVC, hyperdynamic left ventricle, and markedly irregular pleura/subpleural consolidations, accompanied by B-lines (Figure 8). These observations leaned more towards an infectious or inflammatory lung condition rather than acute decompensated heart failure contrary to what was inferred from the CXR. Generally, the pleural line is smooth and regular in cases of cardiogenic pulmonary edema unless the patient has pre-existing lung or pleural disease.
POCUS guided management: The diuretics were discontinued, and intravenous fluids were administered, resulting in the serum creatinine returning to within normal limits within 48 hours. Antibiotics were titrated to cover possible pneumonia.
How did POCUS help? In this instance, the patient's medical history and clinical examination didn't strongly indicate CHF. However, the CXR report and recent hospitalization for acute pulmonary edema caused diagnostic uncertainty, resulting in the incorrect treatment approach for the patient's AKI. Although the data gathered through POCUS wasn't sufficient to definitively rule out CHF, the observed features pointed towards a more probable alternative diagnosis in this clinical context, guiding a more suitable intervention. Unlike radiologists, who frequently interpret images with minimal clinical context provided to them, POCUS, being clinician-performed, allows for immediate integration of relevant clinical information with the images.
An elderly woman underwent bowel surgery and experienced symptoms of nausea, vomiting, and fatigue one day following the procedure. She was normotensive and showed no signs of respiratory distress. Lung auscultation revealed clear breath sounds, and there was no peripheral edema observed (clinically euvolemic). Laboratory results showed a serum sodium level of 118 mmol/L. Urine sodium was 40 mmol/L, urine osmolality was 516 mOsm/kg, and serum osmolality was 256 mOsm/kg. Her sodium level was within the normal range four days prior to admission. Based on this information, it appeared to be a straightforward case of postoperative syndrome of inappropriate antidiuretic hormone secretion (SIADH).
POCUS enhanced physical examination findings: POCUS revealed severe enlargement of the left atrium suggestive of elevated left sided filling pressures, along with mild enlargement of the right ventricle. Lung examination showed diffuse B-lines indicating elevated extravascular lung water. The IVC appeared plethoric, and the portal vein exhibited more than 50% pulsatility, indicating elevated RAP and venous congestion (Figure 9).
POCUS guided management: Considering the findings, the diagnosis of concurrent CHF was established. The patient received treatment with intravenous diuretics and tolvaptan, resulting in improvement of sodium levels. A follow-up POCUS examination performed 48 hours after treatment demonstrated almost complete resolution of B-lines. Additionally, improvements were observed in both IVC and portal venous pulsatility.
How did POCUS help? This case initially seemed to indicate a typical postoperative SIADH scenario. With the POCUS-assisted identification of heart failure, the treatment approach shifted from salt tablets/urea supplements to tolvaptan and furosemide. While furosemide may also help with SIADH, the case likely involved a mixed etiology, and POCUS played a crucial role in diagnosing clinically silent heart failure, potentially averting the need for rehospitalization.
Here are some of the common concerns nephrologists may have when considering the integration of POCUS into their practice. We aim to offer reasoned explanations and practical suggestions based on our experience for each of these barriers.
Firstly, not every patient requires a POCUS-enhanced physical examination. Moreover, once a certain level of proficiency is achieved, the additional time required is often minimal. For instance, assessing the lungs for effusions or pulmonary edema, examining the IVC, and ruling out hydronephrosis can be accomplished in about 5 minutes[8]. Surprisingly, in some instances, POCUS can even expedite the examination process! Consider the following scenarios to illustrate this point: To diagnose a pleural effusion conventionally involves auscultating for reduced breath sounds, eliciting reduced vocal/tactile fremitus, and assessing dullness to percussion, which is more time-consuming than simply placing an ultrasound probe at the dependent lung zone and visualizing an effusion. Similarly, diagnosing ascites typically requires demonstrating shifting dullness or a fluid thrill, which also takes more time than merely positioning the ultrasound probe on the abdomen to identify fluid between the liver and the kidney. Thus, while it may initially appear time-consuming, POCUS can ultimately streamline the examination process.
Our suggestion: Our recommendation is to begin practicing POCUS examinations initially on hospitalized patients with their consent. They are easier to position in a hospital bed, and there is less time pressure compared to clinic settings. Start with simpler examinations such as lung, IVC, and ruling out hydronephrosis, as they require less technical expertise. These examinations can be performed almost immediately after attending a well-structured POCUS workshop and watching a few online tutorials. The findings can be compared with the formal imaging report, where available. Clinical integration should not be a hurdle for practicing physicians in most scenarios. For instance, a plethoric IVC presents a similar diagnostic implication as an elevated jugular venous pressure, and a B-line pattern is analogous to crackles on auscultation, albeit more sensitive. Once comfortable with these basic POCUS applications, one can gradually integrate more complex examinations such as cardiac ultrasound and Doppler studies.
