Published online Sep 25, 2025. doi: 10.5527/wjn.v14.i3.109400
Revised: May 28, 2025
Accepted: July 10, 2025
Published online: September 25, 2025
Processing time: 131 Days and 6 Hours
This case report describes myeloperoxidase-anti-neutrophil cytoplasmic antibody associated vasculitis with kidney involvement in a patient with relapsing polychondritis, which was successfully treated with Avacopan. Although relapsing polychondritis has been associated with anti-neutrophil cytoplasmic antibody-associated vasculitis, overlap can result in severe organ involvement, particularly renal damage progressing to end-stage kidney disease. This case presents a unique opportunity to evaluate the potential role of Avacopan as an alternative therapeutic option in managing myeloperoxidase-anti-neutrophil cytoplasmic antibody-associated vasculitis in the context of relapsing polychondritis highlighting a positive renal response despite treatment challenges.
This is a case of a 69-year-old Caucasian woman who presented to our hospital’s emergency department with a 4 week history of inflammatory polychondritis affecting the auricular cartilage, accompanied by acute kidney injury. On admission, serum creatinine was elevated at 4.0 mg/dL, which progressively increased to 6.07 mg/dL on day 6. The renal biopsy revealed necrotizing and crescentic glomerulonephritis affecting more than 50% of the glomeruli. She was treated with a total of 2500 mg intravenous methylprednisolone over 3 days followed by oral prednisone. Induction treatment included intravenous cyclophosphamide induction, with plans for a total of 2 doses followed by transition to rituximab. However the patient was unable to tolerate rituximab due to allergic reaction so intravenous cyclophosphamide was continued for a total of 6 doses (cumulative dose 3000 mg). In the setting of persistent acute kidney injury, Avacopan was added to the regimen 3 months after diagnosis. Maintenance therapy included azathioprine in addition to Avacopan. Prednisone gradually tapered off at 6 months.
Avacopan may be beneficial in treating anti-neutrophil cytoplasmic antibody-associated vasculitis with coexisting relapsing polychondritis, especially in cases where preservation of kidney function is critical. Further research will be essential to validate these findings and refine treatment protocols for such complex cases.
Core Tip: This case report presents a rare overlap of relapsing polychondritis and myeloperoxidase anti-neutrophil cytoplasmic antibody associated vasculitis, complicated by severe acute kidney injury. The patient demonstrated sustained renal improvement following treatment with Avacopan, a complement 5a receptor antagonist, after intolerance to rituximab. This case highlights the potential role of Avacopan in preserving renal function and achieving disease control in complex autoimmune syndromes, especially when conventional immunosuppressive regimens are contraindicated or poorly tolerated.
- Citation: Nguyen JT, Anand M. Avacopan in the treatment of relapsing polychondritis with myeloperoxidase-antineutrophil cytoplasmic antibody associated vasculitis: A case report. World J Nephrol 2025; 14(3): 109400
- URL: https://www.wjgnet.com/2220-6124/full/v14/i3/109400.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i3.109400
Relapsing polychondritis is a rare autoimmune disorder characterized by recurrent inflammation of cartilaginous tissues, which can lead to progressive anatomical deformation and functional impairment[1]. While it is primarily known for affecting the ears, nose, and respiratory tract, it may also occur in association with other autoimmune diseases, including anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. ANCA-associated vasculitis is a systemic autoimmune condition marked by small-vessel inflammation, often resulting in significant end-organ damage particularly renal involvement, which can progress to end-stage kidney disease[2].
The current standard of care for ANCA-associated vasculitis includes high-dose glucocorticoids in combination with immunosuppressive agents such as cyclophosphamide or rituximab. While rituximab is effective in inducing remission and managing symptoms of the disease, it seems to have a limited ability in improving kidney function, particularly relevant in patients with severe acute kidney injury (AKI). In contrast, recent studies have shown that Avacopan not only induces remission but also leads to more rapid reduction in proteinuria, a key marker of kidney function, providing a promising alternative for preserving renal health in patients with ANCA-associated vasculitis[3].
Avacopan is a novel oral complement 5a receptor antagonist that modulates the inflammatory response by blocking C5a-mediated neutrophil activation. Clinical trials have also demonstrated that Avacopan is non-inferior to prednisone in achieving disease remission at 12 and 26 weeks, with superior outcomes in sustaining remission and reducing relapse rates over 52 weeks[4,5]. Importantly, Avacopan offers a potential steroid sparing approach and may serve as an alternative to other immunosuppressive agents, particularly in patients who are intolerant to standard therapies.
We present a rare case of ANCA-associated glomerulonephritis in a patient with a history of relapsing polychondritis, successfully managed with Avacopan following intolerance to rituximab.
A 69-year-old Caucasian woman was admitted to the emergency department with a 3-4 weeks history of inflammatory polychondritis affecting the auricular cartilage, accompanied by AKI.
Inflammatory polychondritis which was treated with a 2 weeks course of doxycycline and ciprofloxacin without any improvement.
The patient had a negative history of infections, and was up to date on all vaccinations.
Medical history was notable for diet controlled type 2 diabetes mellitus, hypertension for which she had received hydralazine more than 2 years prior to this presentation and infiltrating ductal carcinoma of the right breast status post radiation therapy, resection and 5 years treatment with Arimidex.
The patient did not display respiratory, cardiac or neurological manifestations of ANCA vasculitis.
On admission (day 0), serum creatinine was elevated at 4.0 mg/dL, which progressively increased to 6.07 mg/dL (Day 6). Urine analysis revealed microscopic hematuria along with red cell casts and urine protein-to-creatinine ratio of 1.7 g/g.
Additional serological investigations were notable for a positive myeloperoxidase-anti-neutrophil cytoplasmic antibody with a titer of 1:640 and elevated anti-myeloperoxidase antibody levels (> 8.0) along with elevated C-reactive protein at 98.6 mg/L and erythrocyte sedimentation rate at 53 mm/hour, normal C3 and C4, rheumatoid factor, Hepatitis B/C, human immunodeficiency virus, and Quantiferon TB Gold were negative. Antinuclear antibody testing was positive (1:80) with a homogenous pattern, but a comprehensive ANA panel with titer was negative.