As physicians, our commitment to lifelong learning is fundamental. While certain procedures may be best handled by specialists with dedicated training (e.g., dialysis access interventions, kidney biopsy), the essence of physical examination remains a cornerstone of our practice. Therefore, it's essential that we embrace the POCUS-enhanced physical examination to provide better patient care. However, not everyone needs to master advanced techniques like stroke volume assessment or diastology. Simple POCUS applications such as estimating RAP through IVC POCUS and lung ultrasound offer significant benefits and are relatively easy to learn. Learning any new skill requires time commitment, but the motivation to serve our patients better should drive us to adopt POCUS into our practice. Additionally, many private practice physicians play a role in teaching trainees at community hospitals. Incorporating POCUS could reignite interest in nephrology as a career choice, which currently faces some challenges. Studies have shown that POCUS can enhance the interest of medical students in pursuing nephrology electives[9].
Our suggestion: Take advantage of freely available online resources, such as NephroPOCUS.com. Attend live workshops whenever feasible to gain hands-on experience. Given that the scope of nephrologist-performed POCUS aligns with that of hospitalists and intensivists, it's acceptable to participate in workshops and certification programs designed for these physicians until dedicated nephrology courses become more widespread[10]. Some larger private practice groups arrange for POCUS experts from academic institutions to conduct exclusive workshops for their members. This setup allows the workshop to utilize the equipment that the group is already using or familiar with and ensures a favorable learner-to-faculty ratio. Consequently, physicians can benefit from specialized training without the need to travel to attend conventional workshops elsewhere. Additionally, some ultrasound devices feature image-sharing capabilities, allowing experts to provide feedback on your scans. Certain devices also offer artificial intelligence-based real-time image guidance, which can be beneficial for novice users. If you have your own handheld device, consider scanning yourself at home to further refine your skills.
Advancements in ultrasound technology in recent years have led to the development of smaller, more portable devices with improved image quality and ergonomics. The availability of handheld ultrasound devices, in particular, has revolutionized portability, which is crucial for private practice nephrologists who may need to travel to multiple facilities to see patients. These devices typically range in price from approximately $4000 to $12000, depending on factors such as image quality and options like spectral Doppler. While cart-based machines generally offer superior image quality compared to handheld devices, they may be more suitable for advanced users who have less need for mobility and primarily see patients in specific wards or clinics. For instance, qualified users can perform comprehensive hemodynamic scans with high-quality Doppler and EKG guidance using such machines in the nephrology clinic[11]. POCUS-certified physicians with appropriate credentials can bill for these studies, which helps offset the equipment costs. The reimbursement rates vary depending on factors such as insurance type and the specific scans performed, as detailed elsewhere[3,12,13].
Our suggestion: Discuss with your practice or hospital leadership the potential acquisition of handheld or cart-based ultrasound machines tailored to your imaging requirements. Highlight the ability to bill for POCUS procedures, which can lead to financial reimbursement. Most commercially available handheld devices can be integrated with the hospital's picture archiving and communication system, ensuring secure storage of patient data, which is necessary for billing purposes. If your practice offers a continuing medical education allowance, inquire about using it to procure an ultrasound machine. Additionally, many ultrasound companies provide financing options to mitigate the initial cost. However, it's essential to exercise caution and acknowledge the limitations of the device being used, while also avoiding overestimation of one's skills.
Regrettably, this perspective is prevalent among physicians who harbor skepticism about incorporating POCUS into their practice. However, it's important to recognize that while POCUS serves as a diagnostic aid, its impact on mortality is contingent upon being tied together with treatments that can enhance patient survival. As a diagnostic tool, POCUS is expected to offer superior diagnostic accuracy compared to traditional methods, which it has shown to do in multiple clinical settings[2,14,15]. Yet, its findings significantly influence various clinically relevant outcomes such as time to diagnosis, need for additional imaging, healthcare expenditure, and patient satisfaction. For example, in a systematic review involving 5393 dyspneic patients, those managed with POCUS experienced notably shorter times to correct diagnosis and treatment compared to conventional care recipients (mean difference -63 min and -27 min, respectively). Moreover, the odds of receiving appropriate therapy were substantially higher in the POCUS group, with a corresponding reduction in intensive care unit length of stay[16]. This scenario holds relevance for nephrologists tasked with addressing dyspnea linked to fluid overload, underscoring the importance of promptly discerning its cause to guide interventions effectively. Similarly, POCUS-guided management has proven effective in reducing subsequent imaging needs, including chest radiographs, echocardiograms, and CT scans, thereby positively impacting healthcare expenditure[17]. In hemodialysis patients, POCUS-based ultrafiltration titration has resulted in notable reductions in left ventricular filling pressures, cardiac dimensions, and ambulatory blood pressure, indicating its efficacy as a treatment guiding tool[18,19]. Regarding patient-reported outcomes, mounting evidence suggests that POCUS enhances patient satisfaction and facilitates better comprehension of their diagnosis[20,21], a particularly relevant aspect for nephrologists tasked with conveying dietary restrictions and medication adherence to asymptomatic patients. In this context, sharing and discussing POCUS images with patients could be beneficial.