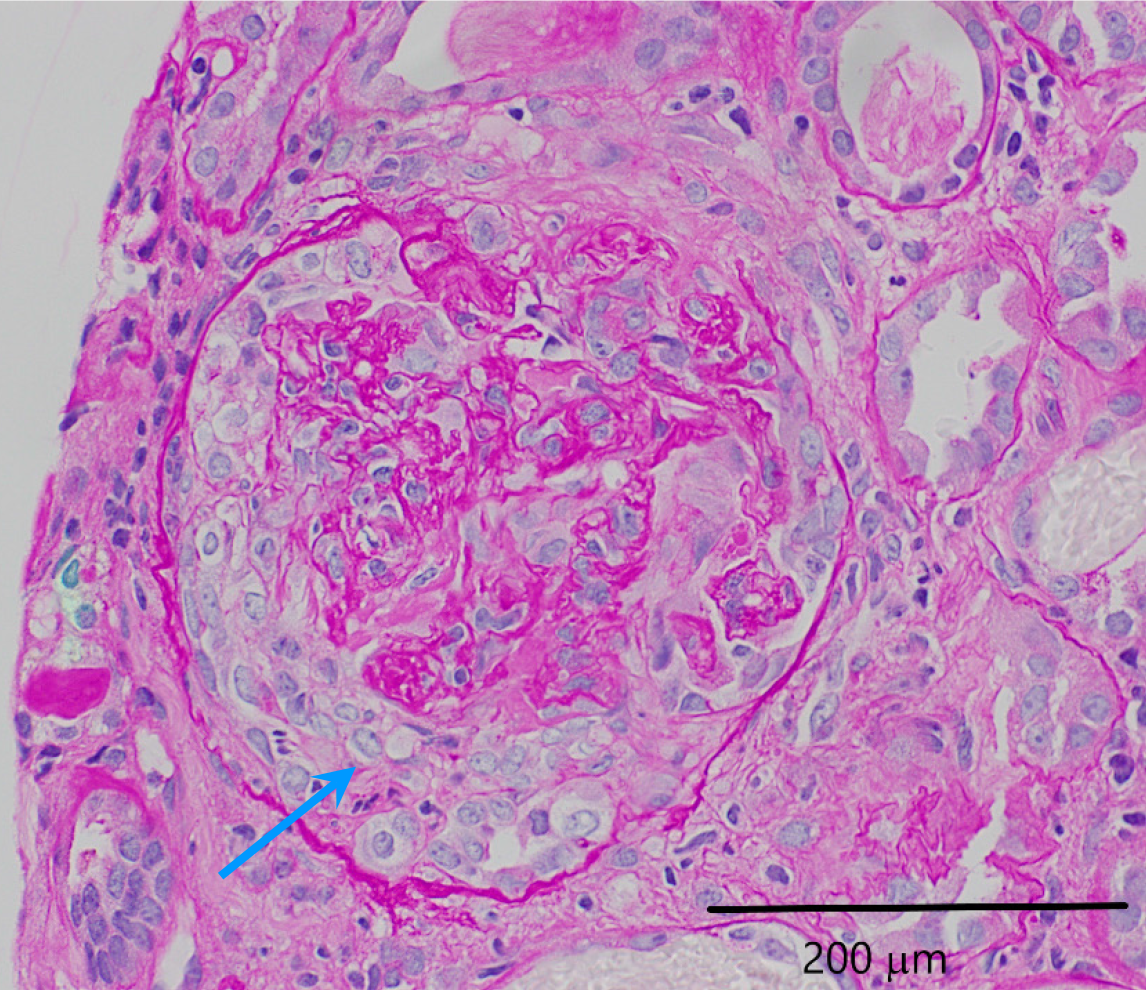
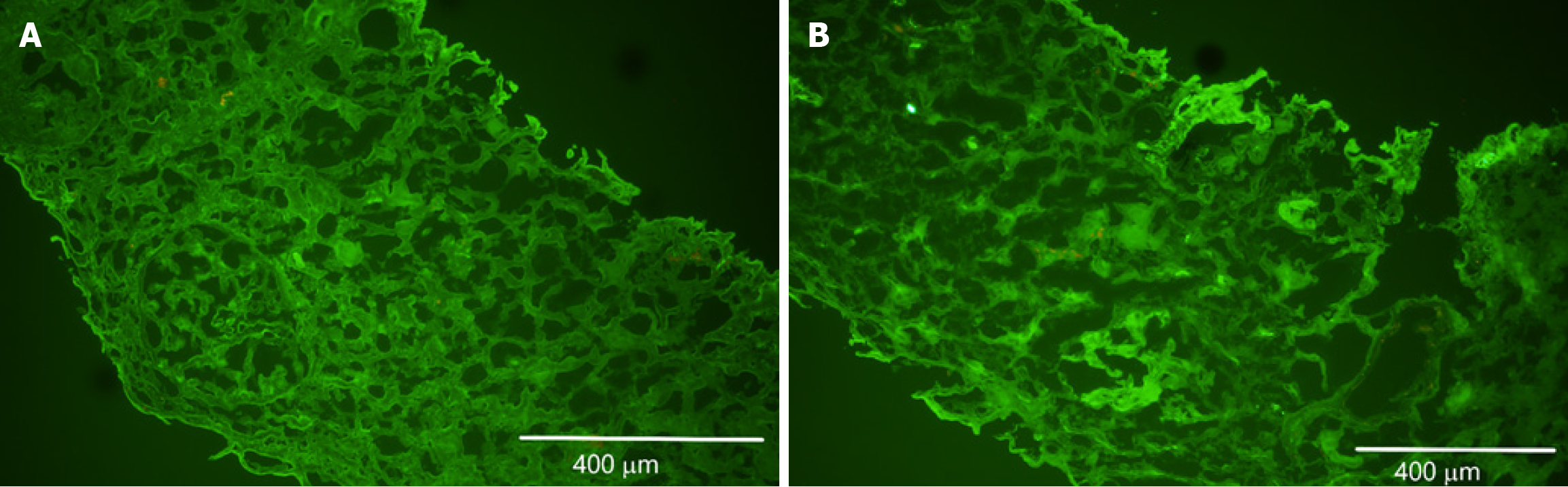
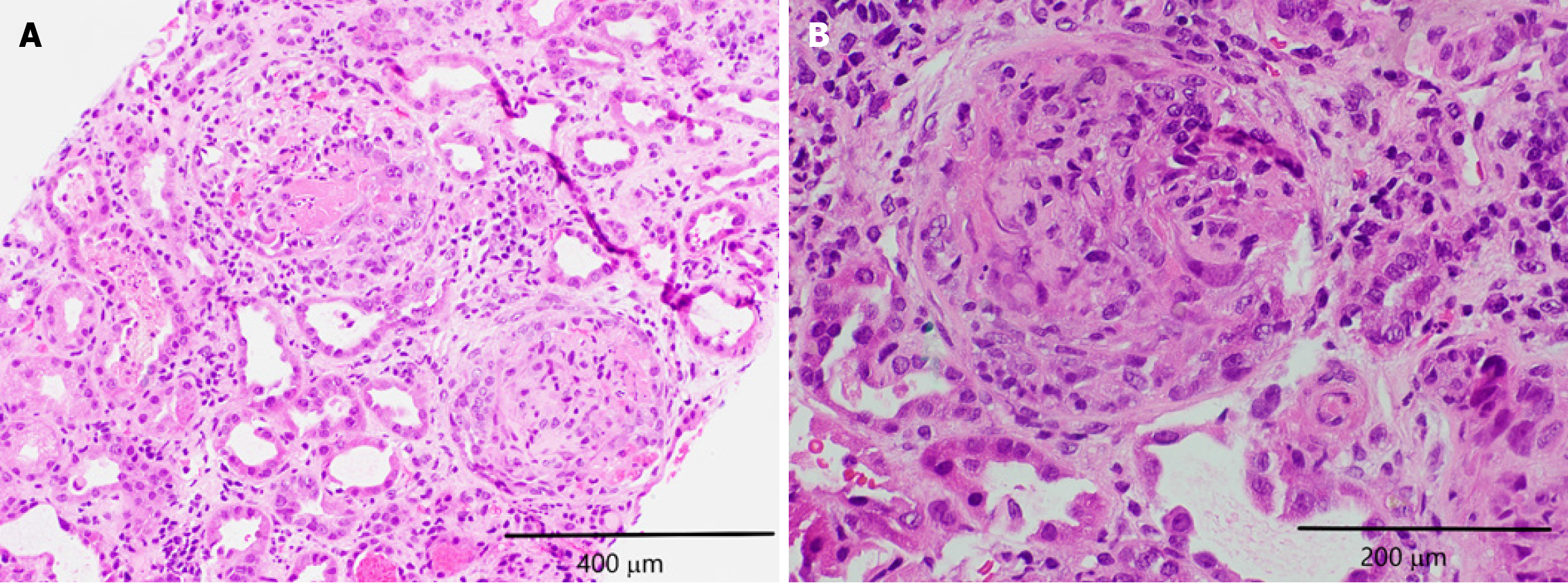
Renal ultrasound suggested possible right-sided medical renal disease. The renal biopsy revealed necrotizing and crescentic glomerulonephritis affecting more than 50% of the glomeruli as seen in Figure 1. Fibrocellular crescents were observed in 20 out of 27 glomeruli, 1 glomerulus (4%) was globally sclerotic. Approximately 10%-15% of the cortex had interstitial fibrosis and tubular atrophy. The renal vasculature showed medial thickening, consistent with moderate arteriolar nephrosclerosis. Immunofluorescence was negative for immunostaining as seen in Figure 2. Hematoxylin and eosin staining revealed glomeruli with fragments of necrotic nuclear contents due to karyorrhexis as seen in Figure 3. Electron microscopy did not show any electron dense deposits.
The clinical, serological, and histopathological findings were consistent with a diagnosis of myeloperoxidase-related ANCA-associated vasculitis with kidney and auricular involvement, in the setting of relapsing polychondritis.
The patient received a total of 2500 mg intravenous methylprednisolone over 3 days followed by oral prednisone. Induction treatment for ANCA vasculitis included intravenous cyclophosphamide induction, with plans for a total of 2 doses followed by transition to rituximab. The patient did not tolerate rituximab due to allergic reaction so IV cyclophosphamide was continued for a total of 6 doses (cumulative dose 3000 mg). The patient also started on Avacopan 30 mg twice daily at 3 months after diagnosis due to persistent severe AKI. Maintenance therapy included azathioprine in addition to Avacopan. Prednisone was gradually tapered off at 6 months.
After 13 months of follow up Cr has improved to 2.3 mg/dL, blood urea nitrogen of 38 mg/dL, and an urine protein-to-creatinine ratio (uPCR) of 1, indicating continued improvement in renal function. No side effects including infectious complications were noted. The patient felt well clinically with improvement in fatigue, appetite and leg swelling. Ear inflammation had also resolved. Laboratory data are detailed in Table 1.
| Day 0 | Day 10 | Day 40 | 3 months | 4 months | 9 months | 11 months | 13 months | |
| Serum creatinine | 4 | 6.16 | 4.06 | 2.6 | 2.72 | 2.42 | 2.52 | 2.32 |
| BUN | 57 | 141 | 61 | 46 | 47 | 46 | 40 | 38 |
| uPCR | 1.7 | NA | NA | 1.91 | 3.28 | N/A | 0.84 | 1 |
| Urine RBC (/hpf) | > 100 | 98 | 12 | 4 | 1 | N/A | 1 | 1 |
| Anti-MPO | > 8 | NA | 6.3 | 3.4 | N/A | N/A | 0.4 | N/A |
| P-ANCA | > 1:640 | NA | > 1:640 | < 1:20 | N/A | N/A | < 1:20 | N/A |
The pathogenesis of ANCA-associated vasculitis is well characterized and typically begins with a loss of self-tolerance to neutrophil antigens, often triggered by environmental stressors. This breakdown in tolerance leads to the production of ANCA antibodies, which promote neutrophil activation and result in endothelial injury[6]. The renal system is particularly vulnerable to damage in this condition due to its dense network of small blood vessels, making it highly susceptible to neutrophil-mediated inflammation. Further studies have shown that neutrophil activation in ANCA-associated vasculitis can also stimulate the release of factors that activate the alternative complement pathway, creating an amplification loop that primes additional neutrophils and exacerbates vascular inflammation[7]. Despite these insights, the association between relapsing polychondritis and ANCA-associated vasculitis remains poorly understood.
One possible mechanism that could be possible is that chronic immune stimulation in relapsing polychondritis may contribute to ANCA production, as has been observed in other autoimmune diseases such as rheumatoid arthritis[8]. This could be explained by the phenomenon called epitope spreading wherein chronic inflammation caused by ANCA-associated vasculitis could lead to development of an enhanced immune response to numerous new antigens leading to the production of autoantibodies to type II collagen commonly found in patients with relapsing polychondritis[9,10].
Another potential mechanism involves the release of neutrophil extracellular traps (NETs) by ANCA-activated neutrophils. These NETs are composed of chromatin fibers embedded with autoantigens such as myeloperoxidase, which can deposit in various organs and may serve as a trigger for vasculitis. Importantly, this inflammatory process may not be limited to blood vessels alone and could extend to cartilaginous tissues, potentially contributing to the inflammation observed in relapsing polychondritis[11].
Standard induction regimens for ANCA-associated vasculitis typically include high-dose glucocorticoids in combination with cyclophosphamide or rituximab. While these therapies are effective in inducing remission, their ability to restore kidney function, particularly in patients with advanced AKI, is often limited[3]. In this case, the patient developed a severe hypersensitivity reaction to rituximab, precluding its use as an induction or maintenance agent. Additionally, prolonged corticosteroid therapy is associated with substantial adverse effects, including weight gain, osteoporosis, and glucose intolerance, further complicating long-term management.