Our suggestion: POCUS complements conventional physical examination, significantly enhancing the accuracy of bedside diagnosis and positively influencing practical outcomes, as outlined above. The primary duty of a physician is to ensure accurate diagnoses by thoughtfully integrating historical and physical examination data. Therefore, it would be irrational to disregard the utilization of enhanced bedside diagnostic tools simply due to the absence of treatments directly impacting mortality.
The concern about misinterpreting or missing important findings, which might result in legal consequences, often acts as a barrier to the integration of POCUS in nephrology practice[22]. Numerous studies have investigated lawsuits related to POCUS across different specialties. However, none of these studies have shown that missed findings on focused ultrasound scans led to legal action against physicians. Rather, the research indicates that legal action is more frequently associated with not conducting POCUS when necessary and in a timely manner[23-27].
Our suggestion: To further reduce the risk of medicolegal action related to the use of POCUS, we recommend the following:
Avoid replacing definitive diagnostic testing with POCUS, unless there is absolutely no indication for further testing.
Ensure thorough documentation of findings and securely archive images.
Recognize your level of competency and understand the capabilities and limitations of the equipment. Acknowledge that while POCUS can be effective for confirming a suspected diagnosis or narrowing the differential, it may not always definitively rule them out. Exercise clinical judgment accordingly.
Seek consultation and discuss images with institutional experts when in doubt. Image archiving facilitates this, as the experts can retrieve the images from the patient’s chart instead of repeating the scan.
Manage patient expectations by clearly explaining the purpose of POCUS and its limitations. For instance, instead of stating "I will perform an ultrasound of your chest," say "This device allows me to examine the surface of your lungs for fluid or infection, but it does not allow me to visualize the entirety of your lungs like a CT scan." Similarly, when discussing findings, refrain from definitive statements like "Your heart looks normal" and instead, provide a nuanced explanation such as "I can observe that your heart is functioning well in terms of squeezing, and the sizes of the heart chambers appear grossly normal. However, for a complete assessment of valve function and pressures inside your heart, a formal echocardiogram is necessary."
POCUS presents significant opportunities to improve daily nephrology practice and enhance patient satisfaction when performed by adequately trained practitioners. In nephrology, a field heavily reliant on laboratory data, POCUS renews our connection with patients at the bedside. Please take a moment to consider this quote: "Like most new technologies, there is a risk that new practitioners will make mistakes based on their erroneous interpretations. This technology, therefore, must be restricted." Interestingly, this statement wasn't referring to POCUS but was a reaction to the invention of the stethoscope, which has become a cornerstone of the physical examination. Another similar instance occurred in the surgical field with the introduction of laparoscopic surgery. In the 1980s, Dr. Kurt Semm faced significant resistance, stating, "Both surgeons and gynecologists were angry with me, they virtually stoned me. All my initial attempts to publish a report on laparoscopic appendectomy were rejected with the comment that such nonsense does not, and will never, belong in general surgery."
Like the stethoscope and laparoscope during their early days, POCUS represents a new and promising technology to aid us in patient care, regardless of location. So, how will today's nephrologists perceive this modern diagnostic tool? Will we embrace the change, or will we reject it due to our familiarity and comfort with the status quo?
| 1. | Díaz-Gómez JL, Mayo PH, Koenig SJ. Point-of-Care Ultrasonography. N Engl J Med. 2021;385:1593-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (1)] |
| 2. | Koratala A, Kazory A. An Introduction to Point-of-Care Ultrasound: Laennec to Lichtenstein. Adv Chronic Kidney Dis. 2021;28:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Koratala A, Olaoye OA, Bhasin-Chhabra B, Kazory A. A Blueprint for an Integrated Point-of-Care Ultrasound Curriculum for Nephrology Trainees. Kidney360. 2021;2:1669-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Ha JW, Ahn JA, Moon JY, Suh HS, Kang SM, Rim SJ, Jang Y, Chung N, Shim WH, Cho SY. Triphasic mitral inflow velocity with mid-diastolic flow: the presence of mid-diastolic mitral annular velocity indicates advanced diastolic dysfunction. Eur J Echocardiogr. 2006;7:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Santus P, Radovanovic D, Saad M, Zilianti C, Coppola S, Chiumello DA, Pecchiari M. Acute dyspnea in the emergency department: a clinical review. Intern Emerg Med. 2023;18:1491-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Reddy YNV, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-Output Heart Failure: A 15-Year Experience. J Am Coll Cardiol. 2016;68:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 7. | Kazory A, Ronco C. Hepatorenal Syndrome or Hepatocardiorenal Syndrome: Revisiting Basic Concepts in View of Emerging Data. Cardiorenal Med. 2019;9:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 8. | Koratala A. Point of Care Ultrasonography Enhanced Physical Examination: A Nephrologist's Perspective. Am J Med. 2020;133:e384-e385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Koratala A, Paudel HR, Regner KR. Nephrologist-Led Simulation-Based Focused Cardiac Ultrasound Workshop for Medical Students: Insights and Implications. AJM Open. 2023;10:100051. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Koratala A. How to get certified in POCUS? NephroPOCUS.com. Last accessed: April 14, 2024. Available from: https://nephropocus.com/2020/11/05/how-to-get-certified-in-pocus/. |
| 11. | Koratala A. Utilizing point-of-care ultrasound in the cardiorenal clinic to enhance patient care. J Bras Nefrol. 2021;43:135-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Zeidan A, Liu EL. Practical Aspects of Point-of-Care Ultrasound: From Billing and Coding to Documentation and Image Archiving. Adv Chronic Kidney Dis. 2021;28:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | GE Healthcare. Reimbursement information for point of care ultrasound procedures. Last accessed: 4/14/24. Available from: https://www.gehealthcare.com/-/jssmedia/gehc/us/files/products/reimbursement/2022-reimbursement-guides/point-of-care-reimbursement-guide.pdf?rev=-1&hash=C51BD6790502ED55314D61AF2E36E7DD. |
| 14. | Bhagra A, Tierney DM, Sekiguchi H, Soni NJ. Point-of-Care Ultrasonography for Primary Care Physicians and General Internists. Mayo Clin Proc. 2016;91:1811-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 15. | Koratala A, Reisinger N. POCUS for Nephrologists: Basic Principles and a General Approach. Kidney360. 2021;2:1660-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Szabó GV, Szigetváry C, Szabó L, Dembrovszky F, Rottler M, Ocskay K, Madzsar S, Hegyi P, Molnár Z. Point-of-care ultrasound improves clinical outcomes in patients with acute onset dyspnea: a systematic review and meta-analysis. Intern Emerg Med. 2023;18:639-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 17. | Brogi E, Bignami E, Sidoti A, Shawar M, Gargani L, Vetrugno L, Volpicelli G, Forfori F. Could the use of bedside lung ultrasound reduce the number of chest x-rays in the intensive care unit? Cardiovasc Ultrasound. 2017;15:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Loutradis C, Papadopoulos CE, Sachpekidis V, Ekart R, Krunic B, Karpetas A, Bikos A, Tsouchnikas I, Mitsopoulos E, Papagianni A, Zoccali C, Sarafidis P. Lung Ultrasound-Guided Dry Weight Assessment and Echocardiographic Measures in Hypertensive Hemodialysis Patients: A Randomized Controlled Study. Am J Kidney Dis. 2020;75:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Loutradis C, Sarafidis PA, Ekart R, Papadopoulos C, Sachpekidis V, Alexandrou ME, Papadopoulou D, Efstratiadis G, Papagianni A, London G, Zoccali C. The effect of dry-weight reduction guided by lung ultrasound on ambulatory blood pressure in hemodialysis patients: a randomized controlled trial. Kidney Int. 2019;95:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Andersen CA, Brodersen J, Rudbæk TR, Jensen MB. Patients' experiences of the use of point-of-care ultrasound in general practice - a cross-sectional study. BMC Fam Pract. 2021;22:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Mathews BK, Miller PE, Olson APJ. Point-of-Care Ultrasound Improves Shared Diagnostic Understanding Between Patients and Providers. South Med J. 2018;111:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Koratala A, Bhattacharya D, Kazory A. Harnessing Twitter polls for multi-specialty collaboration in standardizing point-of-care ultrasonography in nephrology. Clin Nephrol. 2020;94:50-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Blaivas M, Pawl R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med. 2012;30:338-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Stolz L, O'Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Nguyen J, Cascione M, Noori S. Analysis of lawsuits related to point-of-care ultrasonography in neonatology and pediatric subspecialties. J Perinatol. 2016;36:784-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Reaume M, Farishta M, Costello JA, Gibb T, Melgar TA. Analysis of lawsuits related to diagnostic errors from point-of-care ultrasound in internal medicine, paediatrics, family medicine and critical care in the USA. Postgrad Med J. 2021;97:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Russ B, Arthur J, Lewis Z, Snead G. A Review of lawsuits related to point-of-care emergency ultrasound applications. J Emerg Med. 2022;63:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/