Avacopan, an oral C5a receptor antagonist, offers a therapeutic approach by targeting the complement pathway, a critical contributor to neutrophil activation in ANCA-associated vasculitis[12,13]. In this patient, initiation of Avacopan alongside a steroid taper was associated with sustained improvement in renal function, demonstrated by a marked reduction in both serum creatinine and uPCR. These findings are consistent with results from the ADVOCATE trial, which showed that Avacopan was noninferior to prednisone in achieving remission at 12 and 26 weeks, and superior in maintaining remission and reducing relapse rates at 52 weeks[3]. Additionally, a recent analysis of the ADVOCATE trial found that among patients with baseline estimated glomerular filtration rate ≤ 20, those treated with Avacopan experienced greater improvements in estimated glomerular filtration rate compared to those receiving prednisone a trend mirrored in our patient's clinical course[14].
This case highlights the potential utility of Avacopan in managing ANCA-associated vasculitis with coexisting relapsing polychondritis, particularly in severe renal involvement and when standard immunosuppressive regimens are either contraindicated or poorly tolerated. To our knowledge this is the first case report highlighting use of Avacopan in the treatment of relapsing polychondritis and concurrent glomerulonephritis. Further studies are warranted to explore the role of complement inhibition in optimizing renal outcomes in complex autoimmune overlap syndromes.
| 1. | Chauhan K, Surmachevska N, Hanna A. Relapsing Polychondritis. 2023 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 2. | File I, Trinn C, Mátyus Z, Ujhelyi L, Balla J, Mátyus J. Relapsing polychondritis with p-ANCA associated vasculitis: Which triggers the other? World J Clin Cases. 2014;2:912-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Chalkia A, Jayne D. ANCA-associated vasculitis-treatment standard. Nephrol Dial Transplant. 2024;39:944-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | van Leeuwen JR, Bredewold OW, van Dam LS, Werkman SL, Jonker JT, Geelhoed M, Langeveld APM, Remmelts HHF, van den Broecke MM, Ray A, Rabelink TJ, Teng YKO. Compassionate Use of Avacopan in Difficult-to-Treat Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Kidney Int Rep. 2022;7:624-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group. Avacopan for the Treatment of ANCA-Associated Vasculitis. N Engl J Med. 2021;384:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 691] [Article Influence: 138.2] [Reference Citation Analysis (0)] |
| 6. | Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, Kullman J, Lyons PA, Merkel PA, Savage COS, Specks U, Kain R. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020;6:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 638] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 7. | Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 448] [Article Influence: 23.6] [Reference Citation Analysis (9)] |
| 8. | Szilasi M, Mátyus J, File I, Szücs G, Rákóczi E, Pfliegler G, Szabó Z, Végh E, Szekanecz Z. Association of ANCA-associated vasculitis-rheumatoid arthritis overlap syndrome in four patients: rituximab may be the right choice? Autoimmunity. 2012;45:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Vanderlugt CJ, Miller SD. Epitope spreading. Curr Opin Immunol. 1996;8:831-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 286] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Ebringer R, Rook G, Swana GT, Bottazzo GF, Doniach D. Autoantibodies to cartilage and type II collagen in relapsing polychondritis and other rheumatic diseases. Ann Rheum Dis. 1981;40:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 92] [Reference Citation Analysis (0)] |
| 11. | Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1352] [Cited by in RCA: 1318] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 12. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012 . [PubMed] |
| 13. | Moiseev S, Lee JM, Zykova A, Bulanov N, Novikov P, Gitel E, Bulanova M, Safonova E, Shin JI, Kronbichler A, Jayne DRW. The alternative complement pathway in ANCA-associated vasculitis: further evidence and a meta-analysis. Clin Exp Immunol. 2020;202:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Cortazar FB, Niles JL, Jayne DRW, Merkel PA, Bruchfeld A, Yue H, Schall TJ, Bekker P; ADVOCATE Study Group. Renal Recovery for Patients with ANCA-Associated Vasculitis and Low eGFR in the ADVOCATE Trial of Avacopan. Kidney Int Rep. 2023;8:860-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/